Calamintha nepeta (L.) Savi and its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry
Abstract
:1. Introduction
2. Calamintha: Species, Taxonomy, Occurrence and Uses
3. Calamintha Essential Oils
4. Calamintha nepeta (L.) Savi: Taxonomic Characterization, Distribution and Uses
Taxonomic Ambiguity and Synonyms of CN
5. Essential Oil Composition of CN
- (I)
- The first and the most abundant one consists of PUL as the major component associated with other compounds. Two main variants of this chemotype can be defined as follows: (1) the one presented by PUL and menthone and/or isomenthone, menthol and its isomers, and (2) the other where PUL is associated with piperitenone, or piperitone and piperitenone oxides;
- (II)
- The second chemotype can be considered as piperitone oxide or the piperitone oxide/piperitenone oxide one. A piperitone/piperitenone variant of this chemotype was only reported for some Croatian material [17];
- (III)
- Lastly, the rarest one is distinguished by the presence of carvone and 1,8-cineole.
6. Antimicrobial Activity of CN
7. Antioxidant Properties of EOCN
8. Insecticidal Activity of EOCN
9. Phytotoxic Potential of CN
10. Anti-Ulcer Potential of CN
11. Additional Bioactivities of CN
12. Pulegone—The Main EOCN Chemical Compound
13. Alternative Sources of PUL
14. Toxicokinetic Studies on PUL and its Metabolic Pathways
15. Antimicrobial Activity of PUL
16. Spasmolytic and Gastrointestinal-Related Activities of PUL
17. Insecticidal Propertis of PUL
18. Other Bioactivities of PUL
19. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch. Komplement./Res. Complement. Med. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Mahmood, M.S.; Akhtar, M. In vitro and in vivo evaluation of antimicrobial activities of essential oils extracted from some indigenous spices. Pak. Vet. J. 2013, 33, 413–417. [Google Scholar]
- Demirci, B.; Temel, H.E.; Portakal, T.; Kırmızıbekmez, H.; Demirci, F.; Başer, K.H.C. Inhibitory effect of Calamintha nepeta subsp. glandulosa essential oil on lipoxygenase. Turk. J. Biochem. 2011, 36, 290–295. [Google Scholar]
- Alan, S.; Ocak, A. Taxonomical and morphological studies on the genus Calamintha Miller (Lamiaceae) in Turkey. Biol. Divers. Conserv. 2009, 2, 125–143. [Google Scholar]
- Alan, S.; Kürkҫüoglu, M.; Hüsnü, K.; Baser, K. Composition of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta and Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball. Asian J. Chem. 2011, 23, 2357–2360. [Google Scholar]
- Marin, P.D.; Grayer, R.J.; Veitch, N.C.; Kite, G.C.; Harborne, J.B. Acacetin glycosides as taxonomic markers in Calamintha and Micromeria. Phytochemistry 2001, 58, 943–947. [Google Scholar] [CrossRef]
- Ball, P.W.; Getliffe, F. Calamintha Miller. In Flora Europaea; Tutin, T., Heywood, V., Burges, N., Moore, D., Valentine, D., Walters, S., Webb, D., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 3, pp. 166–167. [Google Scholar]
- Šilić, Č. Monografija Rodova Satureja L., Calamintha Miller, Micromeria Bentham, Acinos Miller i Clinopodium L. u Flori Jugoslavije; Zemaljski Muzej: Sarajevo, Bosnia and Herzegovina, 1979; pp. 1–440. [Google Scholar]
- Ćavar, S.; Vidić, D.; Maksimović, M. Volatile constituents, phenolic compounds, and antioxidant activity of Calamintha glandulosa (Req.) Bentham. J. Sci. Food Agric. 2013, 93, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, S. Flora d’Italia 2; Edagricole: Bologna, Italy, 1982; pp. 345–347. [Google Scholar]
- Bacchetta, G.; Brullo, S. Calamintha sandaliotica (Lamiaceae) a new species from Sardinia. An. Jard. Bot. Madr. 2005, 62, 135–141. [Google Scholar] [CrossRef]
- Chevallier, A. Encyclopedia of Medicinal Plants; Dorling Kindersley: London, UK, 2001; pp. 211–212. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present), 2nd ed.; Nobel Tıp Kitabevi: Istanbul, Turkey, 1999; pp. 1–371. [Google Scholar]
- Bown, D. The Herb Society of America—New Encyclopedia of Herbs & Their Uses; Dorling Kindersley: New York, NY, USA, 2001; pp. 1–448. [Google Scholar]
- Formisano, C.; Rigano, D.; Napolitano, F.; Senatore, F.; Apostolides, A.N.; Piozzi, F.; Rosselli, S. Volatile constituents of Calamintha origanifolia Boiss. growing wild in Lebanon. Nat. Prod. Commun. 2007, 2, 1253–1256. [Google Scholar]
- Mancini, E.; De Laura, M.; Malova, H.; De Vincenzo, F. Chemical composition and biological activities of the essential oil from Calamintha nepeta plants from the wild in southern Italy. Nat. Prod. Commun. 2013, 8, 139–142. [Google Scholar] [PubMed]
- Burzo, I.; Mihaescu, D.; Dobrescu, A.; Ambăruş, S.; Fălticeanu, M.; Bădulescu, L. Contribution to the Knowledge of the Composition of the Essential Oils from Five Calamintha Species Cultivated in Romania. Alexandru Ioan Cuza University of Iaşi, “Al. I. Cuza” din Iasi 2006, 52, 39–42. [Google Scholar]
- Small, E. Culinary Herbs; NRC Research Press: Ottawa, ON, Canada, 2006; pp. 1–236. [Google Scholar]
- Sarac, M.; Ugur, A. The in vitro antimicrobial activities of the essential oils of some Lamiaceae species from Turkey. J. Med. Food 2009, 12, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Branković, S.V.; Kitić, D.V.; Radenković, M.M.; Veljković, S.M.; Golubović, T.D. Calcium blocking activity as a mechanism of the spasmolytic effect of the essential oil of Calamintha glandulosa Šilić on the isolated rat ileum. Gen. Physiol. Biophys. 2009, 28, 174–178. [Google Scholar] [PubMed]
- De Ortiz Urbina, A.V.; Martín, M.L.; Montero, M.J.; Morán, A.; San Román, L. Sedating and antipyretic activity of essential oil of Calamintha sylvatica subsp. ascendens. J. Ethnopharmacol. 1989, 25, 165–171. [Google Scholar] [CrossRef]
- Viney, D.E. An Illustrated Flora of North Cyprus; Koeltz Scientific Books: Koenigstein, Germany, 1994; pp. 514–515. [Google Scholar]
- Iqbal, T.; Hussain, A.I.; Chatha, S.A.S.; Naqvi, S.A.R.; Bokhari, T.H. Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani flora. J. Anal. Methods Chem. 2013, 2013, 536490. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Trabelsi, N.; Noumi, E.; Snoussi, M.; Fallah, H.; Ksouri, R.; Bakhrouf, A. Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J. Microbiol. Biotechnol. 2009, 25, 2227–2238. [Google Scholar] [CrossRef]
- Kitić, D.; Stojanović, G.; Palić, R.; Ranđelović, V. Chemical composition and microbial activity of the essential oil of Calamintha nepeta (L.) Savi ssp. nepeta var. subisodonda (Borb.) Hayek from Serbia. J. Essent. Oil Res. 2005, 17, 701–703. [Google Scholar]
- Baldovini, N.; Ristorcelli, D.; Tomi, F.; Casanova, J. Intraspesific variability of the essential oil of Calamintha nepeta from Corsica (France). Flavour Fragr. J. 2000, 15, 50–54. [Google Scholar] [CrossRef]
- Cook, C.M.; Lanaras, T.; Kokkini, S. Essential oils of two Calamintha glandulosa (Req.) Bentham chemotypes in a wild population from Zakynthos, Greece. J. Essent. Oil Res. 2007, 19, 534–539. [Google Scholar] [CrossRef]
- Riela, S.; Bruno, M.; Formisano, C.; Rigano, D.; Rosselli, S.; Saladino, M.L.; Senatore, F. Effects of solvent-free microwave extraction on the chemical composition of essential oil of Calamintha nepeta (L.) Savi compared with the conventional production method. J. Sep. Sci. 2008, 31, 1110–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, E.; Sefidkon, F. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Eucalyptus sargentii. J. Agric. Sci. Technol. 2012, 14, 1035–1042. [Google Scholar]
- Wong, Y.C.; Ahmad-Mudzaqqir, M.Y.; Wan-Nurdiyana, W.A. Extraction of essential oil from cinnamon (Cinnamomum zeylanicum). Orient. J. Chem. 2014, 30, 37–47. [Google Scholar] [CrossRef]
- Pereira, C.G.; Gualtieri, I.P.; Maia, N.B.; Meireles, M.A.A. Supercritical extraction to obtain vetiver (Vetiveria zizanioides L. Nash) extracts from roots cultivated hydroponically. J. Agric. Sci. Technol. 2008, 2, 45–50. [Google Scholar]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Teles, S.; Pereira, J.A.; Santos, C.H.B.; Menezes, R.V.; Malheiro, R.; Lucchese, A.M.; Silva, F. Effect of geographical origin on the essential oil content and composition of fresh and dried Mentha × villosa Hudson leaves. Ind. Crops Prod. 2013, 46, 1–7. [Google Scholar] [CrossRef]
- Tibaldi, G.; Fontana, E.; Nicola, S. Postharvest management affects spearmint and calamint essential oils. J. Sci. Food Agric. 2013, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Irchhaiya, M.; Singh, R.; Kailasiya, P.P.; Kanaujia, V. Studies on antiulcer activity of essential oil of Calamintha officinalis Moench. Int. J. Res. Pharm. Sci. 2011, 2, 2733–2736. [Google Scholar]
- Ristorcelli, D.; Tomi, F.; Casanova, J. Essential oils of Calamintha nepeta subsp. nepeta and subsp. glandulosa from Corsica (France). J. Essent. Oil Res. 1996, 8, 363–366. [Google Scholar] [CrossRef]
- Şarer, E.; Pançalı, S.S. Composition of the essential oil from Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) R.W. Ball. Flavour Fragr. J. 1998, 13, 31–32. [Google Scholar] [CrossRef]
- De Pooter, H.L.; De Buyck, L.F.; Schamp, N.M. The volatiles of Calamintha nepeta subsp. glandulosa. Phytochemistry 1986, 25, 691–694. [Google Scholar] [CrossRef]
- De Pooter, H.L.; Schamp, N.M. Comparison of the volatile composition of some Calamintha/Satureja species. In Progress in Essential Oil Research, Proceedings of the International Symposium on Essential Oils, Holzminder/Neuhaus, Germany, 18–21 September, 1985; Brunke, E.J., Ed.; Walter de Gruyter: Berlin, Germany, 1986; pp. 139–150. [Google Scholar]
- De Pooter, H.L.; Goetghebeur, P.; Schamp, N. Variability in composition of the essential oil of Calamintha nepeta. Phytochemistry 1987, 26, 3355–3356. [Google Scholar] [CrossRef]
- Souleles, C.; Argyriadou, N.; Philianos, S. Constituents of the essential oil of Calamintha nepeta. J. Nat. Prod. 1987, 50, 510–511. [Google Scholar] [CrossRef]
- Akgül, A.; De Pooter, H.L.; De Buyck, L.F. The essential oils of Calamintha nepeta subsp. glandulosa and Ziziphora clinopodioides from Turkey. J. Essent. Oil Res. 1991, 3, 7–10. [Google Scholar]
- Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological assays of essential oils of Calamintha nepeta (L.) Savi subsp. nepeta (Lamiaceae). Nat. Prod. Res. 2010, 24, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Notarnicola, S.; De Bellis, L.; Miceli, A. Intraspecific variability of the essential oil of Calamintha nepeta subsp. nepeta from Southern Italy (Apulia). Nat. Prod. Res. 2013, 27, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Amira, S.; Dade, M.; Schinella, G.; Ríos, J.L. Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak. J. Pharm. Sci. 2012, 25, 65–72. [Google Scholar] [PubMed]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F.; Uzunov, D.; Solimene, U.; Menichini, F. Comparative chemical composition and antioxidant activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Nyman and Calamintha grandiflora (L.) Moench (Labiatae). Nat. Prod. Res. 2012, 25, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and antimicrobial properties of essential oils of four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef]
