Porous Hydrogen-Bonded Organic Frameworks
Abstract
:1. Introduction
2. The Main Content of This Review
2.1. Hydroxyl or Amine Groups as Hydrogen-Bonded Motifs for HOFs
2.2. Carboxylic Groups or Pyrazole Moieties as Hydrogen-Bonded Motifs for HOFs
2.3. Amide or Urea Groups as Hydrogen-Bonded Motifs for HOFs
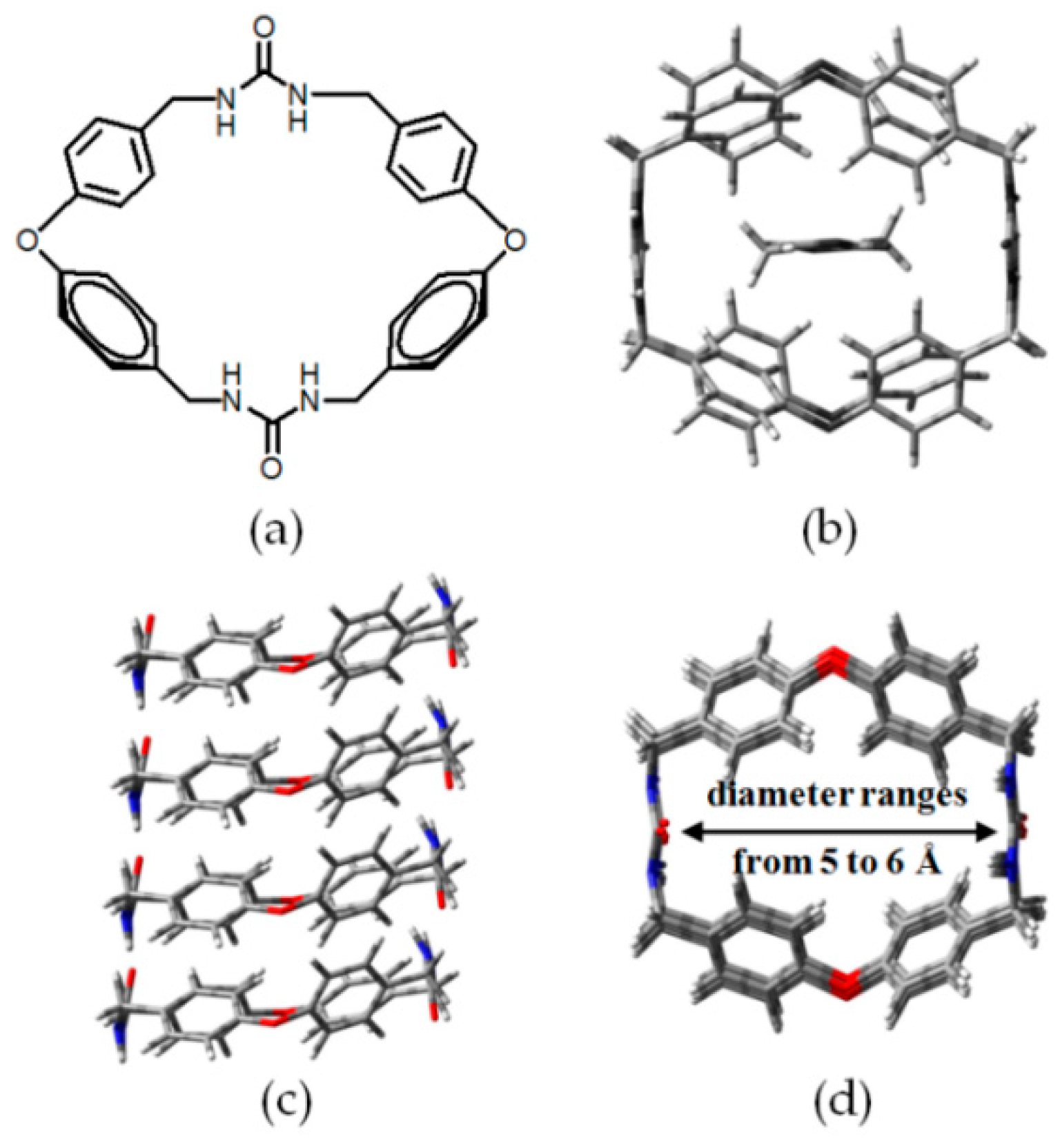
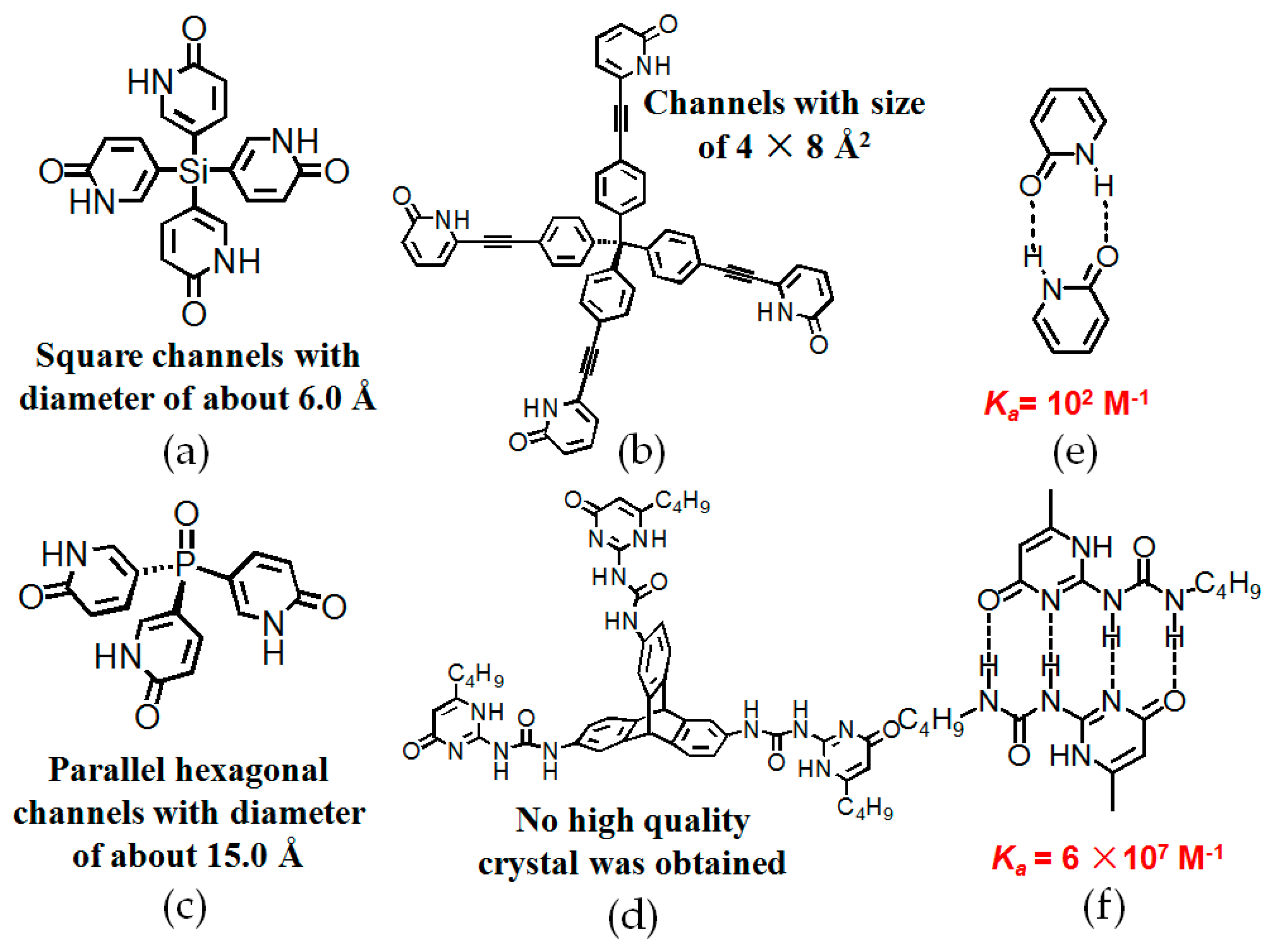
2.4. Macrocyclic Receptors as Hydrogen-Bonded Motifs for HOFs
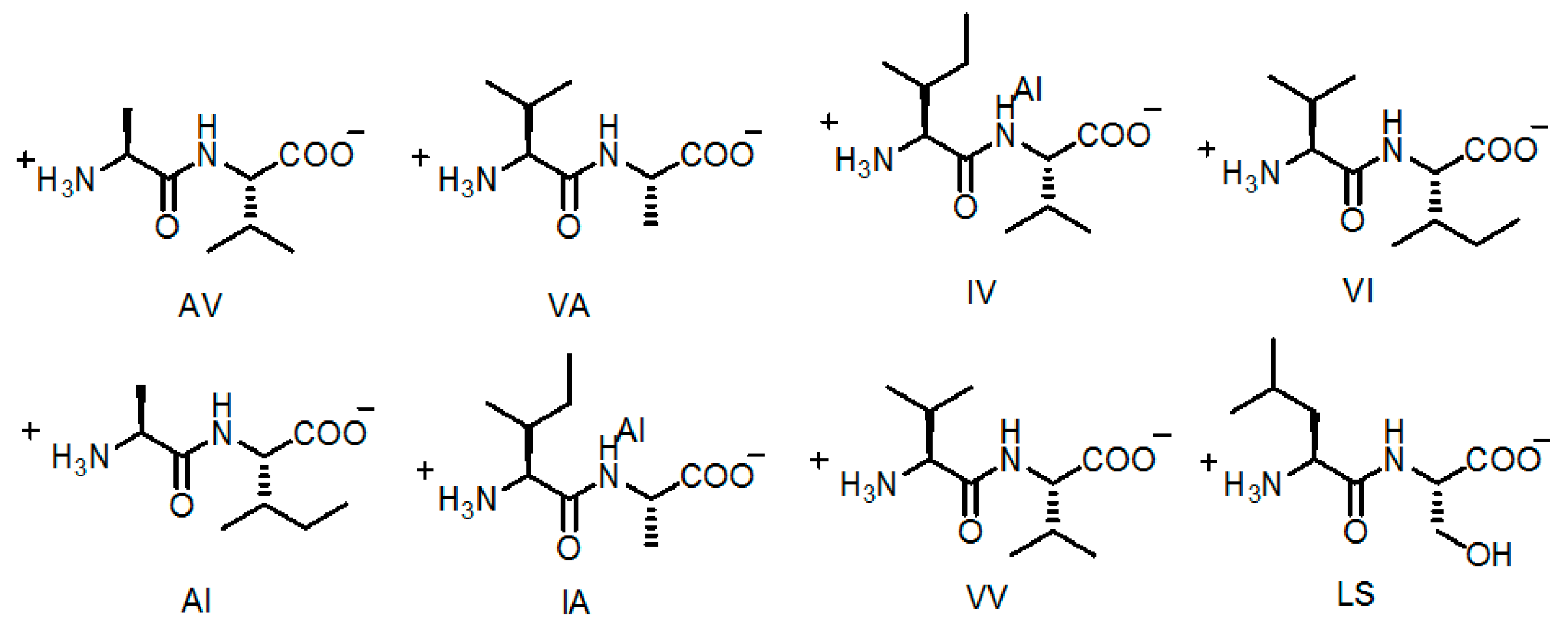
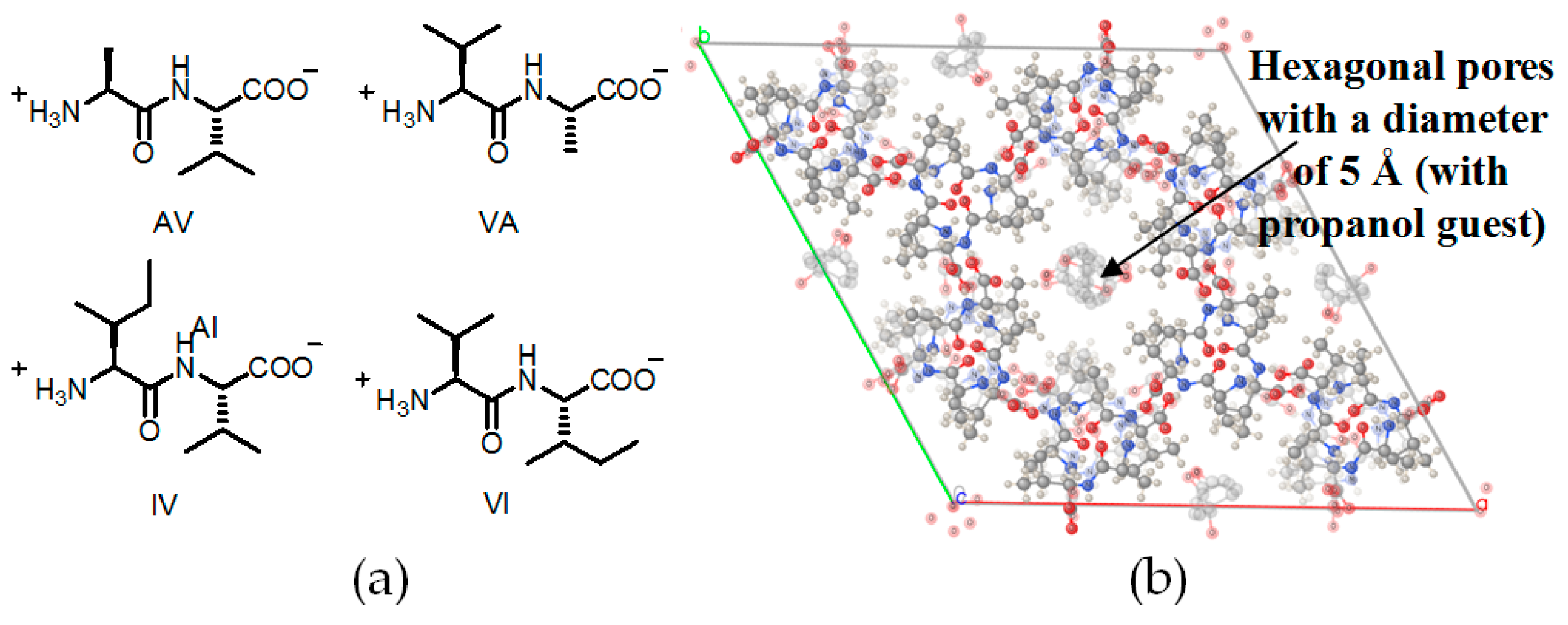
2.5. Linear Dipeptide as Hydrogen-Bonded Building Motifs for HOFs
2.6. Pyridone or UPy Moieties as Hydrogen-Bonded Motifs for HOFs
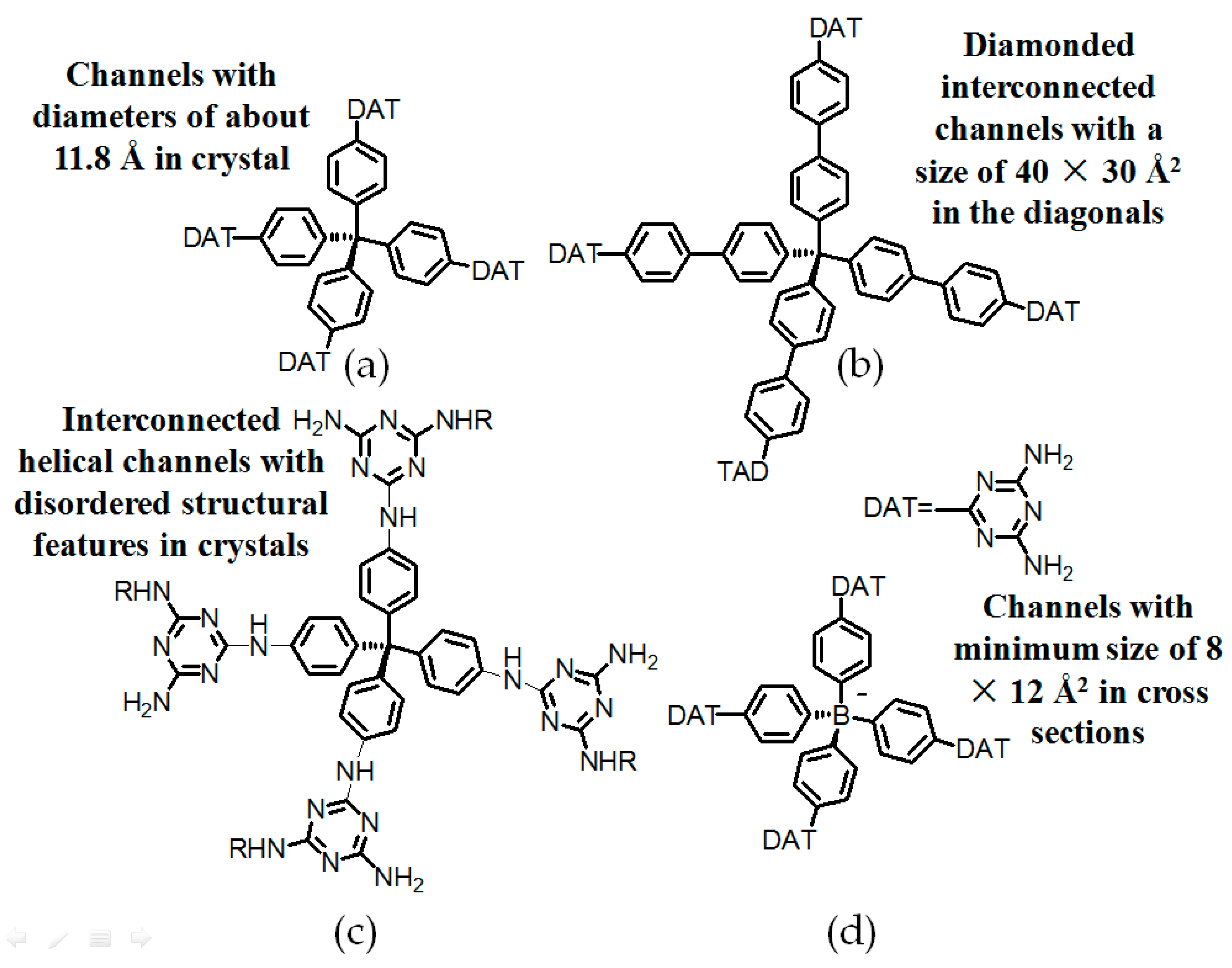
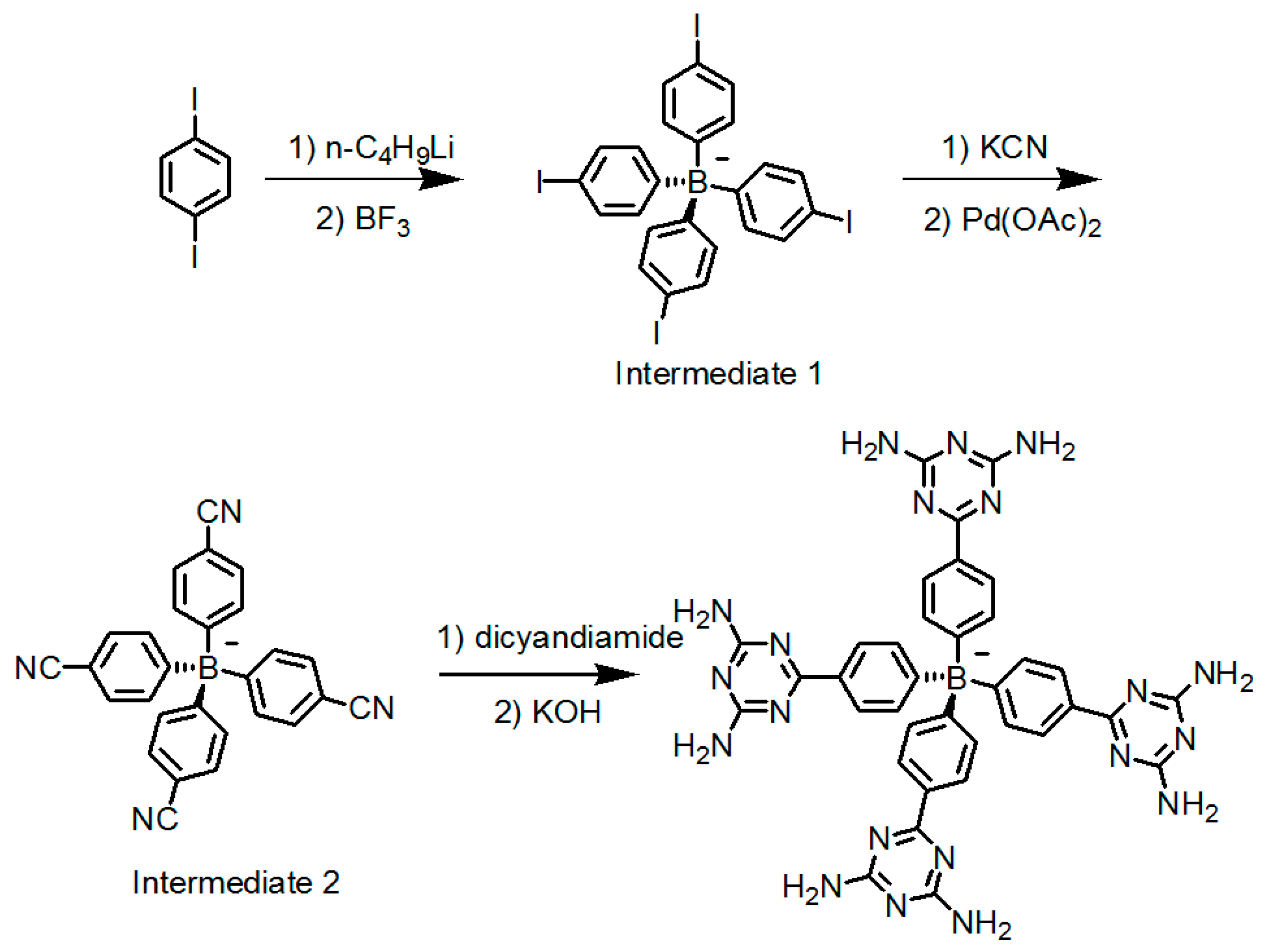
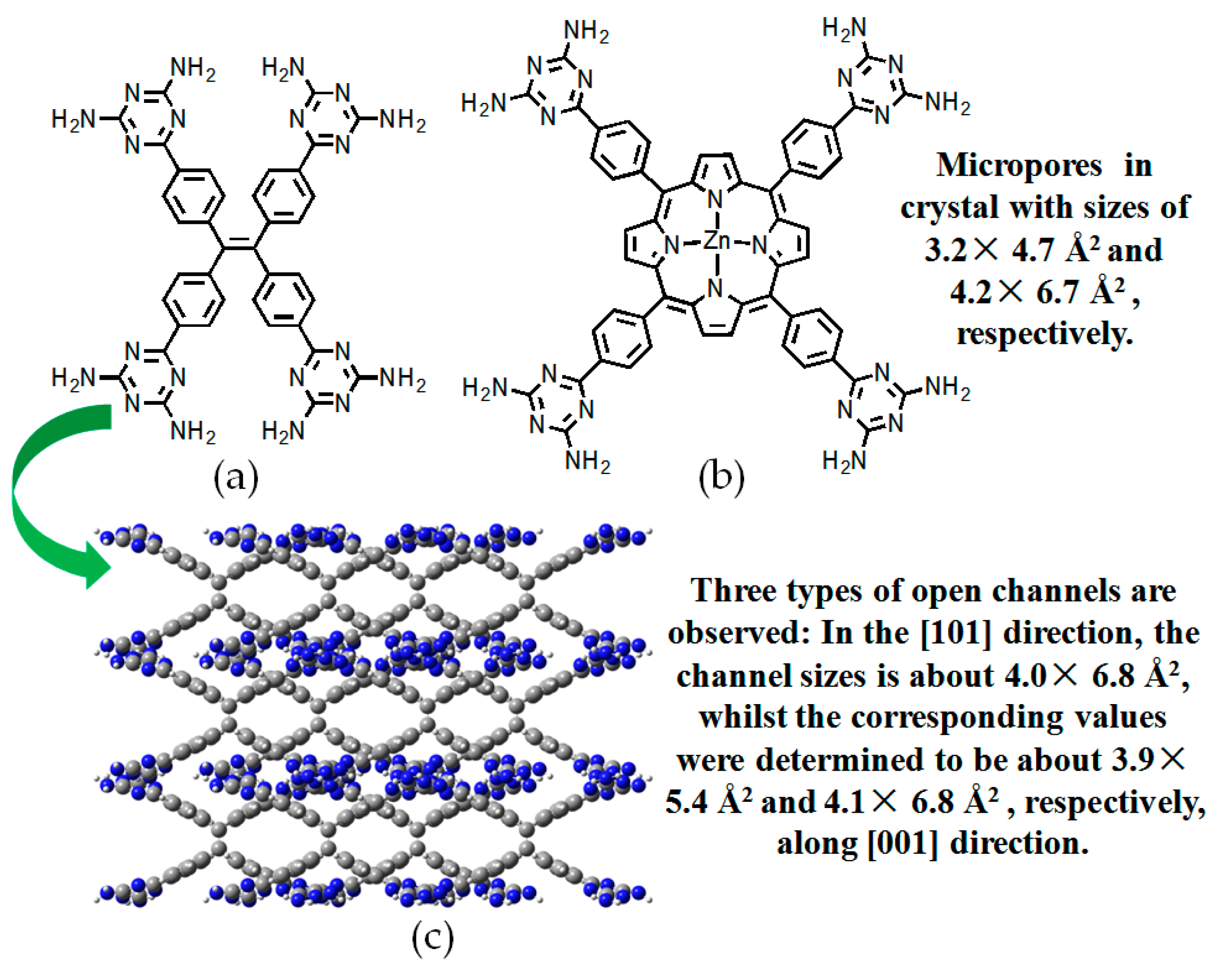
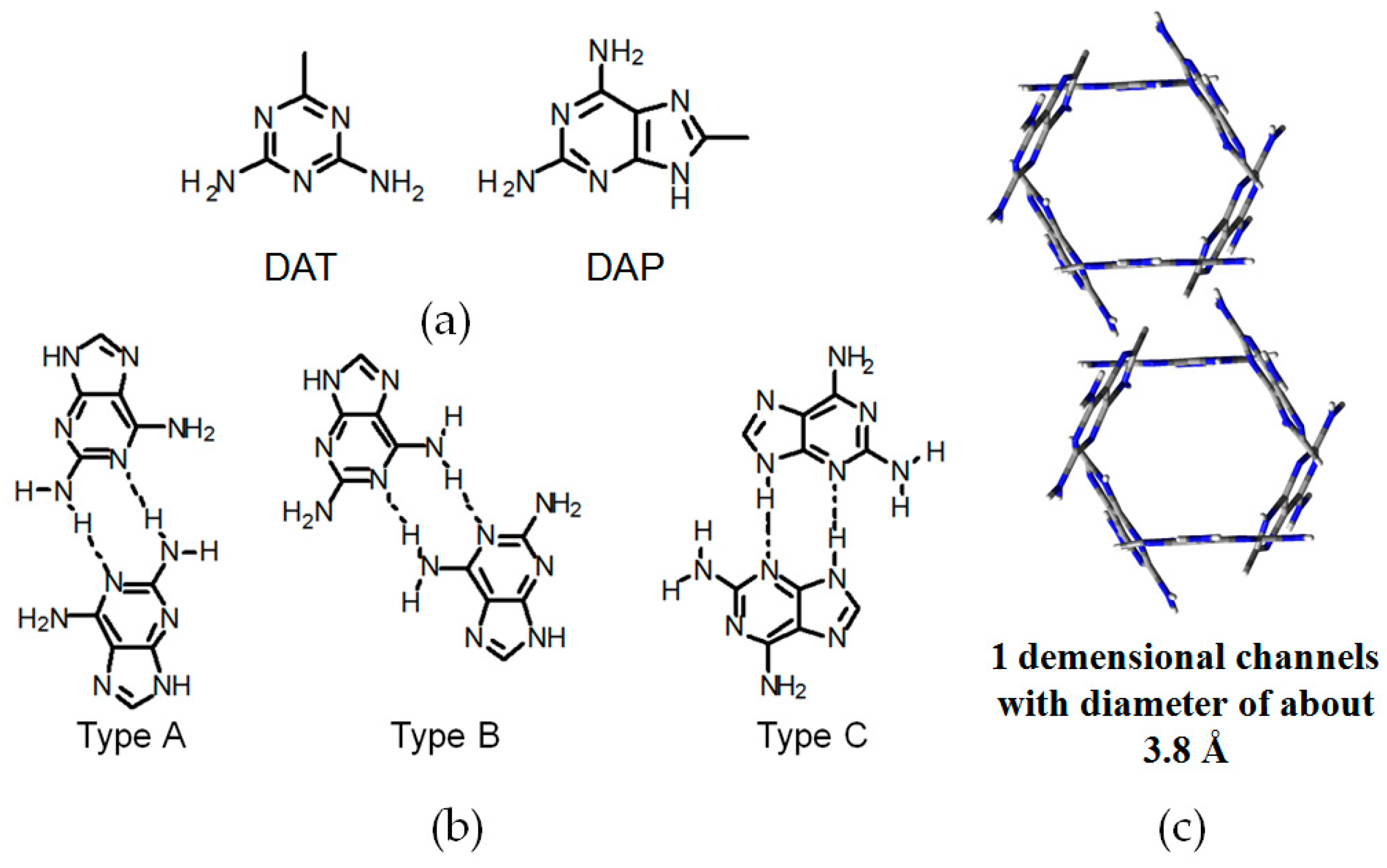
2.7. DAT or DAP Moieties as Hydrogen-Bonded Motifs for HOFs
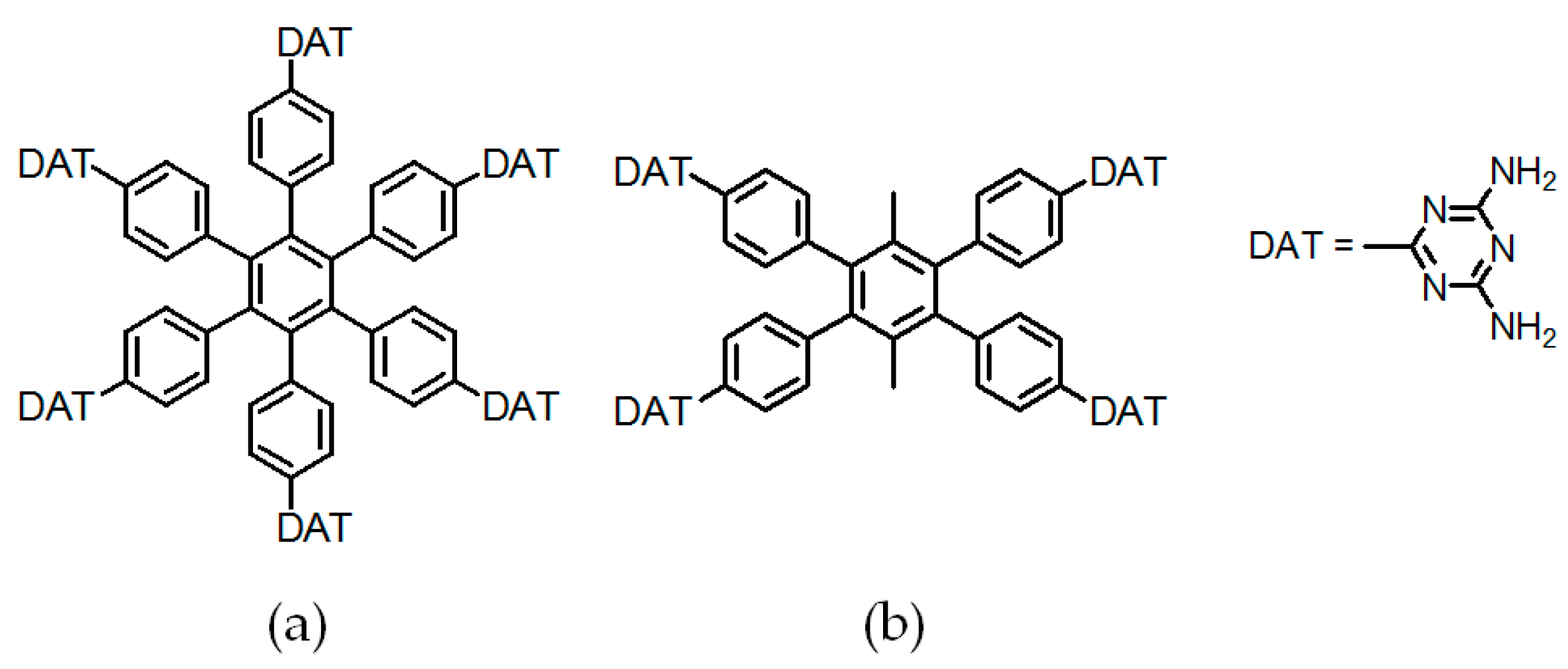
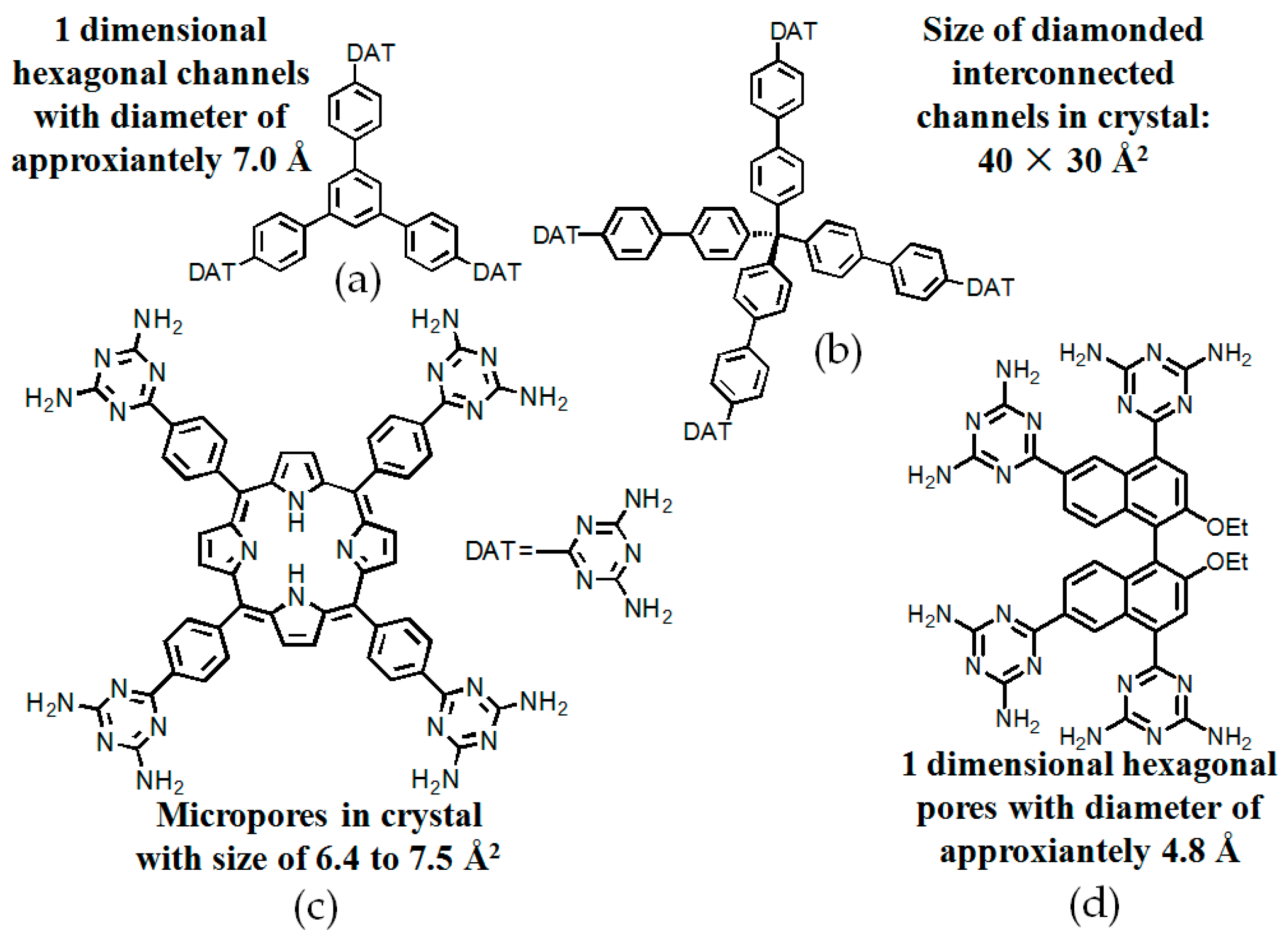
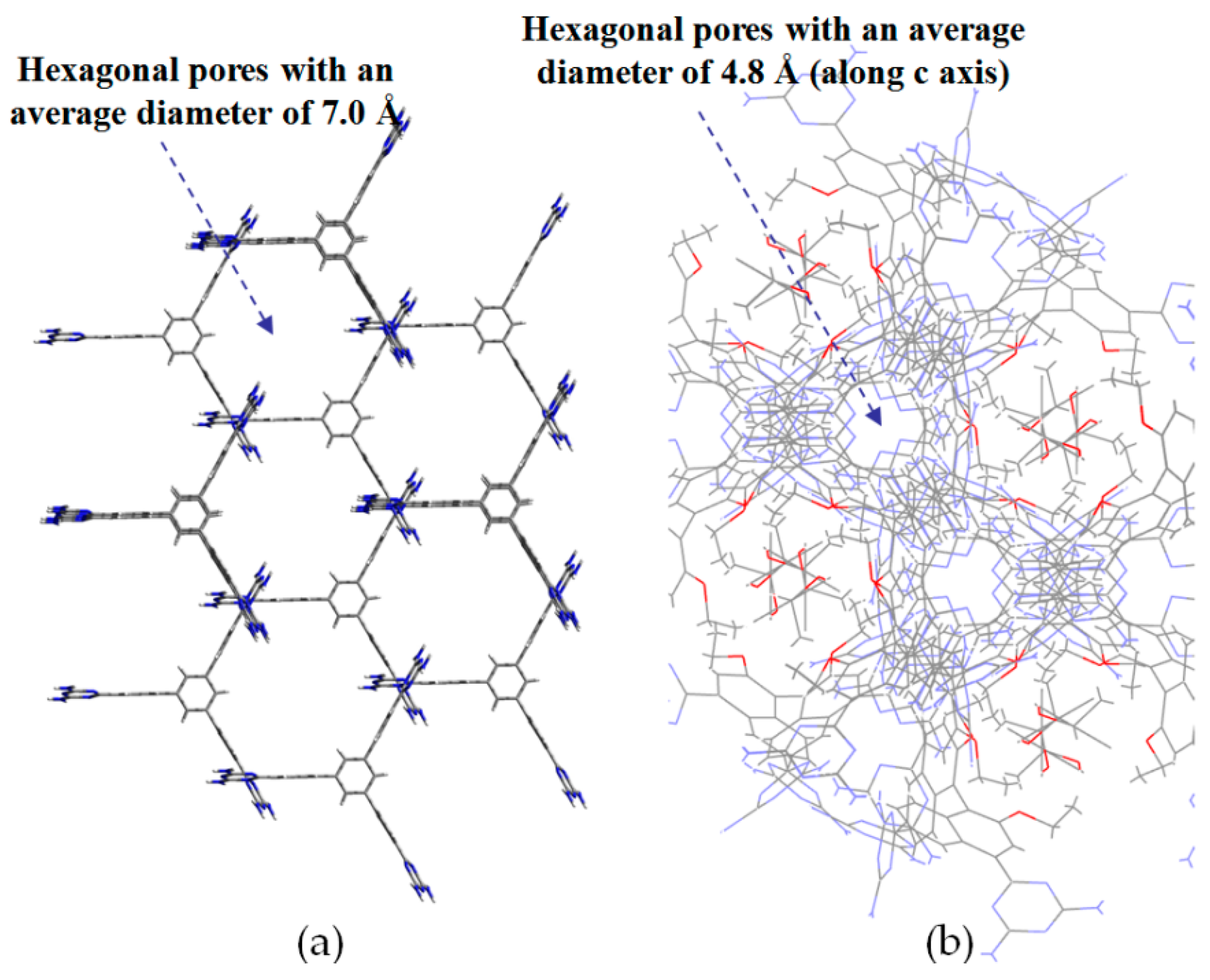
2.7.1. Tetrahedral DAT-Based Building Blocks
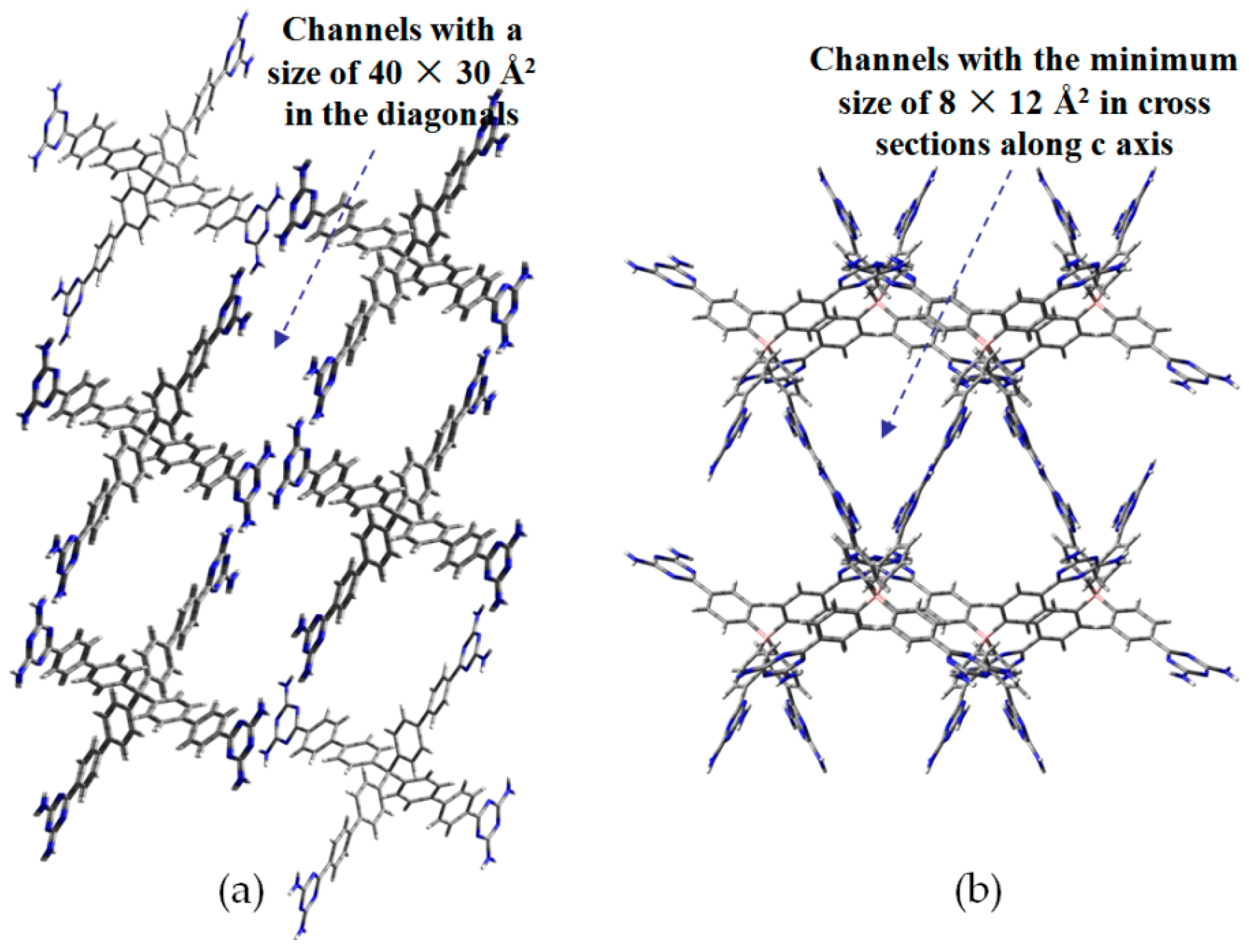
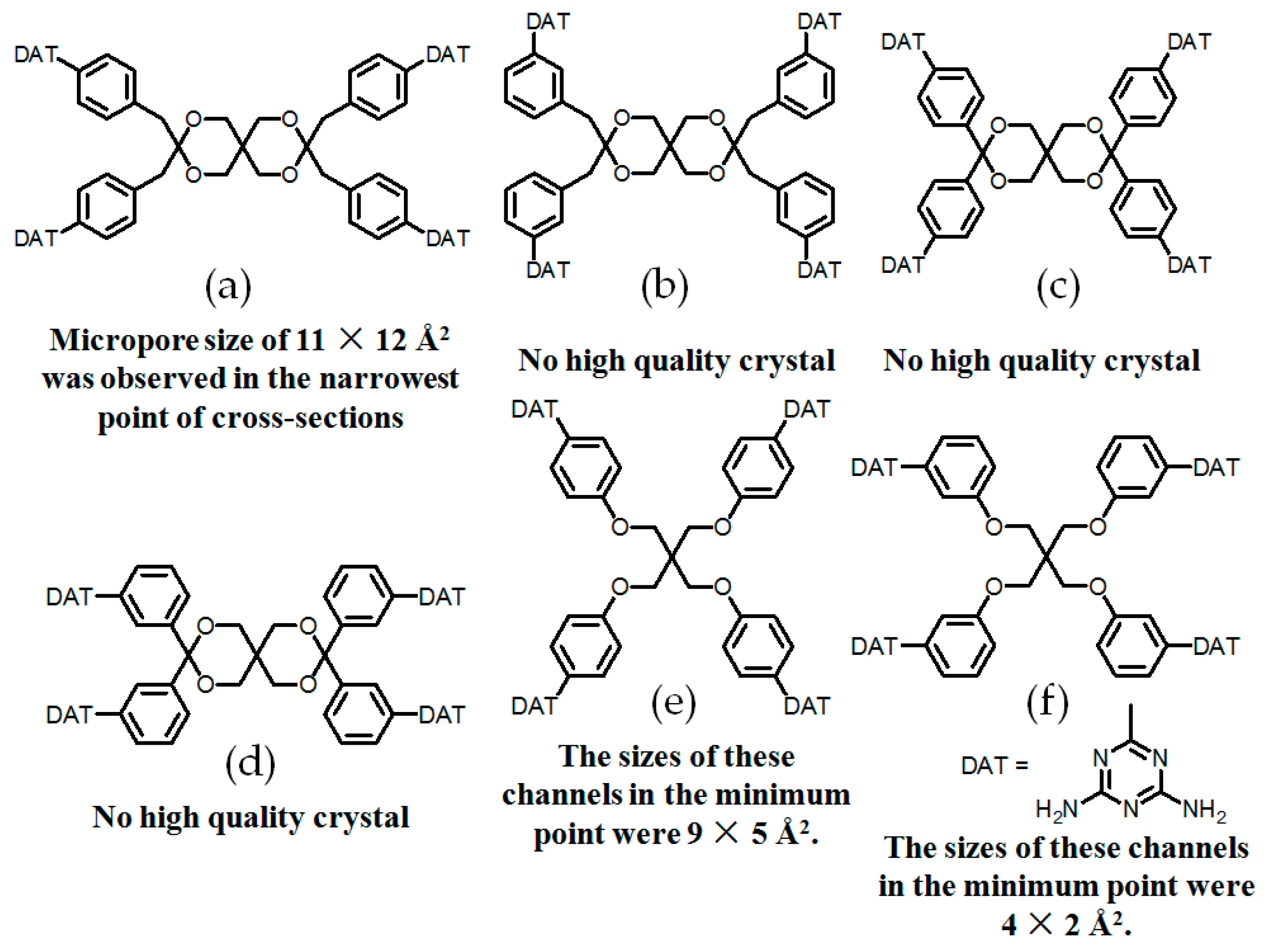
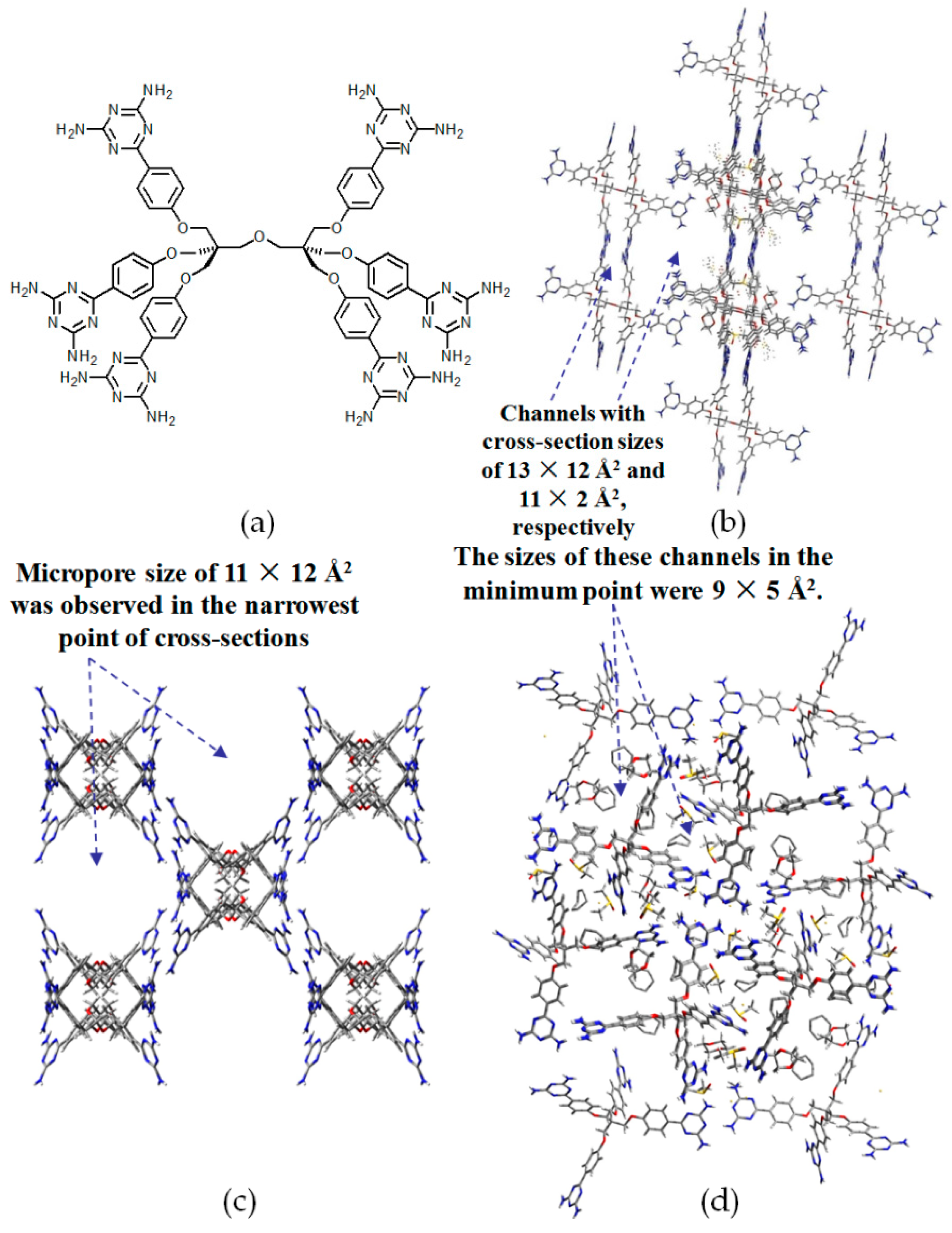
- (a)
- For neutral building motifs, the best position of a DAT motif generally lies in the phenyl para-position rather than ortho- and meta-positions, since a DAT motif in a para-position is more likely to enable scaffolds to separate from each other as far as possible when the building motifs are in close proximity to each other. As for charged building motifs, their complexation manners are rather difficult to predict precisely due to the subtle equilibrium between electrostatic and hydrogen bonding interactions.
