Physicochemical Characterization and Biological Activities of the Triterpenic Mixture α,β-Amyrenone
Abstract
:1. Introduction
2. Results and Discussion
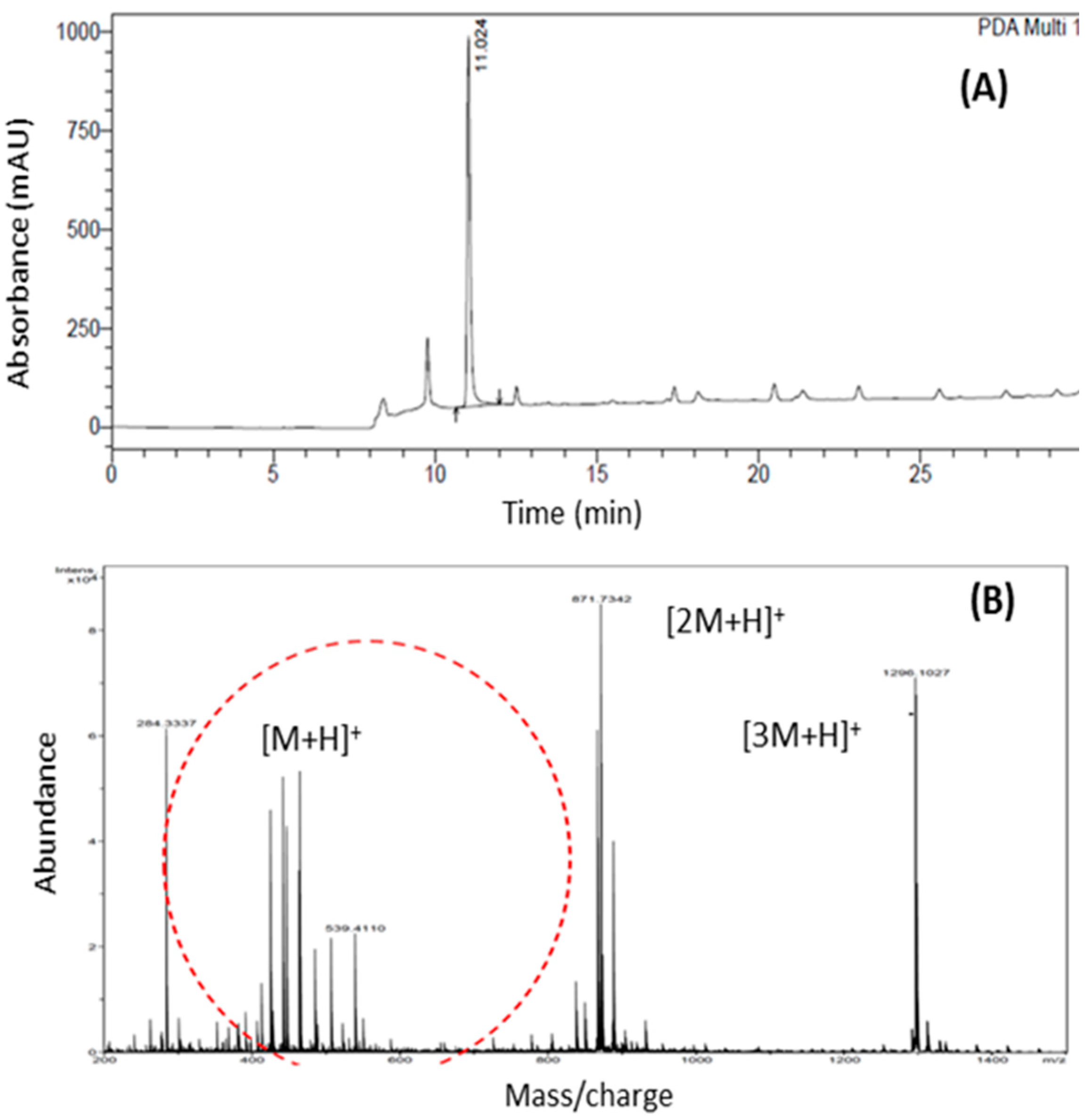
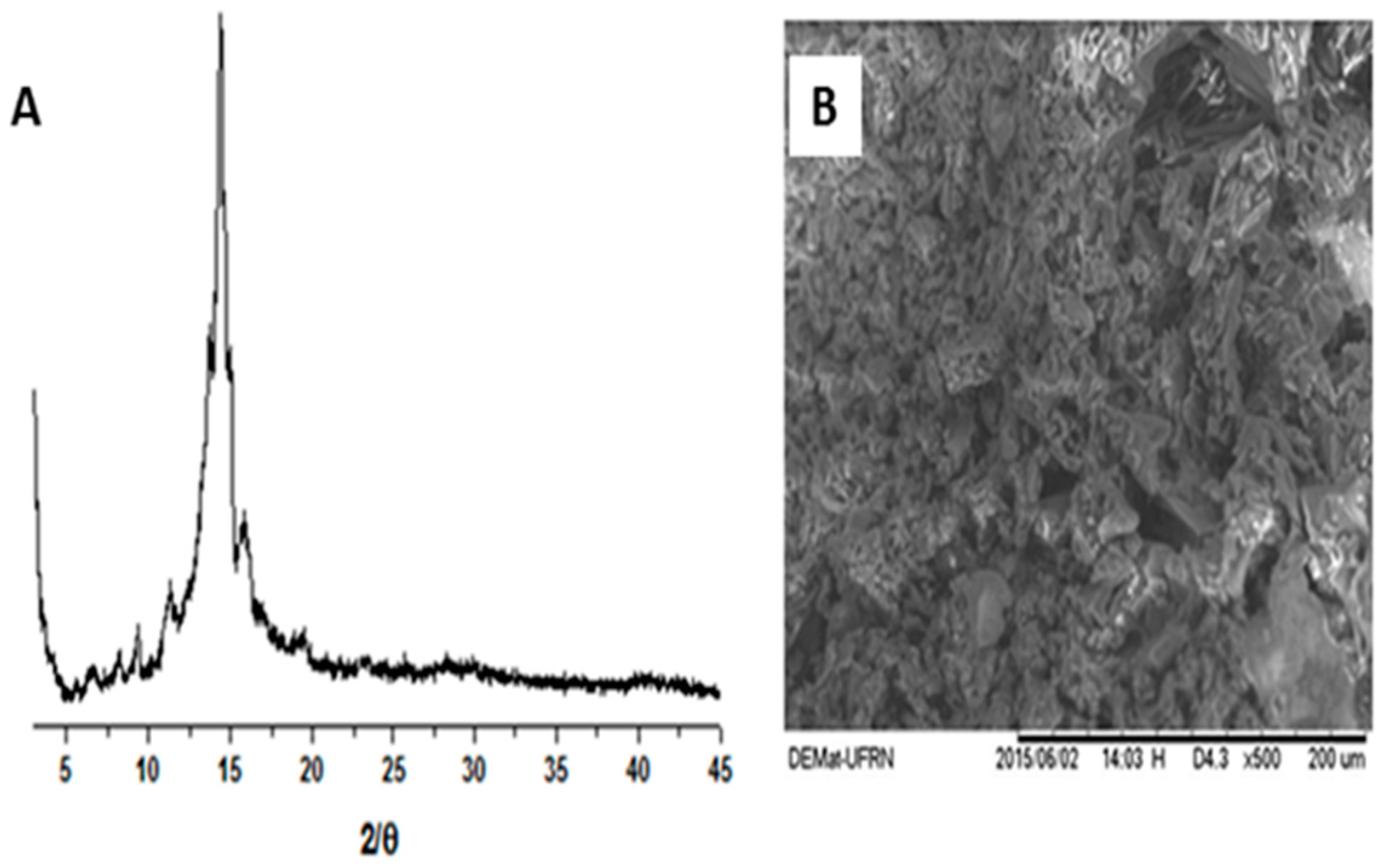
2.1. Physicochemical Characterization of α,β-Amyrenone
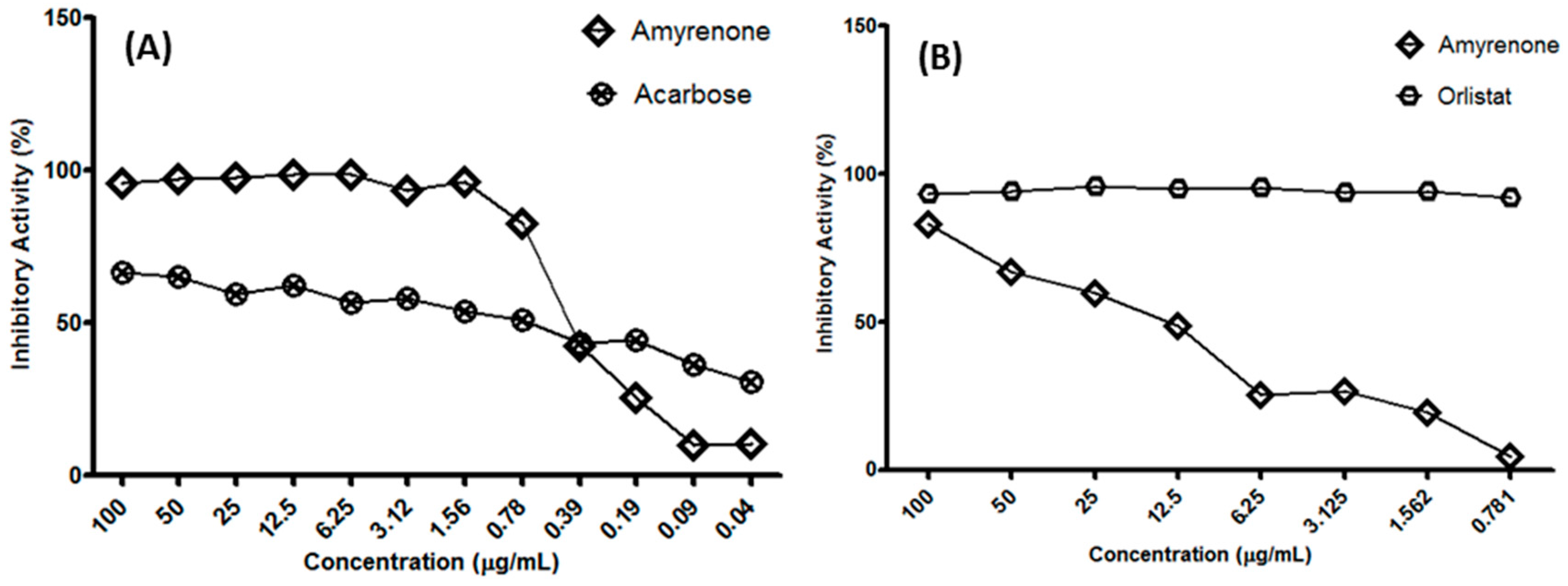
2.2. Biological Assays
3. Materials and Methods
3.1. General Information
3.2. Physico-Chemical Characterization
3.3. Enzymatic Inhibition Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rüdiger, A.L. Estudo Fitoquímico e citotóxico de Oleorresinas de Burseraceae. Ph.D. Thesis, Universidade Federal do Amazonas (UFAM), Manaus, Brazil, 2012. [Google Scholar]
- Siani, A.C. Chemical composition of South American burseraceae non-volatile oleoresins and preliminary solubility assessment of their commercial blend. Phytochem. Anal. 2012, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Buthani, K.K.; Kapoor, R.; Atal, C.K. Two unsymmetric tetracyclic tritrepenoids from Cissus quandrangularis. Phytochemistry 1984, 23, 407–410. [Google Scholar] [CrossRef]
- Verma, R.K.; Gupta, M.M. Lipid constituents of Tridax procumbens. Phytochemistry 1988, 27, 459–463. [Google Scholar] [CrossRef]