- Perrucci, S.; Mancianti, F.; Cioni, P.L.; Flamini, G.; Morelli, I.; Macchioni, G. In vitro antifugal activity of essential oils against some isolated of Microsporum canis and Microsporum gypseum. Planta Med. 1994, 60, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.L.; Puleio, R.; Morelli, I.; Panizzi, L. Antimicrobial activity of the essential oil of Calamintha nepeta and its constituent pulegone against bacteria and fungi. Phyther. Res. 1999, 13, 349–351. [Google Scholar] [CrossRef]
- Kitić, D.; Jovanović, T.; Ristić, M.; Palić, R.; Stojanović, G. Chemical composition and antimicrobial activity of the essential oil of Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) P.W. Ball from Montenegro. J. Essent. Oil Res. 2002, 14, 150–152. [Google Scholar]
- Miladinović, D.L.; Ilić, B.S.; Mihajilov-Krstev, T.M.; Nikolić, N.D.; Miladinović, L.C.; Cvetković, O.G. Investigation of the chemical composition-antibacterial activity relationship of essential oils by chemometric methods. Anal. Bioanal. Chem. 2012, 403, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Ceker, S.; Agar, G.; Alpsoy, L.; Nardemir, G.; Kizil, H.E. Protective role of essential oils of Calamintha nepeta L. on oxidative and genotoxic damage caused by Alfatoxin B1 in vitro. Fresenius Environ. Bull. 2013, 22, 3258–3263. [Google Scholar]
- Grieve, M.M. Calamint. In A Modern Herbal; Leyel, C.F., Ed.; Hafner Publishing: New York, NY, USA; London, UK, 1967; pp. 152–153. [Google Scholar]
- Johnson, T. Ethnobotany Desk Reference, 1st ed.; CRC Press: London, UK, 1991; p. 37. [Google Scholar]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal herbs for the treatment of rheumatic disorders—A survey of European herbals from the 16th and 17th century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Turolli, F. Erbe Vitali di Casa Nostra. In Tecniche Alimentari Alternative Di Prevenzione E Cura; Mursia, U., Ed.; Mursia (Gruppo Editoriale): Milan, Italy, 1981; pp. 205–206. [Google Scholar]
- Monforte, M.T.; Tzakou, O.; Nostro, A.; Zimbalatti, V.; Galati, E.M. Chemical composition and biological activities of Calamintha officinalis Moench essential oil. J. Med. Food 2011, 14, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, G. Plants for Human Consumption; Koeltz Scientific Books: Koenigstein, Germany, 1984; p. 234. [Google Scholar]
- Engler, H.G.A.; Prantl, K.A.E. Die Natürlichen Pflanzenfamilien, Teil 4, Abt. 3a; Wilhelm Engelmann: Leipzig, Germany, 1986; pp. 302–303. [Google Scholar]
- De Candole, A.P. Prodromus Systematis Naturalis Regni Vegetabilis, 12th ed.; Treuttel and Wurz: Paris, France, 1848; pp. 208–234. [Google Scholar]
- Boissier, P.E. Flora Orientalis; H. Georg: Basel/Geneva, Switzerland, 1879; Volume 4, pp. 562–583. [Google Scholar]
- Pagni, A.M.; Catalano, S.; Cioni, P.L.; Coppi, C.; Morelli, I. Etudes morpho-anatomiques et phytochimiques de Calamintha nepeta (L.) Savi (Labiétes). Plant. Med. Phytothér. 1990, 24, 203–213. [Google Scholar]
- Adzet, T.; Passet, J. Chemotaxonomie du genre Satureja-Calamintha. Riv. Ital. Essenz. EPPOS 1972, 54, 482–486. [Google Scholar]
- Velasco Neguerela, A.; Pérez Alonso, M.J. Estudio químico del aceite esencial de diversas Saturejae iberícas. An. Jord. Bot. Madr. 1983, 40, 107–118. [Google Scholar]
- Fraternale, D.; Giamperi, L.; Ricci, D.; Manunta, A. Composition of the essential oil as a taxonomic marker for Calamintha nepeta (L.) Savi ssp. Nepeta. J. Essent. Oil Res. 1998, 10, 568–570. [Google Scholar] [CrossRef]
- Garbari, F.; Jarvis, C.E.; Pagni, A.M. Typification of Melissa calamintha L, M. nepeta L, and Thymus glandulosus Req (Lamiaceae), with some systematic observations. Taxon 1991, 40, 499–504. [Google Scholar] [CrossRef]
- Karousou, R.; Hanlidou, E.; Lazari, D. Essential-oil diversity of three Calamintha species from Greece. Chem. Biodivers. 2012, 9, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Božović, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagno, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-Candida activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball—New approaches. Molecules 2017, 22, 203. [Google Scholar]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Statti, G.A.; Abenavoli, M.R. Phytotoxic activity of foliar volatiles and essential oils of Calamintha nepeta (L.) Savi. Nat. Prod. Res. 2012, 27, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Bellomaria, A.; Valentini, G. Composition of the essential oil of Calamintha nepeta ssp. Glandulosa. G. Bot. Ital. 1985, 119, 237–245. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Cioni, P.L.; Bader, A.; Marino, A.; Alonzo, V. Preservative properties of Calamintha officinalis essential oil with and without EDTA. Lett. Appl. Microbiol. 2002, 35, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, F.; Fellous, R.; Vernin, G.; Parkanyi, C. GC/MS analysis of the volatile constituents of Calamintha nepeta (L.) Savi ssp. nepeta from Southeastern France. J. Essent. Oil Res. 2000, 12, 481–486. [Google Scholar] [CrossRef]
- Kokkalou, E.; Stefanou, E. The volatile oil of Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) Ball, endemic to Greece. Flavour Fragr. J. 1990, 5, 23–26. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O. Essential oil of Calamintha nepeta subsp. glandulosa from Greece. J. Essent. Oil Res. 2001, 13, 11–12. [Google Scholar] [CrossRef]
- Kirimer, N.; Baser, K.H.C.; Özek, T.; Kürkçüoglu, M. Composition of the essential oil of Calamintha nepeta subsp. Glandulosa. J. Essent. Oil Res. 1992, 4, 189–190. [Google Scholar] [CrossRef]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Sahin, F. Chemical composition and antibacterial activity of essential oils of two species of Lamiaceae against phytopathogenic bacteria. Pol. J. Microbiol. 2015, 64, 121–127. [Google Scholar] [PubMed]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Yasar, S.; Fakir, H.; Erbas, S.; Karakus, B. Volatile constituents of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball. and Calamintha nepeta (L.) Savi subsp. nepeta from Mediterranean Region in Turkey. Asian J. Chem. 2011, 23, 3765–3766. [Google Scholar]
- Perez-Alonso, J.; Velasco-Negueruela, A.; Saez, J.A.L. The volatiles of two Calamintha species growing in Spain, Calamintha sylvatica Bromf. and C. nepeta (L.) Savi. Acta Hortic. 1992, 335, 255–260. [Google Scholar] [CrossRef]
- Popović, A.; Šućur, J.; Orčić, D.; Štrbac, P. Effects of esssential oil formulations on the adult insect Tribolium castaneum (herbst) (Col., Tenebrionideae). J. Cent. Eur. Agric. 2013, 14, 181–193. [Google Scholar]
- Stanić, G.; Blažević, N.; Brkić, D.; Lukač, G. The composition of essential oils of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball and Calamintha sylvatica Bromf. subsp. Sylvatica. Acta. Pharm. 1999, 49, 107–112. [Google Scholar]
- Mastelić, J.; Miloš, M.; Kuštrak, D.; Radonić, A. The essential oil and glycosidically bound volatile compounds of Calamintha nepeta (L.) Savi. Croat. Chem. Acta 1998, 71, 147–154. [Google Scholar]
- Nickavar, B.; Mojab, F. Hydrodistilled volatile constituents of Calamintha officinalis Moench from Iran. J. Essent. Oil-Bear. Plants 2005, 8, 23–27. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Akbarzadeh, M. Essential oil composition of Calamintha officinalis Moench from Iran. J. Essent. Oil-Bear. Plants 2007, 10, 494–498. [Google Scholar] [CrossRef]
- Bouchra, C.; Achouri, M.; Hassani, L.M.I.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar] [CrossRef]
- Satrani, B.; Abdellah, F.; Fechtal, M.; Talbi, M.; Blaghen, M.; Chaouch, A. Composition chimique et activité antimicrobienne des huiles essentielles de Satureja calamintha et Satureja alpine du Maroc. Ann. Falsif. Exp. Chim. 2001, 94, 241–250. [Google Scholar]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagán, R.; Laglaoui, A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Labiod, R.; Aouadi, S.; Bouhaddouda, N. Chemical composition and antifungal activity of essential oil from Satureja Calamintha nepeta against phytopathogens fungi. Int. J. Pharm. Pharm. Sci. 2015, 7, 208–211. [Google Scholar]
- Kerbouche, L.; Hazzit, M.; Baaliouamer, A. Essential oil of Satureja calamintha subsp. nepeta (L.) Briq. from Algeria: Analysis, antimicrobial and antioxidant activities. J. Biol. Act. Prod. Nat. 2013, 3, 266–272. [Google Scholar]
- Velasco-Negueruela, A.; Perez-Alonso, M.J.; Esteban, J.L.; Garcia Vallejo, M.C.; Zygadlo, J.A.; Guzman, C.A.; Ariza-Espinar, L. Essential oils of Calamintha nepeta (L) Savi and Mentha aff. suaveolens Ehrh. grown in Coroba, Argentina. J. Essent. Oil Res. 1996, 8, 81–84. [Google Scholar] [CrossRef]
- Thoppil, J.E. A menthone chemotype in Calamintha nepeta. J. Med. Aromat. Plant Sci. 1997, 19, 5–6. [Google Scholar]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Horváth, G.; Kovács, K.; Kocsis, B.; Kustos, I. Effect of Thyme (Thymus vulgaris L.) essential oil and its main constituents on the outer membrane protein composition of Erwinia strains studied with microfluid chip technology. Chromatographia 2009, 70, 1645–1650. [Google Scholar] [CrossRef]
- Kotan, R.; Cakir, A.; Dadasoglu, F.; Aydin, T.; Cakmakci, R.; Ozer, H.; Kordali, S.; Mete, E.; Dikbas, N. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J. Sci. Food Agric. 2010, 90, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Gormez, A.; Bozari, S.; Yanmis, D.; Gulluce, M.; Agar, G.; Sahin, F. Antibacterial activity and chemical composition of essential oil obtained from Nepeta nuda against phytopathogenic bacteria. J. Essent. Oil Res. 2013, 25, 149–153. [Google Scholar] [CrossRef]
- İşcan, G.; Ki̇ri̇mer, N.; Kürkcüoǧlu, M.; Başer, K.H.C.; Demi̇rci̇, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Simić, D.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Đarmati, Z.; Jankov, R.M. New assay system for detecting bioantimutagens in plant extracts. Arch. Biol. Sci. 1994, 46, 81–85. [Google Scholar]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary approach to determine the optimal time and period for extracting the essential oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef] [PubMed]
- Szalek, J.; Grzeskowiak, E.; Kozielczyk, E. Interactions between herbal and synthetic drugs, advantages and risks. Herba Pol. 2006, 52, 153–157. [Google Scholar]
- Kürkçüoglu, M.; Iscan, G.; Ozek, T.; Baser, K.H.C.; Alan, S. Composition and antimicrobial activity of the essential oils of Calamintha betulifolia Boiss. et Bal. J. Essent. Oil Res. 2007, 19, 285–287. [Google Scholar] [CrossRef]
- Bensouici, C.; Benmerache, A.; Chibani, S.; Kabouche, A.; Abuhamdah, S.; Semra, Z.; Kabouche, Z. Antibacterial activity and chemical composition of the essential oil of Satureja calamintha ssp. sylvatica from Jijel, Algeria. Pharm. Lett. 2013, 5, 224–227. [Google Scholar]
- Ortiz De Urbina, A.V.; Martín, M.L.; Montero, M.J.; Carón, R.; San Román, L. Pharmacologic screening and antimicrobial activity of the essential oil of Calamintha sylvatica subsp. Ascendens. J. Ethnopharmacol. 1988, 23, 323–328. [Google Scholar] [PubMed]