- (b)
- Even though high porosities may be achieved from the elaborate design of rigid building blocks, it is essential to “provide” DAT moieties or backbones with appropriate degree of flexibility for the purpose of rendering building motifs more adaptable to well-defined supramolecular networks;
- (c)
- Despite the fact that enlarged building motifs are more likely to generate porous HOFs with better performance in terms of gas absorption or isolation [71], but it by no means indicates that HOFs with enlarged building motifs unambiguously result in remarkable enhancements in porosity. On the basis of these empirical principles, we can qualitatively elucidate the relationship between building motifs and porosities of following HOFs.
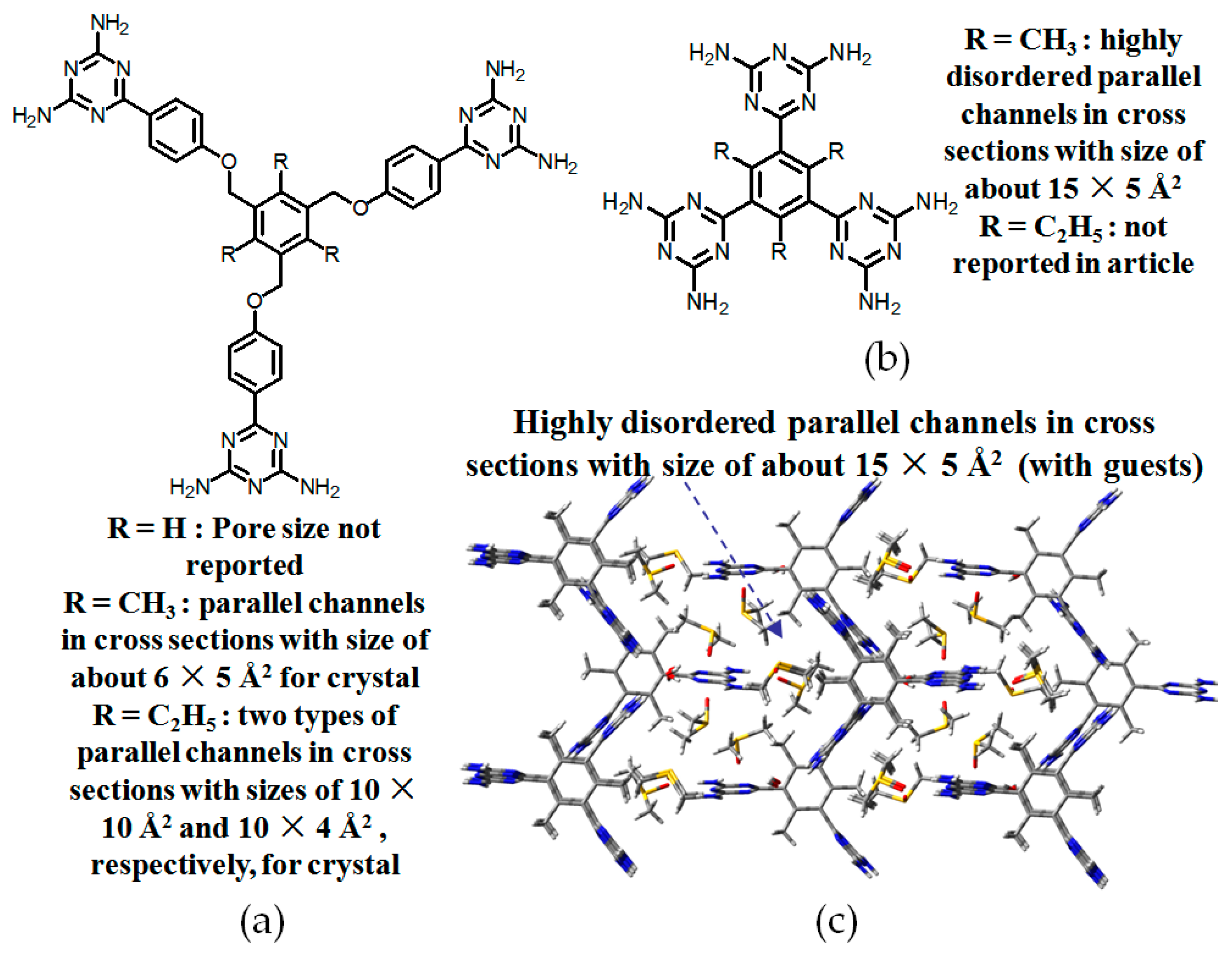
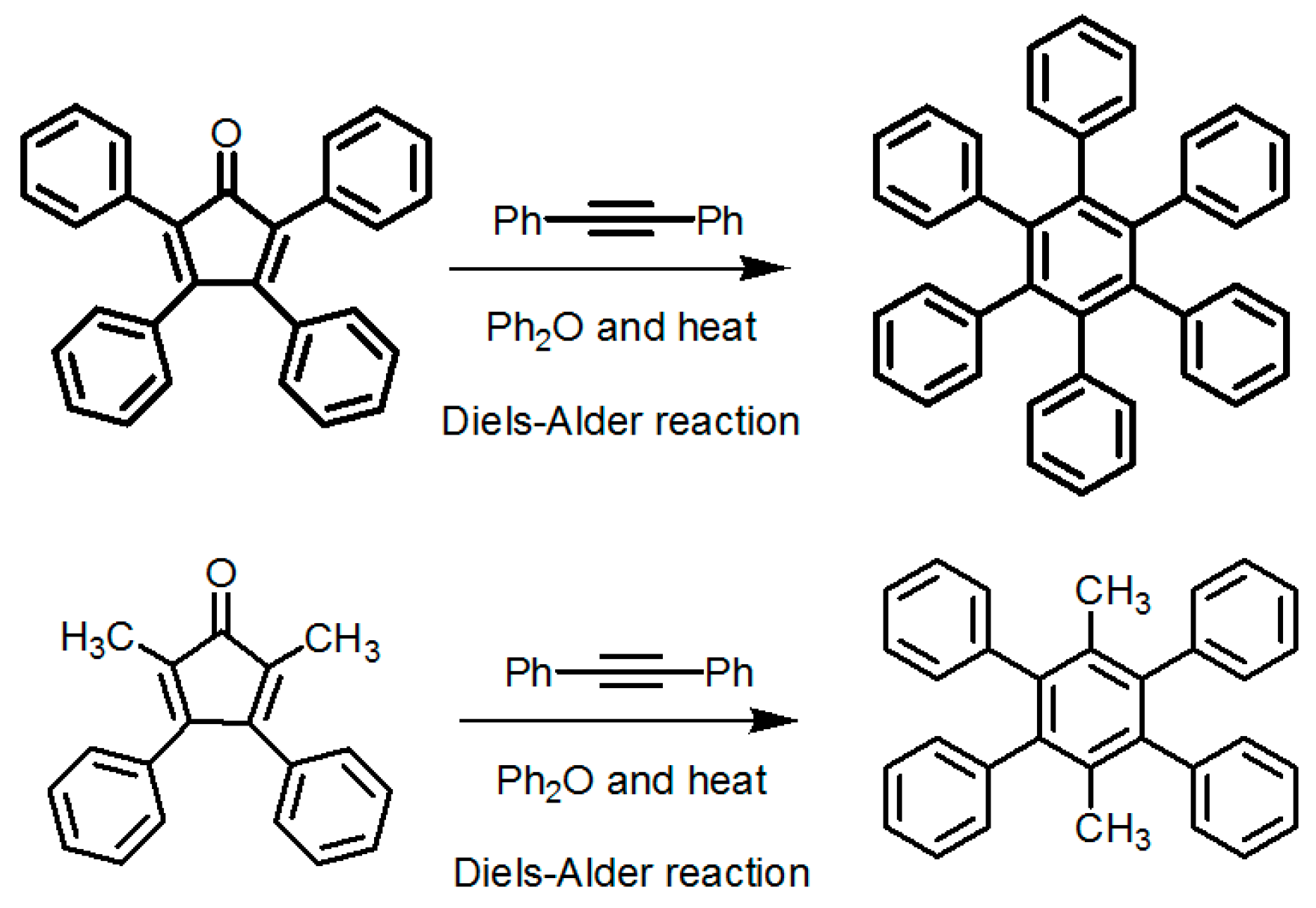
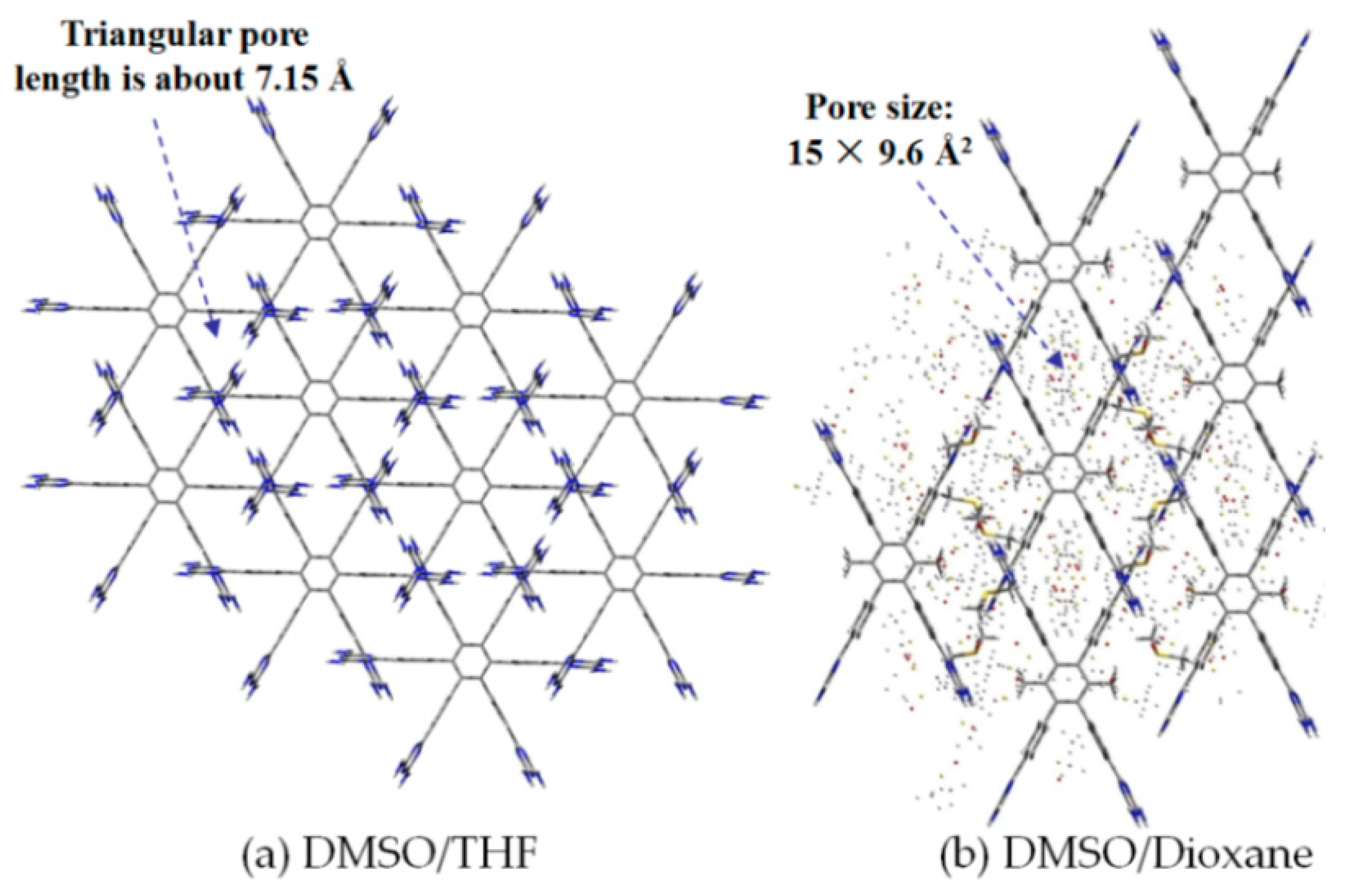
2.7.2. Planar DAT-Based Building Blocks
2.7.3. DAP Moieties as Hydrogen-Bonded Motifs for HOFs
2.7.4. Potential Applications of DAT-Based HOFs
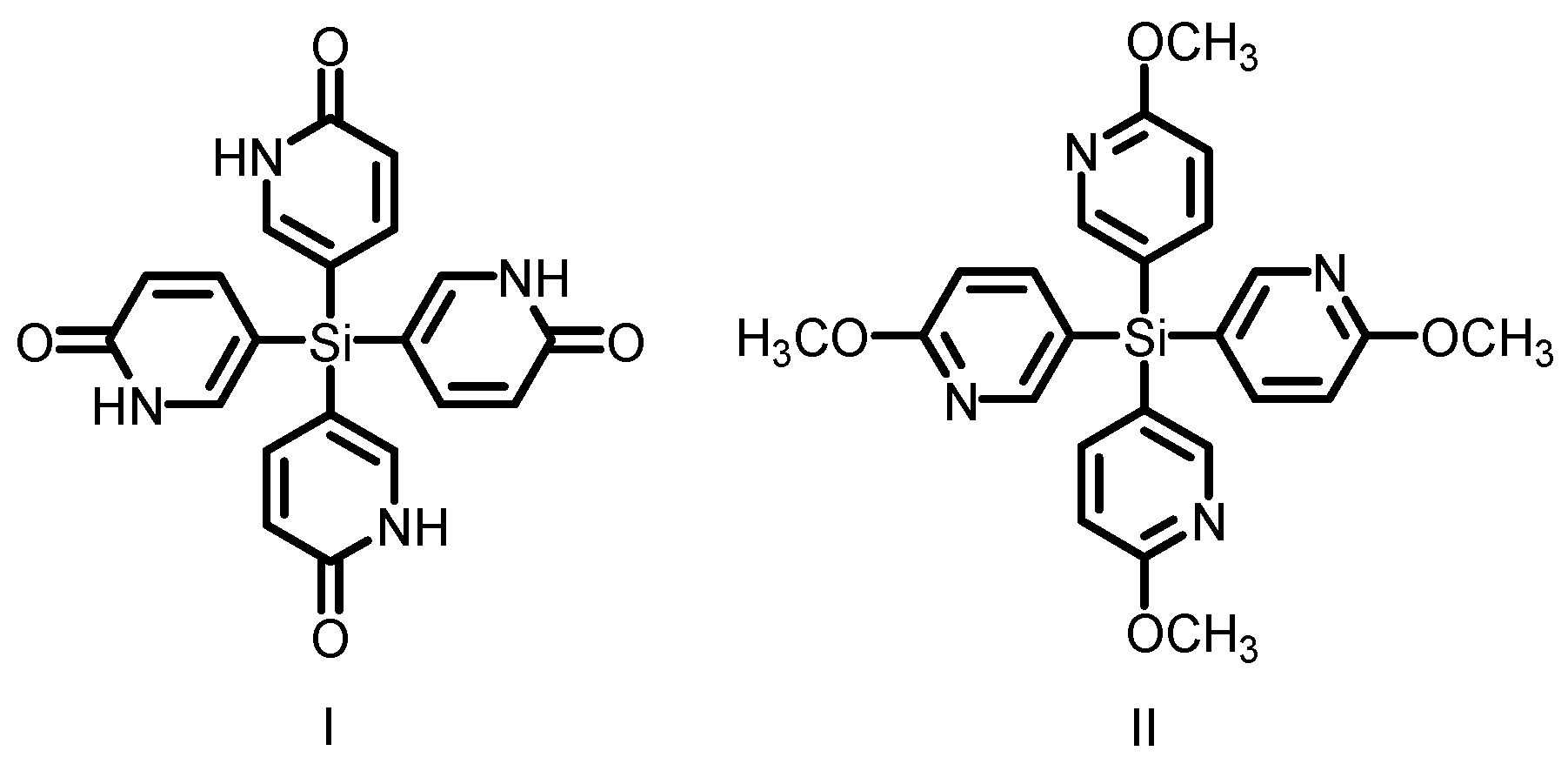
2.8. Charge-Assisted Hydrogen Bonds for HOFs
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endo, K.; Koike, T.; Sawaki, T.; Hayashida, O.; Masuda, H.; Aoyama, Y. Catalysis by organic solids. Stereoselective Diels-Alder reactions promoted by microporous molecular crystals having an extensive hydrogen-bonded network. J. Am. Chem. Soc. 1997, 119, 4117–4122. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, P.; Bracco, S.; Comotti, A.; Ferretti, L.; Simonutti, R. Methane and carbon dioxide storage in a porous van der waals crystal. Angew. Chem. Int. Ed. 2005, 44, 1816–1820. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Ren, H.; Ma, S.Q.; Cao, D.P.; Lan, J.H.; Jing, X.F.; Wang, W.C.; Xu, J.; Simmons, J.M.; Qiu, S.L.; et al. Targeted synthesis of porous aromatic framework with high stability and exceptionally high surface areas. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef] [PubMed]
- Dalgarno, S.J.; Thallapally, P.K.; Barbour, L.J.; Atwood, J.L. Engineering void space in organic van der waals crystals: Calixarenes lead the way. Chem. Soc. Rev. 2007, 36, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Valence-Tautomeric Metal-Organic Nanoparticles. Chem. Soc. Rev. 2007, 36, 770–818. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J., IV; Perman, J.A.; Zaworokto, M.J. Design and synthesis of metal-organic using metal-organic polyhedra as supramolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.D.; Liu, D.M.; Lin, W.B. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Mckinlay, A.C.; Morris, R.E.; Horcajada, P.; Férry, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férry, G.; Morrs, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Rexford, R.C.; Rocca, J.D.; Lin, W.B. Metal-organic frameworks as potential drugs carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFS): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Q.; Wei, Z.W.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.W.; Zhang, Q.; Gentle, T., III; et al. Tuning structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature 1993, 365, 499–505. [Google Scholar] [CrossRef]
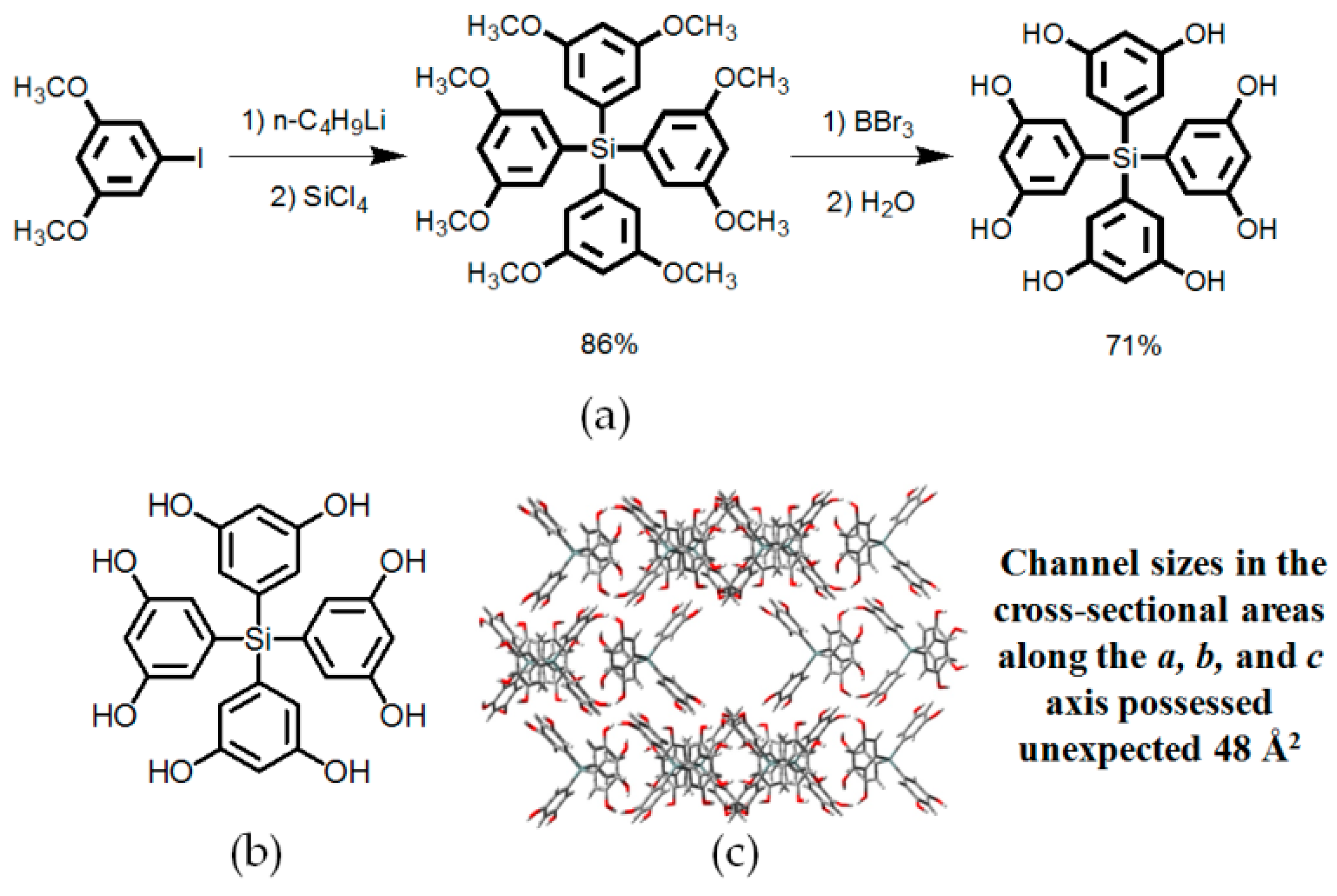
- Saied, O.; Maris, T.; Simard, M.; Wuest, J.D. Tetrakis(2-methoxy-5-pyridyl)silane. Acta Cryst. 2005, E61, 4136–4138. [Google Scholar] [CrossRef]