- Ling, T. New triterpenoids and other constituents from a special microbial-fermented tea—Fuzhuan brick tea. J. Agric. Food Chem. 2010, 58, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Mollataghi, A.; Coudiere, E.; Hamid, A.; Hadi, A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Bruni, S.; Guglielmi, V. Identification of archaeological triterpenic resins by the non-separative techniques FTIR and 13C-NMR: The case of Pistacia resin (mastic) in comparison with frankincense. Spectrochim. Acta A 2014, 121, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yao, H.; Li, W.; Mu, Q.; Li, H.; Hu, H.; Li, Y.; Huang, H. δ-Amyrone, a specific inhibitor of cyclooxygenase-2, exhibits anti-inflammatory effects in vitro and in vivo mice. Int. Immunopharmacol. 2014, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.M. Contribution of α,β-amyrenone to the anti-inflammatory and antihypersensitivity effects of Aleurites moluccana (L.) Willd. BioMed Res. Int. 2014. [Google Scholar] [CrossRef]
- Almeida, P.D.O.; Boleti, A.P.A.; Rüdiger, A.L.; Lourenço, G.A.; Veiga Junior, V.F.; Lima, E.S. Anti-inflammatory activity of triterpenes isolated from Protium paniculatum oil-resins. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef]
- Soldi, C.; Pizzolatti, M.G.; Luiz, A.P.; Marcon, R.; Meotti, F.C.; Mioto, L.A.; Santos, A.R.S. Synthetic derivatives of the alpha- and beta-amyrin triterpenes and their antinociceptive properties. Bioorg. Med. Chem. 2008, 16, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Li, B.; Lin, L.; Tundis, R.; Loizzo, M.R.; Zheng, B.; Xiao, J. Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato. Molecules 2016, 21, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Winley: New York, NY, USA, 2005. [Google Scholar]
- Anghel, M.; Vlase, G.; Bilanin, M.; Vlase, T.; Albu, P.; Fuliaş, A.; Tolan, I.; Nicolae, D. Comparative study on the thermal behavior of two similar triterpenes from birch. J. Therm. Anal. Calorim. 2013, 113, 1379–1385. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mazumdar, B. Feasibility and characterization of gummy exudates of Cochlospermum religiosum as pharmaceutical excipient. Ind. Crops Prod. 2013, 50, 776–786. [Google Scholar] [CrossRef]
- Brandão, F.C.; Tagiari, M.P.; Silva, M.A.S.; Berti, L.F.; Stulzer, H.K. Structure of chemical compounds, methods of analysis and process control. Pharm. Chem. J. 2008, 42, 368–376. [Google Scholar] [CrossRef]
- Rüdiger, A.L. Estudo Fitoquímico do óleo-Resina Exsudado de Espécies de Burseraceae. Master Thesis, Universidade Federal do Amazonas (UFAM), Manaus, Brazil, 2008. [Google Scholar]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTPe1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar]