- Singh, P.P.; Jha, S.; Irchhaiya, R. Antidiabetic and antioxidant activity of hydroxycinnamic acids from Calamintha officinalis Moench. Med. Chem. Res. 2012, 21, 1717–1721. [Google Scholar] [CrossRef]
- Rabah, A.; Karima, K.; Nassima, L.; Hakim, B.; Besma, H.; Hacene, B. Effect of essential oils extracted from Satureja calamintha, Mentha pulegium and Juniperus phoenicea on in vitro methanogenesis and fermentation traits of vetch-oat hay. Afr. J. Environ. Sci. Technol. 2013, 7, 140–144. [Google Scholar]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.J.; Yáñez-Ruiz, D.R.; Duval, S.M.; McEwan, N.R.; Newbold, C.J. Plant extracts to manipulate rumen fermentation. Anim. Feed Sci. Technol. 2008, 147, 8–35. [Google Scholar] [CrossRef]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant bioactives for ruminant health and productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, L.; Calsamiglia, S.; Ferrer, A.; Losa, R. Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim. Feed Sci. Technol. 2005, 19, 29–41. [Google Scholar] [CrossRef]
- Macheboeuf, D.; Papon, Y.; Artuso-Schaan, M.; Mousset, J.L.; Cherel, R. Utilisation d’extraits végétaux (huiles essentielles et extrait de polyphénols) pour diminuer la dégradation ruminale des protéines-Étude in vitro. Rencontres Rech. Rumin. 2006, 13, 69–72. [Google Scholar]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Lorán, S.; Rota, C.; Pagán, R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov. Food Sci. Emerg. Technol. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Loran, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagan, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Espina, E.; Cherrat, L.; Hassani, M.; Laglaoui, A.; Conchello, P.; Pagán, R. Synergistic combination of essential oils from Morocco and physical treatments for microbial inactivation. Innov. Food Sci. Emerg. Technol. 2012, 16, 283–290. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Lorán, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea and Cyperus longus essential oils from Morocco. Food Res. Int. 2012, 45, 313–319. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Poolman, B.; Smid, E.J. S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crops Prod. 1995, 4, 23–31. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Kumar, T.R.S.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L. Role of the outer membrane of Escherichia coli AG100 and Pseudomonas aeruginosa NCTC 6749 and resistance/susceptibility to monoterpenes of similar chemical structure. J. Essent. Oil Res. 2001, 13, 380–386. [Google Scholar] [CrossRef]
- Neto, A.C.; Netto, J.C.; Pereira, P.S.; Pereira, A.M.S.; Taleb-Contini, S.H.; França, S.C.; Marques, M.O.M.; Beleboni, R.O. The role of polar phytocomplexes on anticonvulsant effects of leaf extracts of Lippia alba (Mill.) N.E. Brown chemotypes. J. Pharm. Pharmacol. 2009, 61, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. A multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flavour Fragr. J. 2013, 28, 144–154. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.J.; Haroutounian, S.A. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Ilić, B.S.; Miladinović, D.L.; Mihajilov-Krstev, T.M.; Nikolić, D.M.; Marković, M.S. Assessing essential oils food protection by chemometric analysis of antibacterial activity. In Proceedings of the International conference “Medicinal and Aromatic Plants in Generating of New Values in 21st Century”, Sarajevo, Bosnia and Herzegovina, 9–12 November 2011.
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the action of selected essential oil components on gram negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [PubMed]
- Lakušić, B.; Slavkovska, V.; Pavlović, M.; Milenković, M.; Stanković, J.A.; Couladis, M. Chemical composition and antimicrobial activity of the essential oil from Chaerophyllum aureum L. (Apiaceae). Nat. Prod. Commun. 2009, 4, 115–118. [Google Scholar] [PubMed]
- Conner, D.E.; Beuchat, L.R. Effects of essential oils from plants on growth of food spoilage yeasts. J. Food Sci. 1984, 49, 429–434. [Google Scholar] [CrossRef]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Vanden Berghe, D.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Petretto, G.L.; Fancello, F.; Zara, S.; Foddai, M.; Mangia, N.P.; Sanna, M.L.; Omer, E.A.; Menghini, L.; Chessa, M.; Pintore, G. Antimicrobial Activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. J. Food Sci. 2014, 79, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. Longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Sticher, O. Plant mono-, di- and sesquiterpenoids with pharmacological or therapeutical activity. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity; Wagner, H., Wolff, P., Eds.; Springer: Berlin, Germany, 1977; pp. 137–176. [Google Scholar]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Duru, M.E.; Öztürk, M.; Uǧur, A.; Ceylan, Ö. The constituents of essential oil and in vitro antimicrobial activity of Micromeria cilicica from Turkey. J. Ethnopharmacol. 2004, 94, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Foto, E.; Zilifdar, F.; Yeșilyurt, E.; Biypi, B.; Diril, N. The in vitro antibacterial activity of some extracts and fractions of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) P.W. Ball (Lamiaceae). In Proceedings of the 11th International Symposium on Pharmaceutical Sciences, Ancara, Turkey, 9–12 June 2015.
- Karaarsalan, D. Determining the Antibacterial, Antifungal and Antioxidant Activities of the Petroleum Ether, Ethanol and Methanol Extracts of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball. Master’s Thesis, Balıkesir University, Institute of Science, Balıkesir, Turkey, 2010. [Google Scholar]
- Berber, İ.; Avşar, C.; Çine, N.; Bozkurt, N.; Elmas, E. Determination of antibacterial and antifungal activities of methanolic extracts of some plants growing in Sinop. Karaelmas Sci. Eng. J. 2013, 3, 10–16. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Musolino, A.D.; Scuderi, F.; Pizzimenti, F.; Alonzo, V. Efficiency of Calamintha officinalis essential oil as preservative in two topical product types. J. Appl. Microbiol. 2004, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [PubMed]
- Russell, A.D.; Chopra, I. Understanding Antibacterial Action and Resistance, 2nd ed.; Hellis Horwood: Chichester, UK, 1996; pp. 105–107. [Google Scholar]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Orafidiya, L.O.; Oyedele, A.O.; Shittu, A.O.; Elujoba, A.A. The formulation of an effective topical antibacterial product containing Ocimum gratissimum leaf essential oil. Int. J. Pharm. 2001, 224, 177–183. [Google Scholar] [CrossRef]
- Brannan, D.K.; Dille, J.C.; Kaufman, D.J. Correlation of in vitro challenge testing with consumer use testing for cosmetic products. Appl. Environ. Microbiol. 1987, 53, 1827–1832. [Google Scholar] [PubMed]
- Ramarathnam, N.; Osawa, T.; Ochi, H.; Kawakishi, S. The contribution of plant food antioxidants to human health. Trends Food Sci. Technol. 1995, 6, 75–82. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Ahmad, I.; Abbasi, B.H. Free radical scavenging (DPPH) potential in nine Mentha species. Toxicol. Ind. Health 2012, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 47, 484–489. [Google Scholar]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- El Guiche, R.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimie, A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the south of Marocco. Int. J. New Technol. Res. 2015, 1, 7–11. [Google Scholar]
- Younes, N.; Siegers, C.P. Inhibitiry action of some flavonoids on enhanced spontaneous lipid peroxidation following glutathione depletion. Planta Med. 1981, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Das, N.P.; Pereira, T.A. Effects of flavonoids on thermal autoxidation of palm oil: Structure-activity relationships. J. Am. Oil Chem. Soc. 1990, 67, 255–258. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Arantes, S.; Piçarra, A.; Candeia, M.F.C.; Vaz, A.N.; Tinoco, M.T.; Cruz-Morais, J.; Martins, M.R. Antioxidant properties and analgesic and anti-inflammatory activities of Calamintha nepeta and Foeniculum vulgare. Port. Exp. Pathol. Assoc. 2015, 7, 57. [Google Scholar]
- Bougandoura, N.; Bendimerad, N. Evaluation de l’activité antioxydante des extraits aqueux et méthanolique de Satureja calamintha ssp. nepeta (L.) Briq. Nat. Technol. 2013, 9, 15. [Google Scholar]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, G. Piante Aromatiche. Profumi, Aromi, Sostanze Odorose Dermofunzionali per Uso Farmaceutico, Alimentare, Cosmetico ed Erboristico; Sepem: Milano, Italy, 1995; p. 266. [Google Scholar]
- Monforte, M.T.; Lanuzza, F.; Pergolizzi, S.; Mondello, F.; Tzakou, O.; Galati, E.M. Protective effect of Calamintha officinalis Moench leaves against alcohol-induced gastric mucosa injury in rats. Macroscopic, histologic and phytochemical analysis. Phytother. Res. 2012, 26, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.E.; Watts, B.M. The antioxidant activity of vegetable extracts I. flavone aglycones. J. Food Sci. 1964, 29, 27–33. [Google Scholar] [CrossRef]
- Shams Moattar, F.; Sariri, R.; Giahi, M.; Yaghmaee, P.; Ghafoori, H.; Jamalzadeh, L. Antioxidant and anti-proliferative activity of Calamintha officinalis extract on breast cancer cell line MCF-7. J. Biol. Sci. 2015, 15, 194–198. [Google Scholar]
- Aziz, E.E.; Abbas, M.H. Chemical composition and efficiency of five essential oils against the pulse beetle Callosobruchus maculatus (F.) on vignaradiata seeds. Am. J.Agric. Environ. Sci. 2010, 8, 411–419. [Google Scholar]
- Drapeau, J.; Fröhler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Ayvaz, A.; Sagdic, O.; Karaborklu, S.; Ozturk, I. Insecticidal activity of the essential oils from different plants against three stored-product insects. J. Insect Sci. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.K. Toxicity and Repellency of Essential Oils to the German Cockroach (Dictyoptera: Blattellidae). Master’s Thesis, Graduate Faculty of Auburn University, Auburn, Alabama, 2009. [Google Scholar]
- Phillips, A.K.; Appel, A.G. Fumigant toxicity of essential oils to the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2010, 103, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.K.; Appel, A.G.; Sims, S.R. Topical toxicity of essential oils to the German Cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2010, 103, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and genotoxic activities of oregano essential oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Rodríguez, S.; Reynaldi, F.; Ringuelet, J.; Córdoba, S.; Albo, G. In vitro activity of Laurus nobilis, Calamintha officinalis and Lippia alba against Ascosphaera apis: Evaluation of the potential toxic effects on adults and larvae of Apis mellifera. In Proceedings of the International Congress on Invertebrate Pathology and Microbial Controland the 49th Annual Meeting of the Society for Invertebrate Pathology, Buenos Aires, Argentina, 24–28 July 2012.