- Fournier, J.H.; Maris, T.; Wuest, J.D. Molecular tectonics. Hydrogen-bonded networks built from tetraphenols derived from tetraphenylmethane and tetraphenylsilane. Cryst. Growth Des. 2003, 3, 535–540. [Google Scholar] [CrossRef]
- Laliberté, D.; Maris, T.; Demers, E.; Helzy, F.; Arseneault, M.; Wuest, J.D. Molecular tectonics. Hydrogen-bonded networks built from tetraphenols derived from tetra- and Hex-aanlines. Cryst. Growth Des. 2005, 5, 1451–1456. [Google Scholar] [CrossRef]
- Laliberté, D.; Maris, T.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks built from derivatives of pentaerythrityl tetraphenyl ether. J. Org. Chem. 2004, 69, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.H.; Maris, T.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks built from derivatives of 9,9′-spirobifluorene. J. Org. Chem. 2004, 69, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Maly, K.E.; Maris, T.; Gagnon, E.; Wuest, J.D. Inclusion compounds of hexakis(4-cyanophenyl)benzene: Open networks maintained by C–H⋯π interactions. Cryst. Growth Des. 2006, 6, 461–466. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shirasaka, T.; Stao, A.; Horn, E.; Furukawa, N. Self-assembly of a radially functionalized hexagonal molecule: Hexakis(4-hydroxyphenyl)benzene. Angew. Chem. Int. Ed. 1999, 38, 3483–3486. [Google Scholar] [CrossRef]
- Kobayashi, K.; Stao, A.; Sakamoto, S.; Yamaguchi, K. Solvent-induced polymorphism of three-dimensional hydrogen-bonded networks of hexakis(4-carbamoylphenyl)benzene. J. Am. Chem. Soc. 2003, 125, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Shirasaka, T.; Horn, E.; Furukawa, N. Two-dimensional hexagonal hydrogen-bonded network with triangle0like large cavities: Hexakis(4-carboxyphenyl)benzene. Tetrahedron Lett. 2000, 41, 89–93. [Google Scholar] [CrossRef]