- Dias, M.O.; Hamerski, L.; Pinto, A.C. Separação semipreparativa de α and β-amirina por cromatografia líquida de alta eficiência. Quim. Nova 2011, 34, 704–706. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Alpha glicosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J. Ethnopharmacol. 2008, 116, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janes, D.; Strukelj, B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Zaini, A.M.; Sadikun, A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.G.S.; Silva Júnior, W.F.; Veiga Junior, V.F.; Lima, Á.A.N.; Lima, E.S. Physicochemical Characterization and Biological Activities of the Triterpenic Mixture α,β-Amyrenone. Molecules 2017, 22, 298. https://doi.org/10.3390/molecules22020298
Ferreira RGS, Silva Júnior WF, Veiga Junior VF, Lima ÁAN, Lima ES. Physicochemical Characterization and Biological Activities of the Triterpenic Mixture α,β-Amyrenone. Molecules. 2017; 22(2):298. https://doi.org/10.3390/molecules22020298
Chicago/Turabian StyleFerreira, Rosilene G. S., Walter F. Silva Júnior, Valdir F. Veiga Junior, Ádley A. N. Lima, and Emerson S. Lima. 2017. "Physicochemical Characterization and Biological Activities of the Triterpenic Mixture α,β-Amyrenone" Molecules 22, no. 2: 298. https://doi.org/10.3390/molecules22020298