- Cheng, A.X.; Lou, Y.G.; Mao, Y.B.; Lu, S.; Wang, L.J.; Chen, X.Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.R.; Singh, H.P. Eucalypt oils for the control of Parthenium (Parthenium hysterophorus L.). Crop Prot. 1998, 17, 119–122. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Ramezani, H.; Kohli, R.K. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 2002, 141, 111–116. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Abenavoli, M.R. Individual and joint activity of terpenoids, isolated from Calamintha nepeta extract, on Arabidopsis thaliana. Nat. Prod. Res. 2013, 27, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Sorgonà, A.; Lupini, A.; Abenavoli, M.R. Screening of mediterranean wild plant species for allelopathic activity and their use as bio-herbicides. Allelopath. J. 2012, 29, 107–124. [Google Scholar]
- Araniti, F.; Lupini, A.; Mercati, F.; Statti, G.A.; Abenavoli, M.R. Calamintha nepeta L.(Savi) as source of phytotoxic compounds: Bio-guided fractionation in identifying biological active molecules. Acta Physiol. Plant. 2013, 35, 1979–1988. [Google Scholar] [CrossRef]
- Holm, L.; Plucknett, D.; Pancho, J.; Herberger, J. The World’s Worst Weeds. Distribution and Biology; Krieger Publishing Company: Malabar, FL, USA, 1977; Volume 32, p. 609. [Google Scholar]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Can. J. Plant Sci. 2004, 84, 631–668. [Google Scholar] [CrossRef]
- Talbert, R.E.; Burgos, N.R. History and management of herbicide-resistant barnyardgrass (Echinochloa crus-galli) in Arkansas rice. Weed Technol. 2007, 21, 324–331. [Google Scholar] [CrossRef]
- Goswami, M.; Kulshreshtha, M.; Rao, C.V.; Yadav, S.; Yadav, S. Anti-ulcer potential of Lawsonia inermis L. leaves against gastric ulcers in rats. J. Appl. Pharm. Sci. 2011, 1, 69–72. [Google Scholar]
- Rao, C.V.; Sairam, K.; Goel, R.K. Experimental evaluation of Bacopa monniera on rat gastric ulceration and secretion. Indian J. Physiol. Pharmacol. 2000, 44, 435–441. [Google Scholar] [PubMed]
- Sairam, K.; Rao, C.V.; Babu, M.D.; Goel, R.K. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine 2001, 8, 423–430. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kirimer, N.; Tümen, G. Pulegone-rich essential oils of Turkey—Review. J. Essent. Oil Res. 1998, 10, 1–8. [Google Scholar] [CrossRef]
- Özek, T. Composition of the Essential Oil of Micromeria congesta. Master’s Thesis, Anadolu University, Eskisehir, Turkey, 1990. [Google Scholar]
- Parmar, N.S.; Ghosh, M.N. Gastric antiulcer activity of (+)cyanidanol-3, a histidine decarboxylase inhibitor. Eur. J. Pharmacol. 1981, 69, 25–32. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Forest, F.; Hiramatsu, K.; Sugimoto, M. Effect of pear (Pyrus communis L.) procyanidins on gastric lesions induced by HCl/ethanol in rats. Food Chem. 2007, 100, 255–263. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hasegawa, C.; Kawasuji, T.; Suzuki, H.; Saito, H.; Sagioka, T.; Takahashi, R.; Tsukamoto, H.; Morikawa, T.; Akiyama, T. Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol. Pharm. Bull. 2000, 23, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Sertié, J.A.A.; Carvalho, J.C.; Panizza, S. Antiulcer activity of the crude extract from the leaves of Casearia sylvestris. Pharm. Biol. 2000, 38, 112–119. [Google Scholar] [CrossRef]
- Murakami, S.; Muramatsu, M.; Tomisawa, K. Inhibition of gastric H+,K+-ATPase by flavonoids: A structure-activity study. J. Enzym. Inhib. 1999, 14, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on vascular smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [PubMed]
- Santos, M.; Carvalho, A.; Medeiros, I.; Alves, P.; Marchioro, M.; Antoniolli, A. Cardiovascular effects of Hyptis fruticosa essential oil in rats. Fitoterapia 2007, 78, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zilifdar, F.; Foto, E.; Yesilyurt, E.B.; Biyik, B.; Diril, N. Comparative study of in vitro cytotoxic and anti-cytotoxic activities of extracts of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball. (Lamiaceae). In Proceedings of the 11th International Symposium on Pharmaceutical Sciences, Ankara, Turkey, 9–12 June 2015.
- Savill, J.; Haslett, C. Granulocytes. In Apoptosis and Inflammation; Winkler, J.D., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 53–84. [Google Scholar]
- Nascimento, N.C.; Fragoso, V.; Moura, D.J. Antioxidant and antimutagenic effects of the crude foliar extract and the alkaloid brachycerine of Psychotria brachyceras. Environ. Mol. Mutagen. 2007, 48, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, V.; Nascimento, N.C.; Moura, D.J. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell. Toxicol. In Vitro 2008, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lemhadri, A.; Zeggwagh, N.A.; Maghrani, M.; Jouad, H.; Michel, J.B.; Eddouks, M. Hypoglycaemic effect of Calamintha officinalis Moench. in normal and streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2004, 56, 795. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Clark, Y.L. Changes in body temperature after administration of antipyretics, LSD, D9-THC, CNS depressants and stimulants, hormones, inorganic ions, gases, 2,4-DNP and miscellaneous agents. Neurosci. Biobehav. Rev. 1981, 5, 1–136. [Google Scholar] [CrossRef]
- Phillipson, J.D. Phytochemistry and medicinal plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Carlini, E.A. Plants and the central nervous system. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef]
- Koo, B.; Park, K.; Ha, J.; Park, J. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biol. Pharm. Bull. 2003, 26, 9778–9782. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Raphael, E.; Brocksom, U.; Brocksom, T.J. Sedative effect of monoterpene alcohols in mice: A preliminary screening. Z. Naturforsch. Sect. C J. Biosci. 2007, 62, 563–566. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jäger, W.; Gruber, A.; Dietrich, H. R-(+)- and S-(−)-carvone: Influence of chirality on locomotion activity in mice. Flavour Fragr. J. 2005, 20, 686–689. [Google Scholar] [CrossRef]
- De Sousa, D.P.; De Farias Nóbrega, F.F.; De Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Mint: The Genus Mentha; Taylor Fr. Group: Boca Raton, FL, USA, 2006; p. 576. [Google Scholar]
- Barceloux, D.G. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 563–567. [Google Scholar]
- Benlarbi, K.H.; Elmtili, N.; Macías, F.A.; Galindo, J.C.G. Influence of in vitro growth conditions in the production of defence compounds in Mentha pulegium L. Phytochem. Lett. 2014, 8, 233–244. [Google Scholar] [CrossRef]
- Bandini, P.; Pacchiani, M. Constituents, properties and use of Calamintha nepeta. Essenze Deriv. Agrum. 1981, 51, 325–330. [Google Scholar]
- Croteau, R. Biosynthesis and catabolism of monoterpenoids. Chem. Rev. 1987, 87, 929–954. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Sá, I.C.G.; Duarte, L.P.; Oliveira, F.F. Constituintes voláteis de Mentha pulegium L. e Plectranthus amboinicus (Lour.) Spreng. Rev. Bras. Plantas Med. 2011, 13, 165–169. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Croteau, R.B. Menthofuran regulates essential oil biosynthesis in peppermint by controlling a downstream monoterpene reductase. Proc. Natl. Acad. Sci. USA 2003, 100, 14481–14486. [Google Scholar] [CrossRef] [PubMed]
- Nair, B. Final report on the safety assessment of Mentha Piperita (Peppermint) Oil, Mentha Piperita (Peppermint) Leaf Extract, Mentha Piperita (Peppermint) Leaf, and Mentha Piperita (Peppermint) Leaf Water. Int. J. Toxicol. 2001, 20, 61–73. [Google Scholar] [PubMed]
- Hinou, J.B.; Harvala, C.E.; Hinou, E.B. Antimicrobial activity screening of 32 common constituents of essential oils. Pharmazie 1989, 44, 302–303. [Google Scholar] [PubMed]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Scortichini, M.; Rossi, M.P. Preliminary in vitro evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora (Burrill) Winslow et al. J. Appl. Bacteriol. 1991, 71, 109–112. [Google Scholar] [CrossRef]
- Kalođera, Z.; Pepeljnjak, S.; Vladimir, S.; Blažević, N. Antimicrobial activity of essential oil from Micromeria thymifolia (Scop.) Fritsch. Pharmazie 1994, 49, 376–377. [Google Scholar] [PubMed]
- Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R.; Lima, E.O. Preliminary study of the antimicrobial activity of Mentha × villosa Hudson essential oil, rotundifolone and its analogues. Culture 2006, 16, 307–311. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Bhardwaj, S.; Dhar, K.L. Synthesis and characterization of novel pulegone derivatives as substitutes of 4-(1,1 dimethylethyl) cyclohexan-1-ol acetate. J. Pharm. Res. 2011, 4, 19–21. [Google Scholar]
- González-Chávez, M.M.; Cárdenas-Ortega, N.C.; Méndez-Ramos, C.A.; Pérez-Gutiérrez, S. Fungicidal properties of the essential oil of Hesperozygis marifolia on Aspergillus flavus Link. Molecules 2011, 16, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- De Urbina, A.V.O.; Martin, M.L.; Montero, M.J.; Carron, R.; Sevilla, M.A.; San Roman, L. Antihistaminic activity of pulegone on the guinea-pig ileum. J. Pharm. Pharmacol. 1990, 42, 295–296. [Google Scholar] [CrossRef]