- Bhyrappa, P.; Wilson, S.R.; Suslick, K.S. Hydrogen-boned porphyrinicsolids: Supramolecular networks of octahydroxyporphyrins. J. Am. Chem. Soc. 1997, 119, 8492–8502. [Google Scholar] [CrossRef]
- Sawaki, T.; Endo, K.; Kobayashi, K.; Hayashida, O.; Aoyama, Y. Catalysis by organic solids. Stereoselective intramolecular ene reaction of citronellal promoted by microporous molecular crystals having an extensive hydrogen-bonded network. Bull. Chem. Soc. Jpn. 1997, 70, 3075–3079. [Google Scholar] [CrossRef]
- Saied, O.; Maris, T.; Wang, X.; Simard, M.; Wuest, J.D. Submaximal interpenetration and bicontinuous three-dimensional channels in porous molecular networks. J. Am. Chem. Soc. 2005, 127, 10008–10009. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.H.; Maris, T.; Wuest, J.D.; Guo, W.Z.; Galoppini, E. Molecular tectonics use of the hydrogen bonding boronic acids to direct supramolecular construction. J. Am. Chem. Soc. 2003, 125, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Durka, K.; Jarzembska, K.N.; Kamiński, R.; Luliński, S.; Serwatowski, J.; Woźniak, K. Nanotubular hydrogen-bonded organic framework architecture of 1,2-phenylenediboronic acid hosting ice clusters. Cryst. Growth Des. 2013, 13, 4181–4185. [Google Scholar] [CrossRef]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer research and applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Ermer, O. Five-fold diamond structure of adamantine-1,3,5,7-tetracarboxylic acid. J. Am. Chem. Soc. 1988, 110, 3747–3754. [Google Scholar] [CrossRef]
- Duchampe, D.J.; Marsh, R.E. The crystal structure of trimesic acid. Acta Crystallogr. Sect. B 1969, 25, 5–19. [Google Scholar] [CrossRef]