- Wenzel, D.G.; Ross, C.R. Central stimulating properties of some terpenones. J. Am. Pharm. Assoc. 1957, 46, 77–82. [Google Scholar] [CrossRef]
- Umezu, T. Evidence for dopamine involvement in ambulation promoted by pulegone in mice. Pharmacol. Biochem. Behav. 2010, 94, 497–502. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Nóbrega, F.F.F.; De Lima, M.R.V.; De Almeida, R.N. Pharmacological activity of (R)-(+)-pulegone, a chemical constituent of essential oils. Z. Naturforsch. 2011, 66, 353–359. [Google Scholar] [CrossRef]
- Gordon, W.P.; Forte, A.J.; McMurtry, R.J.; Gal, J.; Nelson, S.D. Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse. Toxicol. Appl. Pharmacol. 1982, 65, 413–424. [Google Scholar] [CrossRef]
- Thorup, I.; Würtzen, G.; Carstensen, J.; Olsen, P. Short term toxicity study in rats dosed with pulegone and menthol. Toxicol. Lett. 1983, 19, 207–210. [Google Scholar] [CrossRef]
- Thomassen, D.; Slattery, J.T.; Nelson, S.D. Menthofuran-dependent and independent aspects of pulegone hepatotoxicity: Roles of glutathione. J. Pharmacol. Exp. Ther. 1990, 253, 567–572. [Google Scholar] [PubMed]
- Watt, M. Natural Toxins in Traditional Medicines Some Myths Removed; Aromatic Thyms: Tulsa, OK, USA, 1995; pp. 22–30. [Google Scholar]
- Bruneton, J. Pharmacognosie: Phytochimie, Plantes Médicinales, 4th ed.; Tec. & Doc. Lavoisier: Paris, France, 2009; p. 1268. [Google Scholar]
- Peixoto, I.T.A.; Furletti, V.F.; Anibal, P.C.; Figueira, G.M.; Sartoratto, A.; de Busato Feiria, S.N.; Duarte, M.C.T.; Höfling, J.F. A survey of essential oils from Mentha spp. as an antimicrobial potential agent against Candida species. Adv. Med. Plant Res. 2016, 4, 58–72. [Google Scholar]
- Imaizumi, K.; Hanada, K.; Mawatari, K.; Sugano, M. Effect of essential oils on the concentration of serum lipids and apolipoproteins in rats. Agric. Biol. Chem. 1985, 49, 2795–2796. [Google Scholar]
- Madyastha, P.; Moorthy, B.; Vaidyanathan, C.S.; Madyastha, K.M. In vivo and in vitro destruction of rat liver cytochrome P-450 by a monoterpene ketone, pulegone. Biochem. Biophys. Res. Commun. 1985, 128, 921–927. [Google Scholar] [CrossRef]
- Lindemann, W. Ueber die Wirkungen des Oleum Pulegii. Naunyn-Schmiedeberg’s Arch. Exp. Pathol. Pharmakol. 1899, 42, 356–374. [Google Scholar] [CrossRef]
- Soares, P.M.G.; Assreuy, A.M.S.; Souza, E.P.; Lima, R.F.; Silva, T.O.; Fontenele, S.R.; Criddle, D.N. Inhibitory effects of the essential oil of Mentha pulegium on the isolated rat myometrium. Planta Med. 2005, 71, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.D. Mechanisms of the formation and disposition of reactive metabolites that can cause acute liver injury. Drug Metab. Rev. 1995, 27, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.R. Evaluation of d-pulegone as an avian repellent. J. Wildl. Manag. 1990, 54, 130–135. [Google Scholar] [CrossRef]
- Lee, S.; Tsao, R.; Peterson, C.; Coats, J.R. Insecticidal activity of monoterpenoids to western corn rootworm (Coleoptera: Chrysomelidae), twospotted spider mite (Acari: Tetranychidae), and house fly (Diptera: Muscidae). J. Econ. Entomol. 1997, 90, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Harwood, S.H.; Moldenke, A.F.; Berry, R.E. Toxicity of peppermint monoterpenes to the variegated cutworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1990, 83, 1761–1767. [Google Scholar] [CrossRef]
- Oka, Y.; Nacar, S.; Putievsky, E.; Ravid, U.; Yaniv, Z.; Spiegel, Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000, 90, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Spencer, G.F. Volatile monoterpenes As potential parent structures for new herbicides. Weed Sci. 1993, 41, 114–119. [Google Scholar]
- Farley, D.R.; Howland, V. The natural variation of the pulegone content in various oils of peppermint. J. Sci. Food Agric. 1980, 31, 1143–1151. [Google Scholar] [CrossRef]
- Weglarz, Z.; Zalecki, R. Investigations of dependence of the crop season of peppermint (Mentha piperita L.) herb upon the crop itself and the quality of the raw material. Herba Pol. 1985, 31, 175–180. [Google Scholar]
- Voirin, B.; Brun, N.; Bayet, C. Effects of day length on the monoterpene. composition of leaves of Mentha × piperita. Phytochemistry 1990, 29, 749–755. [Google Scholar] [CrossRef]
- Kumar, S.; Bahl, J.R.; Bansal, R.P.; Kukreja, A.K.; Garg, S.N.; Naqvi, A.A. Profits of Indian menthol mint Mentha arvensis cultivars at different stages of crop growth in northern plains. J. Med. Aromat. Plant Sci. 2000, 22, 774–786. [Google Scholar]
- Guenther, E. The Essential Oils; D. Van Nostrand Company Inc.: New York, NY, USA, 1949; pp. 1–777. [Google Scholar]
- Smith, D.M.; Levi, L. Essential oils, treatment of compositional data for the characterization of essential oils; Determination of geographical origins of peppermint oils by gas chromatographic analysis. J. Agric. Food Chem. 1961, 9, 230–244. [Google Scholar] [CrossRef]
- Smith, D.M.; Skakum, W.; Levi, L. Determination of botanical and geographical origin of spearmint oils by gas chromatographic and ultraviolet analysis. J. Agric. Food Chem. 1963, 11, 268–276. [Google Scholar] [CrossRef]
- Von Hefendehl, F.W.; Ziegler, E. Analysis of peppermint oil. Deuterob. LEB 1975, 8, 287–290. [Google Scholar]
- Kokkini, S.; Hanlidou, E.; Karousou, R.; Lanaras, T. Variation of pulegone content in pennyroyal (Mentha pulegium L.) plants growing wild in Greece. J. Essent. Oil Res. 2002, 14, 224–227. [Google Scholar] [CrossRef]
- Lorenzo, D.; Paz, D.; Dellacassa, E.; Davies, P.; Vila, R.; Cañigueral, S. Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz. Arch. Biol. Technol. 2002, 45, 519–524. [Google Scholar] [CrossRef]
- Zekri, N.; Amalich, S.; Boughdad, A.; El Belghiti, M.A.; Zair, T. Phytochemical study and fumigant toxicity of Mentha suaveolens Ehrh essential oil from Morocco against adults of S. oryzae (L.). Aust. J. Basic Appl. Sci. 2013, 7, 599–606. [Google Scholar]
- Sardashti, A.; Adhami, Y. Chemical composition of the essential oil of Mentha pulegium L. from Taftan Area by means of gas chromatography/mass spectrometry (GC/MS). J. Med. Plant Res. 2013, 7, 3003–3007. [Google Scholar]
- Rocha, D.; Novo, M.; Matos, O.; Figueiredo, A.C.; Delgado, M.; Cabral, M.D.; Liberato, M.; Moiteiro, C. Potential of Mentha pulegium for mosquito control. Rev. Ciênc. Agrár. 2015, 38, 155–165. [Google Scholar]
- Bellakhdar, J.; Berrada, M.; Holeman, M.; Ilidrissi, A.; Pinel, R. Qualitative and quantitative analysis of the oil from Mentha rotundifolia (L.) Hudson ssp. rotundifolia var. typica. Planta Med. Phytother. 1983, 17, 33–39. [Google Scholar]
- Umemoto, K.; Nagasawa, T. Essential oil of Mentha gentilis L. containing pulegone-methone-neomenthol as major components. Nippon Nogei Kagaku Kaishi 1987, 53, 269–271. [Google Scholar] [CrossRef]
- Von Rudloff, E.; Hefendehl, F.W. Gas-Liquid Chromatography of terpenes: XV. The volatile oil of Mentha arvensis var. glabra Ray. Can. J. Chem. 1966, 44, 2015–2022. [Google Scholar] [CrossRef]
- Schnelle, F.J.; Hörster, H. GLC-Analyse des ätherischen öles von Mentha requinii. Planta Med. 1968, 16, 48–53. [Google Scholar] [CrossRef]
- Fleisher, A.; Fleisher, Z. The essential oils from Mentha longifolia growing in Sinai and lsreal; Aromatic plants of the Holy Land and the Sinai part IV. J. Essent. Oil Res. 1991, 3, 57–58. [Google Scholar] [CrossRef]
- Salman, M.; Abdel-Hameed, E.S.; Bazaid, S.; Dabi, M.M. Chemical composition for hydrodistillation essential oil of Mentha longifolia by gas chromatography-mass spectrometry from north regions in Kingdom of Saudi Arabia. Pharm. Chem. 2015, 7, 34–40. [Google Scholar]
- Virmani, O.P.; Datta, S.C. Oil of spearmint. Perfum. Essent. Oil Res. 1968, 59, 351–362. [Google Scholar]
- Turner, G.W. Organization of monoterpene biosynthesis in Mentha; immunocytochemical localizations of geranyl diphosphate synthase, limonene-6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiol. 2004, 136, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Alankar, S. A review on peppermint oil. Asian J. Pharm. Clin. Res. 2009, 2, 27–33. [Google Scholar]
- Mkaddem, M.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J. Food Sci. 2009, 74, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Boukhebti, H.; Chaker, A.N.; Belhadj, H.; Sahli, F.; Ramdhani, M.; Laouer, H.; Harzallah, D. Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Pharm. Lett. 2011, 3, 267–275. [Google Scholar]
- Benayad, N.; Ebrahim, W.; Hakiki, A.; Mosaddak, M. Chemical characterization and insecticidal evaluation of the essential oil of Mentha suaveolens Ehrh. and Mentha pulegium L. growing in Morocco. Food Ind. 2012, 13, 27–32. [Google Scholar]
- Talbaoui, A.; Jamaly, N.; Aneb, M.; Il Idrissi, A.; Bouksaim, M.; Gmouh, S.; Amzazi, S.; El Moussaouiti, M.; Benjouad, A.; Bakri, Y. Chemical composition and antibacterial activity of essential oils from six Moroccan plants. J. Med. Plants Res. 2012, 6, 4593–4600. [Google Scholar] [CrossRef]