- Hisaki, I.; Nakagawa, S.; Ikenaka, N.; Imamura, Y.; Katouda, M.; Tashiro, M.; Tsuchida, H.; Ogoshi, T.; Sato, H.; Tohnai, N.; et al. A series of layered assemblies of hydrogen-bonded, hexagonal networks of C3-symmetric π-conjugated molecules: A potential motif of porous organic materials. J. Am. Chem. Soc. 2016, 138, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Nakagawa, S.; Tohnai, N.; Miyata, M. A C3-symmetric macrocycle-based, hydrogen-bonded, multiparous hexagonal network as a motif of porous molecular crystals. Angew. Chem. Int. Ed. 2015, 54, 3008–3012. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Chuang, Y.C.; Chen, Y.S.; Daugulis, O.; Jacobson, A.J.; Miljanic, O.S. Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 2015, 5, 5131–5138. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M.; Oppel, I.M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 2012, 51, 5252–5255. [Google Scholar] [CrossRef] [PubMed]
- Zonta, C.; Lucchi, O.D.; Linden, A.; Lutz, M. Synthesis and structure of D3h-symmetric triptycenetrimaleimide. Molecules 2010, 15, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.Z.; Jia, X.J.; Deng, J.H.; Zhong, J.L.; Liu, H.J.; Wang, K.J.; Zhong, D.C. A microporous hydrogen-bonded organic frameworks: Exceptional stability and highly selective adsorption of gas and liquid. J. Am. Chem. Soc. 2013, 135, 11684–11687. [Google Scholar] [CrossRef] [PubMed]
- Adelizzi, B.; Filot, I.A.W.; Palmans, A.R.A.; Meijer, E.W. Unravelling the pathway complexity in conformationally flexible N-centered triarylamine trisamides. Chem. Eur. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.M.J.; Nieuwenhuizen, M.M.L.; Crossman, M.; de Greef, T.F.A.; Schenning, A.P.H.J.; Palamans, A.R.A.; Meijer, E.W. Cooperative two-component self-assembly of mono and ditopic monomers. Macromolecules 2011, 44, 6581–6587. [Google Scholar] [CrossRef]