- Al-Tawaha, A.; Al-Karaki, G.; Massadeh, A. Comparative response of essential oil composition, antioxidant activity and phenolic contents spearmint (Mentha spicata L.) under protected soilless vs. open field conditions. Adv. Environ. Biol. 2013, 7, 902–910. [Google Scholar]
- Muñoz, S.; Collin, G.J.; Gagnon, M.; Ferrufino, J.S. The essential oils of Hedeoma mandoniana Wedd and of Minthostachys andina (Brett) Epling. J. Essent. Oil Res. 1990, 2, 61–66. [Google Scholar] [CrossRef]
- Firmage, D.H. Environmental influences on the monoterpene variation in Hedeoma drummondii. Biochem. Syst. Ecol. 1981, 9, 53–58. [Google Scholar] [CrossRef]
- Souleles, C.; Argyriadou, N. The volatile constituents of Calamintha grandiflora. Planta Med. 1990, 56, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Carnat, A.P.; Cossegros, A.; Lamaison, J.L. The essential oil of Satureja grandiflora (L.) Scheele from France. J. Essent. Oil Res. 1991, 3, 361. [Google Scholar] [CrossRef]
- Baser, K.H.C. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure Appl. Chem. 2002, 74, 527–545. [Google Scholar] [CrossRef]
- Alan, S.; Kürkçüoğlu, M.; Başer, K.H.C. The composition of the essential oils of Calamintha pamphylica subspecies. Turk. J. Biol. 2011, 35, 259–265. [Google Scholar]
- Pavlović, S.; Živanović, P.; Jančić, R.; Kuznetsova, G.; Vujčić, S.; Shevarda, A. Study of the species Acinos majoranifolius (Mill.) Šilić (Lamiaceae) from Orjen Mountain, as a new source of essential oils. Arh. Farm. (Belgrad.) 1984, 34, 27–33. [Google Scholar]
- Pavlović, S.; Kuznetsova, G.; Živanović, P.; Shevarda, A.; Jančić, R.; Vujčić, S. Content and composition of essential oil and some anatomical characteristics of plants of the species Acinos suaveolens (Sibth and SM) G. Don fil. Arh. Farm. (Belgrad.) 1984, 34, 65–71. [Google Scholar]
- Kokkalou, E. Composition of the volatile oil from Acinos suaveolens. Planta Med. 1988, 54, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Kokkalou, E.; Kapetanidis, I. Flavonoids of the aerial parts of Acinos suaveolens (Sibth. et Smith) G. Don Fil. (Lamiaceae). Pharm. Acta Helv. 1988, 63, 170–173. [Google Scholar]
- Tümen, G. The volatile constituents of Acinos suaveolens (Sibt. Et Smith) G. Don fil growing in Turkey. J. Essent. Oil Res. 1991, 3, 191–192. [Google Scholar] [CrossRef]
- Souleles, C.; Katsiotis, S. Study on the essential oil of Acinos arvensis (Lam.) Dandy. Plantes Med. Phytother. 1988, 22, 180–182. [Google Scholar]
- Kaya, A.; Bașer, K.H.; Tümen, G.; Koca, F. The essential oil of Acinos suaveolens (Sm.) G. Don fil., Acinos arvensis (Lam.) Dandy and Acinos rotundifolius Pers. growing wild in Turkey. Flavour Frag. J. 1999, 14, 60–64. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Demetzos, C.; Perdetzoglou, D. Chemical composition and antibacterial activity of the oil of Acinos suaveolens (Sibth. et Sm.) G. Don f. from Greece. J. Essent. Oil Res. 2002, 14, 139–140. [Google Scholar] [CrossRef]
- Puri, H.S.; Pain, S.P. Micromeria capitellata Benth.: A new source of pulegone. Perfum. Cosmet. 1988, 693, 163. [Google Scholar]
- Kirimer, N.; Özek, T.; Bașer, K.H.C.; Harmandar, M. The essential oil of Micromeria fruticosa (L.) Druce subsp. serpyllipholia (Bieb.) P.H. Davis. J. Essent. Oil Res. 1993, 5, 199–200. [Google Scholar] [CrossRef]
- Marinković, B.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Marin, P.D.; Soković, M.; Duletić-Laušević, S. Antibacterial activity of the essential oil of Micromeria thymifolia and M. albanica (Lamiaceae). Bocconea 2003, 16, 1131–1134. [Google Scholar]
- Rojas, L.B.; Usubillaga, A. Composition of the essential oil of Satureja brownei (SW.) Briq. from Venezuela. Flavour Fragr. J. 2000, 15, 21–22. [Google Scholar] [CrossRef]
- Muschietti, L.; Van Baren, C.; Coussio, J.; Vila, R.; Clos, M.; Cañigueral, S.; Adzet, T. Chemical composition of the leaf oil of Satureja odora and Satureja parviflora. J. Essent. Oil Res. 1996, 9, 681–684. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Composition of essential oils from Satureja darwinii (Benth.) Briq. and Satureja multiflora (R.et P.) Briq. (Lamiaceae); relationship between chemotype and oil yiled in Satureja spp. J. Essent. Oil Res. 2010, 22, 477–482. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Sarikardasoglu, S.; Tümen, G. The essential oil of Cyclotrichium niveum (Boiss.) Manden. Et Scheng. J. Essent. Oil Res. 1994, 6, 9–12. [Google Scholar] [CrossRef]
- Bașer, K.H.C.; Kirimer, N.; Kürkçüoglu, M.; Özek, T.; Tümen, G. Essential oil of Cyclotrichium origanifolium (Labill.) Manden. et Scheng. from Turkey. J. Essent. Oil Res. 1996, 8, 569–570. [Google Scholar] [CrossRef]
- Alim, A.; Goze, I.; Cetin, A.; Atas, A.D.; Vural, N.; Donmez, E. Antimicrobial activity of the essential oil of Cyclotrichium niveum (Boiss.) Manden. Et Scheng. Afr. J. Microbiol. Res. 2009, 3, 422–425. [Google Scholar]
- Inan, M.; Tel, A.Z. Determination of Cyclotrichium niveum essential oil and its components at different altitudes. Not. Bot. Horti Agrobot. 2014, 42, 128–131. [Google Scholar] [CrossRef]
- Rojas, L.B.; Usubillaga, A.N. Essential oil of Minthostachys mollis Grisebach from Venezuela. J. Essent. Oil Res. 1995, 7, 211–213. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.A.B.; González-Cervera, T.A.; Güette-Fernandez, J.A.; Jaramillo-Colorado, B.A.B.; Stashenko, E.B. Chemical composition and antioxidant activity of essential oils isolated from Colombian plants. Braz. J. Pharmacogn. 2010, 20, 568–574. [Google Scholar] [CrossRef]
- Svendsen, A.B.; Scheffer, J.J.C.; Looman, A. Composition of the volatile oil of Minthostachys glabrescens Epl. Flavour Fragr. J. 1987, 2, 45–46. [Google Scholar] [CrossRef]
- Rossi, Y.E.; Canavoso, L.; Palacios, S.M. Molecular response of Musca domestica L. to Mintostachys verticillata essential oil, (4R)+-pulegone and menthone. Fitoterapia 2012, 83, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Dzhumaev, K.K.; Zenkevich, I.; Tkachenko, K.G.; Tsibul’skaya, I.A. Essential oils from inflorescences and leaves of Ziziphora brevicalyx. Khimiya Prirodnykh Soedin. 1990, 1, 121–123. [Google Scholar]
- Velasco-Negueruela, A.; Rico, M. The volatile oil of Ziziphora hispanica L. Flavour Fragr. J. 1986, 1, 111–113. [Google Scholar] [CrossRef]
- Sonboli, A.; Mirjalili, M.H.; Hadian, J.; Ebrahimi, S.N.; Yousefzadi, M. Antibacterial activity and composition of the essential oil of Ziziphora clinopodioides subsp. bungeana (Juz.) Rech. f. from Iran. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 677–680. [Google Scholar] [CrossRef]
- Zhaparkulova, K.; Srivedavyasasri, R.; Sakipova, Z.; Ross, S.A. Phytochemical and biological studies on Ziziphora bungeana. Planta Med. 2015, 81, 27. [Google Scholar] [CrossRef]
- Sezik, E.; Tümen, G.; Başer, K.H.C. Ziziphora tenuior L., a new source of pulegone. Flavour Fragr. J. 1991, 6, 101–103. [Google Scholar] [CrossRef]
- Bașer, K.H.C.; Kürkçüoglu, M.; Özek, T.; Tümen, G.; Sezik, E. The volatile constituents of Ziziphora species growing in Turkey. Dŏga Tr. J. Pharm. 1992, 2, 7–16. [Google Scholar]
- Von Poser, G.L.; Menut, C.; Toffoli, M.E.; Vérin, P.; Sobral, M.; Bessière, J.M.; Lamaty, G.; Henriques, A.T. Essential oil compositioon and allelopathic effect of the Brazilian Lamiaceae Hesperozygis ringens (Benth.) Epling and Hesperozygis rhododon Epling. J. Agric. Food Chem. 1996, 44, 1829–1832. [Google Scholar] [CrossRef]
- Svoboda, K.P.; Gough, J.; Hampson, J. Analysis of the essential oils of some Agastache species grown in Scotland from various seed sources. Flavour Fragr. J. 1995, 10, 139–145. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Aguirre Hernández, E.; García-Argáez, A.; Soto Hernández, M.; Linares, E.; Bye, R.; Heinze, G.; Martínez-Vázquez, M. Comparative chemical composition of Agastache mexicana subsp. mexicana and A. mexicana subsp. Xolocotziana. Biochem. Syst. Ecol. 2004, 32, 685–694. [Google Scholar] [CrossRef]
- Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Vostrowsky, R.; Brosche, T.; Ihm, H.; Zintl, R.; Knobloch, K. On the essential oil components of Artemisia absinthium. Z. Naturforsch. 1981, 36, 369–377. [Google Scholar]
- Mustafaeva, S.D. Essential oil of Achillea cuneatiloba. Chem. Nat. Compd. 1991, 27, 251–253. [Google Scholar] [CrossRef]
- Fleisher, Z.; Fleisher, A. Volatiles of Achillea fragrantissima (Forssk.) Sch. Bip. J. Essent. Oil Res. 1993, 5, 211–214. [Google Scholar]
- Asekun, O.T.; Grierson, D.S.; Afolayan, A.J. Characterization of essential oils from Helichrysum odoratissimum using different drying methods. J. Appl. Sci. 2007, 7, 1005–1008. [Google Scholar]
- Shahraki, A.; Ravandeh, M. Comparative survey on the essential oil composition and antioxidant activity of aqueous extracts from flower and stem of Achillea Wilhelmsii from Taftan (Southeast of Iran). HealthSCOPE 2012, 1, 173–178. [Google Scholar] [CrossRef]
- Turner, C.; Elsohly, M.; Boeren, E. Constituents of Cannabis sativa—A review of the natural constituents. J. Nat. Prod. 1979, 45, 169–233. [Google Scholar] [CrossRef]