- Dewal, M.B.; Lufaso, M.W.; Hughes, A.D.; Samuel, S.A.; Pellechia, P.; Shimizu, L.S. Absorption properties a porous organic crystalline apohost formed by a self-assembled bis-urea macrocycle. Chem. Mater. 2006, 18, 4855–4864. [Google Scholar] [CrossRef]
- Shimizu, L.S.; Hughes, A.D.; Simth, M.D.; Davis, M.J.; Zhang, B.P.; Loye, H.-C.Z.; Shimizu, K.D. Self-assembled nanotubes that reversibly bind acetic acid guests. J. Am. Chem. Soc. 2003, 125, 14972–14973. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.Y.; Wei, P.F.; Shi, B.B.; Huang, F.H. A pillar[6]arene-based[2]pseudorotaxane in solution and in the solid state and its photo-responsive self-assembly behavior in solution. Chem. Commun. 2016, 52, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.F.; Ni, M.F.; Hu, X.Y.; Xiao, T.X.; Xiong, S.H.; Chen, L.; Wang, L.Y. Pillar[5]arene-based polymeric architectures constructed by orthogonal supramolecular interactions. Chem. Commun. 2012, 48, 8529–8531. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wu, X.; Duan, Q.P.; Xiao, T.X.; Chen, L.; Wang, L.Y. Novel Pillar[5]arene-based dynamic polyrotaxanes interlocked by the quadruple hydrogen bonding ureidopyrimidone motif. Org. Lett. 2012, 14, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Li, H.W.; Tao, Y.C.; Zhang, S.X.-A.; Wang, B.; Yang, Y.W. Pillar[5]arene-based supramolecular organic frameworks for highly selectiveCO2-capture at ambient conditions. Adv. Mater. 2014, 26, 7027–7031. [Google Scholar] [CrossRef] [PubMed]