- Ashraf, M.; Rafi, A.; Bhatty, M.K. The essential oils of the Pakistani species in the family Umbelliferae: 34, Pimpinella diversifolia seed and stalk oil. Pak. J. Sci. Ind. Res. 1979, 22, 265–266. [Google Scholar]
- Delfini, A.; Retamar, J. The essential oil of Lippia fissicalyx. Essenze Deriv. Agrum. 1974, 44, 23–33. [Google Scholar]
- Horvat, R.J.; Senter, S.D. Comparison of the volatile constituents from rabbiteye blueberries (Vaccinium ashei) during ripening. J. Food Sci. 1985, 50, 429–431. [Google Scholar] [CrossRef]
- Kaiser, R.; Lamparsky, D. Analysis of Buchu leaf oil. J. Agric. Food Chem. 1975, 23, 943–950. [Google Scholar] [CrossRef]
- Holeman, M.; Rombourg, M.; Fechtal, M.; Gorrichon, J.P.; Lassaigne, G. Eucalyptus astringens Maiden, Eucalyptus blakelyi Maiden, and Eucalyptus bosistoana F. Muell.: The same chemotype. Plantes Méd. Phytothér. 1987, 214, 311–316. [Google Scholar]
- Hadjieva, P.; Popov, S.; Budevska, B.; Dyulgerov, A.; Andreev, S. Terpenoids from a Black Sea Bryozoan Conopeum seuratum. Z. Naturforsch. 1987, 42, 1019–1022. [Google Scholar]
- Corey, E.J.; Ensley, H.E.; Suggs, J.W. Convenient synthesis of (S)-(−)-pulegone from (−)-citronellol. J. Org. Chem. 1976, 41, 380–381. [Google Scholar] [CrossRef]
- Bob, L. Enantioselective Total Synthesis of (−)-16-Hydroxitriptolide. Ph.D. Thesis, The University of Hong Kong, Hong Kong, China, 2006. [Google Scholar]
- Scharf, H.D.; Buschmann, H. Large-scale preparation of pure (+)-(1S,2R,5S)-5-methyl-2-(1-methyl-1-phenylethyl) cyclohexanol. Synth. Stuttg. 1988, 10, 827–830. [Google Scholar]
- Black, C.; Buchanan, G.L.; Jarvie, A.W. A synthesis of (±)-pulegone. J. Chem. Soc. 1956, 2971–2973. [Google Scholar] [CrossRef]
- Solodar, J. Asymmetric and regioselective hydrogenation of piperitenone by homogeneous rhodium complexes. J. Org. Chem. 1978, 43, 1787. [Google Scholar] [CrossRef]
- Sayo, N.; Matsumoto, T. Method for Producing 1-Menthol. U.S. Patent 6,342,644 B1, 29 January 2002. [Google Scholar]
- Maux, P.L.; Massonneau, V.; Simonneaux, G. Catalytic asymmetric syntheses. Part III. Asymmetric hydrogenation of piperitenone catalysed by chiral rhutenium hydrides: An example of a catalytic kinetic resolution. Tetrahedron 1988, 44, 1409. [Google Scholar]
- Bartoli, G.; Bosco, M.; Dalpozzo, R.; Giuliani, A.; Marcantoni, E.; Mecozzi, T.; Sambri, L.; Torregiani, E. An efficient procedure for the preparation of (E)-α-alkylidenecycloalkanones mediated by a CeCl(3) × 7H(2)O-NaI system. Novel methodology for the synthesis of (S)-(−)-pulegone. J. Org. Chem. 2002, 67, 9111–9114. [Google Scholar] [CrossRef] [PubMed]
- Grundschober, F. Literature review of pulegone. Perfum. Flavorist 1979, 4, 15–17. [Google Scholar]
- Jalilzadeh-Amin, G.; Maham, M. Antidiarrheal activity and acute oral toxicity of Mentha longifolia L. essential oil. AJP 2015, 5, 128–137. [Google Scholar] [PubMed]
- Anderson, I.B.; Mullen, W.H.; Meeker, J.E.; Khojasteh-Bakht, S.C.; Oishi, S.; Nelson, S.D.; Blanc, P.D. Pennyroyal toxicity: Measurement of toxic metabolite levels in two cases and review of the literature. Ann. Intern. Med. 1996, 124, 726–734. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). NTP techical report on the Toxicology and carcinogenesis studies of pulegone (CAS No. 89-82-7) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2011, 563, 1–201. [Google Scholar]
- Sullivan, J.B.; Rumack, B.H.; Thomas, H.; Peterson, R.G.; Bryson, P. Pennyroyal oil poisoning and hepatotoxicity. JAMA 1979, 242, 2873–2874. [Google Scholar] [CrossRef] [PubMed]
- Bakerink, J.A.; Gospe, S.M.; Dimand, R.J.; Eldridge, M.W. Multiple organ failure after ingestion of pennyroyal oil from herbal tea in two infants. Pediatrics 1996, 98, 944–947. [Google Scholar] [PubMed]
- Gordon, W.P.; Huitric, A.C.; Seth, C.L.; McClanahan, R.H.; Nelson, S.D. The metabolism of the abortifacient terpene, (R)-(+)pulegone, to a proximate toxin, menthofuran. Drug Metab. Dispos. 1987, 15, 589–594. [Google Scholar] [PubMed]
- Madyastha, K.M.; Moorthy, B. Pulegone mediated hepatotoxicity: Evidence for covalent binding of R(+)-[14C]pulegone to microsomal proteins in vitro. Chem. Biol. Interact. 1989, 72, 325–333. [Google Scholar] [CrossRef]
- Nelson, S.D.; McClanahan, R.H.; Thomassen, D.; Gordon, W.P.; Knebel, N. Investigations of mechanisms of reactive metabolite formation from (R)-(+)-pulegone. Xenobiotica 1992, 22, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Madyastha, K.M.; Raj, C.P. Metabolic fate of menthofuran in rats: Novel oxidative pathways. Drug Metab. Dispos. 1992, 20, 295–301. [Google Scholar] [PubMed]
- Madyastha, K.M.; Raj, C.P. Studies on the metabolism of a monoterpene ketone, R-(+)-pulegone—A hepatotoxin in rat: Isolation and characterization of new metabolites. Xenobiotica 1993, 23, 509–518. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, R.H.; Thomasson, D.; Slattery, J.T.; Nelson, S.D. Metabolic activation of (R)-(+)-pulegone to a reactive enonal that covalently binds to mouse liver proteins. Chem. Res. Toxicol. 1989, 2, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lebetkin, E.H.; Burka, L.T. Comparative disposition of (R)-(+)-pulegone in B6C3F1 mice and F344 rats. Drug Metab. Dispos. 2003, 31, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Vadiraja, B.B.; Gaikwad, N.W.; Madyastha, K.M. Hepatoprotective effect of C-phycocyanin: Protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicty in rats. Biochem. Biophys. Res. Commun. 1998, 249, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.H.; Jensen, N.J. Mutagenic investigation of peppermint oil in the Salmonella/mammalian-microsome test. Mutat. Res. 1984, 138, 17–20. [Google Scholar] [CrossRef]
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and genotoxic activities of mint essential oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- Chen, X.W.; Serag, E.S.; Sneed, K.B.; Zhou, S.F. Herbal bioactivation, molecular targets and the toxicity relevance. Chem. Biol. Interact. 2011, 192, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Madyastha, K.M.; Raj, C.P. Biotransformation of R-(+)-pulegone and menthofuran in vitro: Chemical basis for toxicity. Biochem. Biophys. Res. Commun. 1990, 173, 1086–1092. [Google Scholar] [CrossRef]
- Moorthy, B. Toxicity and metabolism of R-(+)-pulegone in rats: Its effects on hepatic cytochrome P450 in vivo and in vitro. J. Indian Inst. Sci. 1991, 71, 76–78. [Google Scholar]
- Khojasteh-Bakht, S.C.; Chen, W.; Koenigs, L.L.; Peter, R.M.; Nelson, S.D. Metabolism of (R)-(+)-pulegone and (R)-(+)-menthofuran by human liver cytochrome P-450s: Evidence for formation of a furan epoxide. Drug Metab. Dispos. 1999, 27, 574–580. [Google Scholar] [PubMed]
- Moorthy, B.; Madyastha, P.; Madyastha, K.M. Metabolism of a monoterpene ketone, R-(+)-pulegone—A hepatotoxin in rat. Xenobiotica 1989, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, D.; Pearson, P.G.; Slattery, J.T.; Nelson, S.D. Partial characterization of biliary metabolites of pulegone by tandem mass spectrometry: Detection of glucuronide, glutathione, and glutathionyl glucuronide conjugates. Drug Metab. Dispos. 1991, 19, 997–1003. [Google Scholar] [PubMed]
- Chen, L.J.; Lebetkin, E.H.; Burka, L.T. Metabolism of (R)-(+)-pulegone in F344 rats. Drug Metab. Dispos. 2001, 29, 1567–1577. [Google Scholar] [PubMed]
- Ferguson, L.J.; Lebetkin, E.H.; Lih, F.B.; Tomer, K.B.; Parkinson, H.D.; Borghoff, S.J.; Burka, L.T. 14C-labeled pulegone and metabolites binding to alpha2u-globulin in kidneys of male F-344 rats. J. Toxicol. Environ. Health A 2007, 70, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Tangarife-Castaño, V.; Roa-Linares, V.; Betancur-Galvis, L.A.; Durán García, D.C.; Stashenko, E.; Mesa-Arango, A.C. Antifungal activity of Verbenaceae and Labiatae families essential oils. Pharmacologyonline 2012, 1, 133–145. [Google Scholar]
- Primo, V.; Rovera, M.; Zanon, S.; Oliva, M.; Demo, M.; Daghero, J.; Sabini, L. Determination of the antibacterial and antiviral activity of the essential oil from Minthostachys verticillata (Griseb.) Epling. Rev. Argent. Microbiol. 2001, 33, 113–117. [Google Scholar] [PubMed]
- De Sousa, D.P.; Júnior, G.A.S.; Andrade, L.N.; Batista, J.S. Spasmolytic activity of chiral monoterpene esters. Rec. Nat. Prod. 2011, 5, 117–122. [Google Scholar]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material; John Wiley & Sons Ltd.: West Sussex, UK, 1996; Volume 1, pp. 25–26. [Google Scholar]
- De Sousa, D.P.; Júnior, G.A.S.; Andrade, L.N.; Calasans, F.R.; Nunes, X.P.; Barbosa-Filho, J.M.; Batista, J.S. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z. Naturforsch. Sect. C J. Biosci. 2008, 63, 808–812. [Google Scholar] [CrossRef]
- Andrade, L.N.; De Sousa, D.P.; Batista, J.S. Action mechanism of the monoterpenes (+)-pulegone and 4-terpinyl acetate in isolated guinea pig ileum. Bol. Latinoam. Caribe Plantas Med. Aromát. 2013, 12, 581–591. [Google Scholar]
- Rasmussen, H.; Barret, Q.P. Calcium messenger system: An integrated view. Phys. Rev. 1994, 64, 938–984. [Google Scholar]
- Magalhães, P.J.; Lahlou, S.; Jucá, D.M.; Coelho-De-Souza, L.N.; Da Frota, P.T.; Da Costa, A.M.; Leal-Cardoso, J.H. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundam. Clin. Pharmacol. 2008, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Mota, M.M.; Barbosa-Filho, J.M.; Viana Dos Santos, M.R.; De Sousa, D.P. Structural relationships and vasorelaxant activity of monoterpenes. DARU J. Pharm. Sci. 2012, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Jalilzadeh-Amin, G.; Mahama, M. Evaluation of pulegone on transit time and castor-oil induced diarrhea in rat. Pharm. Sci. 2013, 19, 77–82. [Google Scholar]
- Abdullahi, A.L.; Agho, M.O.; Amos, S.; Gamaniel, K.S.; Wambebe, C. Antidiarrhoeal activity of the aqueous extract of Terminalia avicennoides roots. Phyther. Res. 2001, 15, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Duarte, A.; Figueiredo, C.; Brito, L.; Teixeira, G.; Moldao, M.; Monteiro, A. Chemical composition and antibacterial activity of the essential oils from the medicinal plant Mentha cervina L. grown in Portugal. Med. Chem. Res. 2012, 21, 3485–3490. [Google Scholar] [CrossRef]
- Watanabe, K.; Shono, Y.; Kakimizu, A.; Okada, A.; Matsuo, N.; Satoh, A.; Nishimura, H. New Mosquito Repellent from Eucalyptus camaldulensis. J. Agric. Food Chem. 1993, 41, 2164–2166. [Google Scholar] [CrossRef]
- Hough-Goldstein, J.A. Antifeedant effects of common herbs on the Colorado porato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1990, 19, 234–238. [Google Scholar] [CrossRef]
- Karr, L.L.; Coats, J.R. Insecticidal properties of d-limonene. J. Pestic. Sci. 1988, 13, 287–290. [Google Scholar] [CrossRef]
- Rice, P.J.; Coats, J.R. Insecticidal properties of several monoterpenoids to the house fly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae), and southern maize rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; Samuelian, J.H.; Evans, C.K.; Brattsten, L.B. Effects of the mint monoterpene pulegone on Spodoptera eridania (Lepidoptera: Noctuidae). Environ. Entomol. 1985, 14, 859–861. [Google Scholar] [CrossRef]
- Duke, S.O. Herbicides from natural compounds. Weed Technol. 1987, 1, 122–128. [Google Scholar]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Lee, S.; Peterson, C.J.; Coats, J.R. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res. 2002, 39, 77–85. [Google Scholar] [CrossRef]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Seo, D.K.; Jang, S.A.; Han, J.H.; Kim, Y.J.; Kim, G.H. Fumigant toxicity of 18 essential oils and their major compounds against adult longicorn beetle, Moechotypa diphysis (Coleoptera: Cerambycidae). J. Appl. Entomol. 2006, 45, 189–194. [Google Scholar]
- Hannin, S.; Bourarach, K.; lsmaïli-Alaoui, M.; Benjilali, B. Activité Insecticide de Mentha pulegium vis-à-vis de Rhyzopertha dominica, Sitophilus oryzea et Tribolium castaneum; Coll. Doc. Sci. Techn.: Rabat, Morocco, 1997; pp. 311–315. [Google Scholar]
- Lahlou, M. Methods to study the phytochemistry and bioactivity of essential oils. Phyther. Res. 2004, 18, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Ngamo, L.S.T.; Hance, T. Diversité des ravageurs des denrées et méthodes alternatives de lutte en milieu tropical. Tropicultura 2007, 25, 215–220. [Google Scholar]
- Ndomo, A.F.; Tapondjou, A.L.; Tendonkeng, F.; Tchouanguep, F.M. Evaluation des propriétés insecticides des feuilles de Callistemon viminalis (Myrtaceae) contre les adultes d’Acanthoscelides obtectus (Say) (Coleoptera; Bruchidae). Tropicultura 2009, 27, 137–143. [Google Scholar]
- Jiang, Z.; Akhtar, Y.; Bradbury, R.; Zhang, X.; Isman, M.B. Comparative toxicity of essential oils of litsea pungens and litsea cubeba and blends of their major constituents against the cabbage looper, trichoplusiani. J. Agric. Food Chem. 2009, 57, 4833–4837. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 Greek Lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F.; Tu, A.T. Toxicologyof plant and fungal compounds. In Handbook of Natural Toxins; Dekke, Marcelr: New York, NY, USA, 1991; Volume 6, pp. 269–296. [Google Scholar]
- Kawata, J.; Kameda, M.; Miyazawa, M. Cyclooxygenase-2 inhibitory effects of monoterpenoids with a p-menthane skeleton. Int. J. Essent. Oil Ther. 2008, 2, 145–148. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Júnior, E.V.M.; Oliveira, F.S.; De Almeida, R.N.; Nunes, X.P.; Barbosa-Filho, J.M. Antinociceptive activity of structural analogues of rotundifolone: Structure-activity relationship. Z. Naturforsch. Sect. C J. Biosci. 2007, 62, 39–42. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Almeida, R.N.; Leite, J.R.; Mattei, R. Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Rev. Bras. Farm. 2007, 1, 170–175. [Google Scholar] [CrossRef]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, J.F.; Silva, M.I.G.; Neto, M.R.; Neto, P.F.; Moura, B.A.; De Melo, C.T.V.; De Araújo, F.L.O.; De Sousa, D.P.; De Vasconcelos, S.P.F.; De Vasconcelos, M.M.; et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.G.; Neto, M.R.; Neto, P.F.; Moura, B.A.; Do Amaral, J.F.; De Sousa, D.P.; Vasconcelos, S.M.M.; De Sousa, F.C.F. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.N.; De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Araújo, D.A.M.; Leite, J.R.; Mattei, R. Anticonvulsant effect of a natural compound α,β-epoxy-carvone and its action on the nerve excitability. Neurosci. Lett. 2008, 443, 51–55. [Google Scholar] [CrossRef] [PubMed]

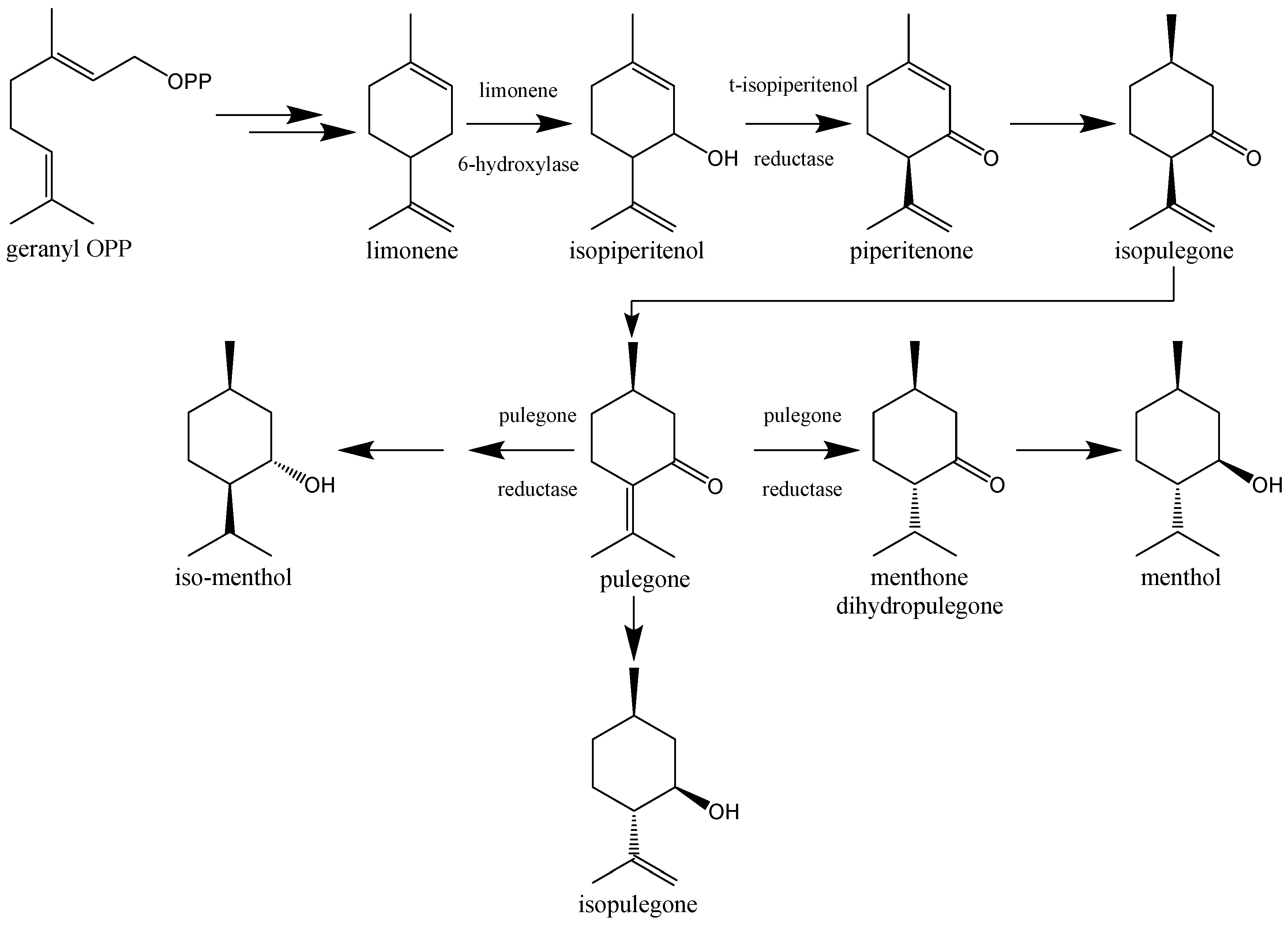
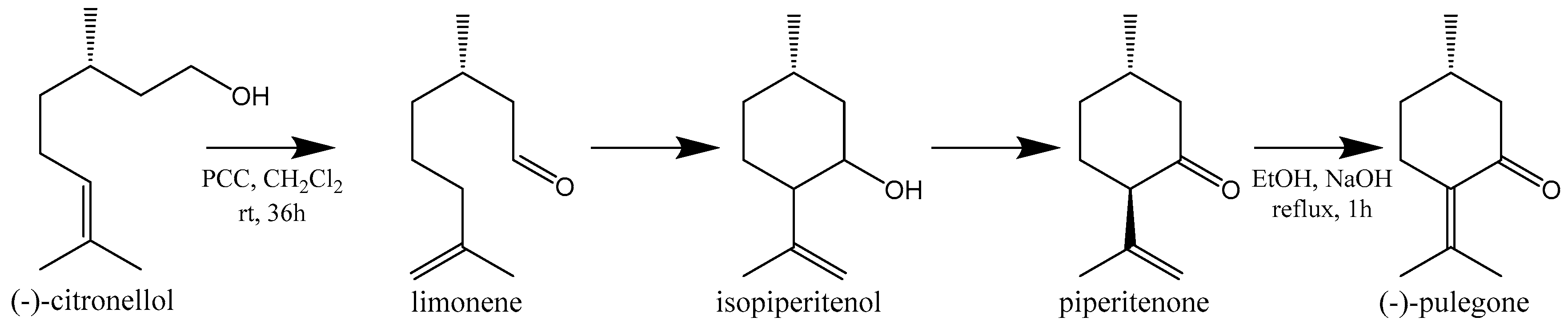
| Species | Subspecies | Synonyms |
|---|---|---|
| Calamintha nepeta (L.) Savi | nepeta L. | C. nepetoides Jord., C. vulgaris Clairv., C. thessala Hausskn., Satureja calamintha subsp. nepetoides (Jord.) Br.-Bl., Satureja nepeta (L.) Scheele, Clinopodium nepeta (L.) Kuntze subsp. nepeta; Melissa nepeta L. |
| glandulosa (Req.) Ball | C. glandulosa (Req.) Bentham, C. officinalis Moench, Thymus glandulosus Req., Clinopodium nepeta (L.) Kuntze subsp. spruneri (Boiss.) Bartolucci et Conti and Satureja calamintha subsp. glandulosa (Req.) Gams, subsp. nepeta sensu Briq., subsp. subnuda (Waldst. et Kit.) Gams |
| Chemical Structure | Chemical Name | MW | CAS Number |
|---|---|---|---|
 | piperitenone | 150.221 | 491-09-8 |
 | piperitenone oxide | 166.220 | 35178-55-3 |
 | piperitone oxide | 168.236 | 5286-38-4 |
 | piperitone | 152.237 | 89-81-6 |
 | pulegone | 152.237 | 89-82-7 |
 | carvone | 150.22 | 6485-40-1 |
 | 1,8-cineole | 154.25 | 470-82-6 |
 | menthone | 154.25 | 14073-97-3 |
 | menthol | 156.27 | 2216-51-5 |
| Constituents | IKa | IKP | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| α-Pinene | 931 | 1022 | 3.2 | 0.5 | 1.0 | 0.6 | 0.7 | 0.3 |
| Camphene | 943 | 1066 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sabinene | 964 | 1120 | 0.6 | 0.1 | 0.3 | 0.2 | 0.3 | 0.1 |
| β-Pinene | 971 | 1110 | 0.6 | 0.4 | 0.8 | 0.4 | 0.6 | 0.2 |
| 3-Octanol | 977 | 1381 | 1.1 | 0.2 | 1.6 | 0.8 | 1.1 | 0.4 |
| Myrcene | 979 | 1159 | 0.5 | 0.3 | 0.9 | 0.4 | 0.5 | 0.2 |
| Limonene | 1020 | 1199 | 5.2 | 3.3 | 12.8 | 5.0 | 6.0 | 2.1 |
| 1,8-Cineole | 1020 | 1209 | 0.5 | 0.4 | 0.7 | 0.5 | 0.4 | 0.2 |
| trans-Sabinene hydrate | 1054 | 1456 | 0.5 | 0.7 | 0.1 | 0.4 | - | - |
| Linalool | 1081 | 1544 | 0.5 | 0.3 | 0.9 | 0.5 | 0.3 | 0.2 |
| Menthone | 1135 | 1456 | 43.4 | 11.5 | 9.3 | 7.2 | 20.0 | 10.4 |
| Isomenthone | 1142 | 1481 | 3.2 | 4.3 | 0.4 | 0.6 | 2.0 | 1.9 |
| Menthol | 1156 | 1637 | 0.3 | 0.7 | 0.2 | 0.4 | 0.1 | 0.3 |
| Neomenthol | 1156 | 1594 | 1.6 | 2.8 | 0.7 | 0.8 | 0.4 | 1.1 |
| Terpinen-4-ol | 1161 | 1600 | 0.4 | 0.5 | 0.2 | 0.5 | 0.1 | 0.1 |
| α-Terpineol | 1172 | 1700 | 0.3 | 0.3 | 0.3 | 0.3 | - | - |
| Dihydrocarveol | 1178 | 1750 | 0.3 | 0.4 | 0.3 | 0.7 | 0.1 | 0.2 |
| Pulegone | 1216 | 1645 | 18.9 | 8.9 | 12.4 | 10.0 | 55.6 | 12.2 |
| Piperitone oxide I | 1230 | 1700 | 0.6 | 1.8 | 2.5 | 4.3 | 0.3 | 0.6 |
| Piperitone oxide II | 1230 | 1722 | 8.3 | 10.1 | 30.5 | 12.6 | 1.2 | 2.7 |
| Piperitone | 1232 | 1730 | 3.3 | 4.1 | 0.8 | 1.0 | 0.4 | 0.6 |
| Thymol | 1266 | 2189 | - | - | 0.2 | 0.2 | - | - |
| Dihydrocarvyl acetate | 1307 | 1657 | 1.8 | 2.4 | 2.1 | 1.7 | 1.2 | 1.3 |
| Piperitenone | 1315 | 1909 | 1.5 | 2.1 | 1.0 | 1.9 | 2.1 | 1.5 |
| α-Terpinyl acetate | 1332 | 1681 | 0.5 | 1.3 | 0.4 | 0.4 | 0.1 | 0.1 |
| Piperitenone oxide | 1333 | 1945 | 0.8 | 1.3 | 12.5 | 6.5 | 0.6 | 1.2 |
| β-Caryophyllene | 1419 | 1571 | 0.4 | 0.2 | 0.7 | 0.7 | 0.2 | 0.2 |
| Germacrene D | 1480 | 1704 | 0.4 | 0.2 | 0.9 | 0.8 | 0.4 | 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božović, M.; Ragno, R. Calamintha nepeta (L.) Savi and its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules 2017, 22, 290. https://doi.org/10.3390/molecules22020290
Božović M, Ragno R. Calamintha nepeta (L.) Savi and its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules. 2017; 22(2):290. https://doi.org/10.3390/molecules22020290
Chicago/Turabian StyleBožović, Mijat, and Rino Ragno. 2017. "Calamintha nepeta (L.) Savi and its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry" Molecules 22, no. 2: 290. https://doi.org/10.3390/molecules22020290