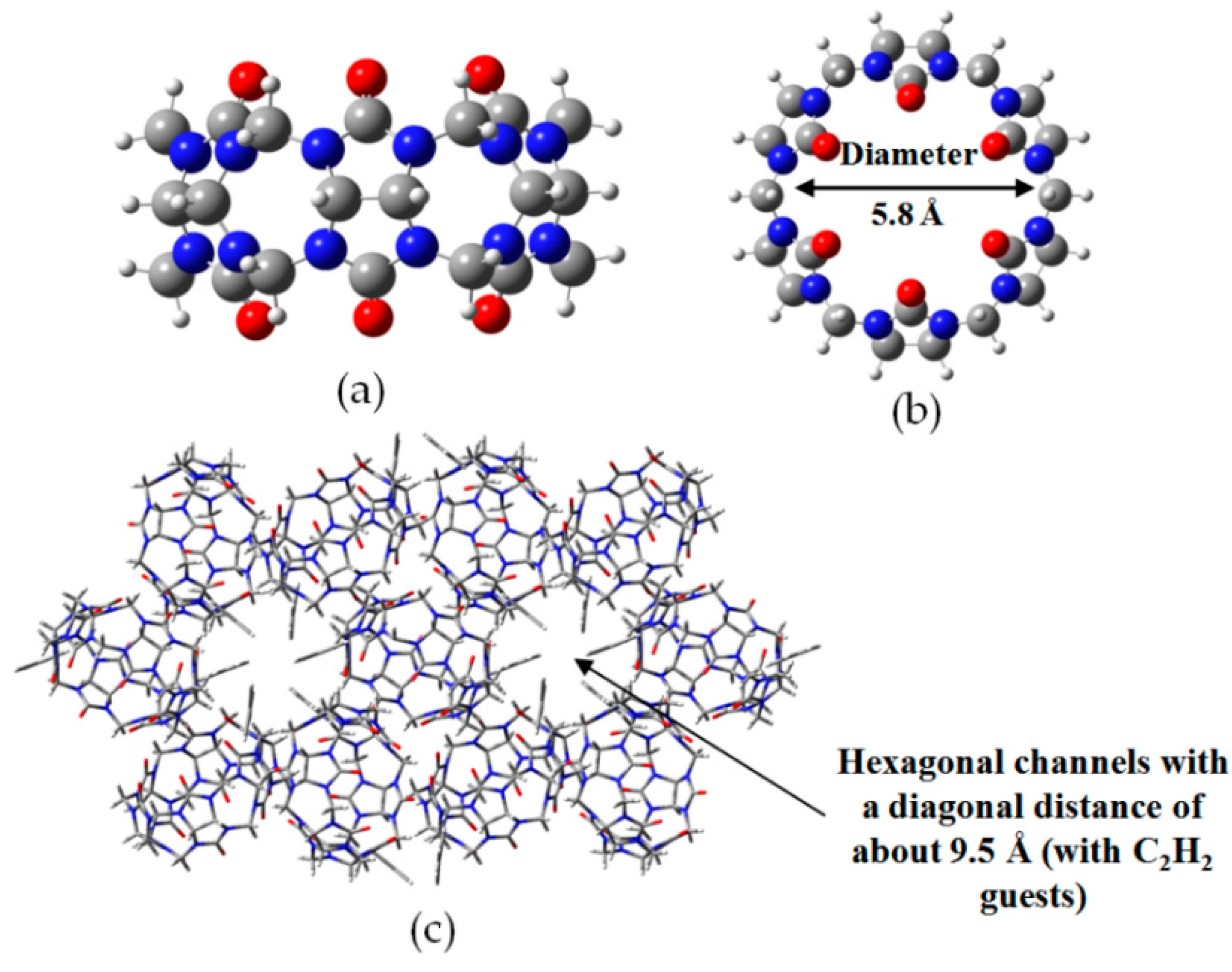
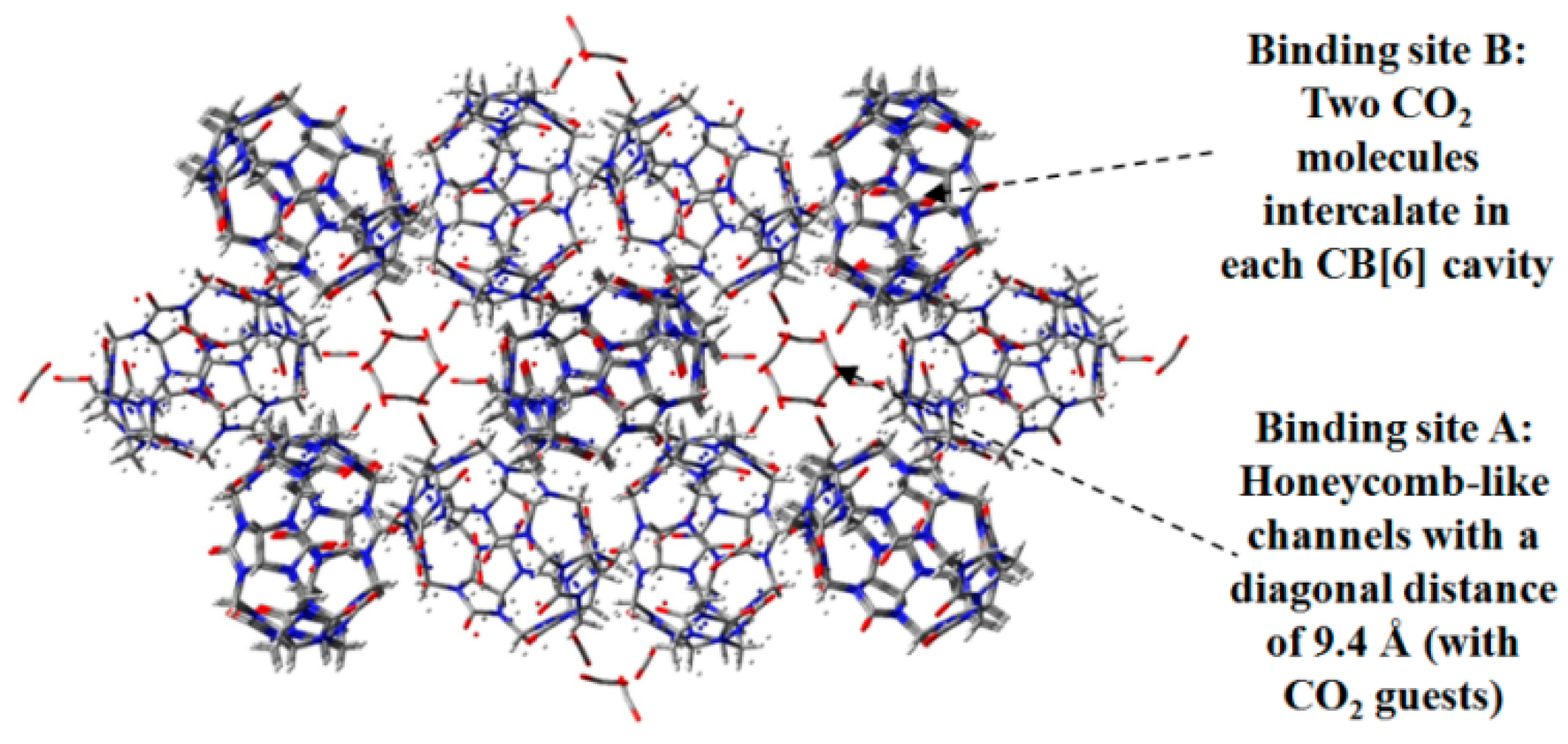
- Lim, S.; Kim, H.; Selvapalam, K.; Kim, J.; Cho, S.J.; Seo, G.; Kim, K. Cucurbit[6]uril: Organic molecular porous material with permanent porosity, exceptional stability, and acetylene sorption properties. Angew. Chem. Int. Ed. 2008, 120, 3400–3403. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Yoon, M.; Lim, S.; Park, S.M.; Seo, G.; Kim, K. Highly selective carbon dioxide sorption in an organic molecular porous material. J. Am. Chem. Soc. 2010, 132, 12200–12202. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, L.-L.; Jia, P.; Li, Q.-L.; Sun, Y.-L.; Zhang, J.; Ning, Y.-Q.; Yu, J.H.; Yan, Y.-W. Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nonaorods. Chem. Sci. 2014, 5, 2804–2808. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zhou, Y.; Li, Q.-L.; Yang, Y.-W. Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]arene [2]pseudorotaxanes for controlled cargo release. Chem. Commun. 2013, 49, 9033–9035. [Google Scholar] [CrossRef] [PubMed]
- Tsue, H.; Ishibashi, K.; Tokita, S.; Takahashi, H.; Matsui, K.; Tamura, R. Azacalix[6]arenehexamethyl ether: Synthesis, structure, and selective uptake of carbon dioxide in the solid state. Chem. Eur. J. 2008, 14, 6125–6134. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, D.V.; Moudrakovski, I.L.; Grachev, E.V.; Ripmeester, J.A. Micropores in crystalline dipeptides as seen from the crystal structure He pycnometry, and 129Xe NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Comotti, A.; Bracco, S.; Distefano, G.; Sozzani, P. Methane, carbon dioxide and hydrogen storage in nanoporous dipeptide-based materials. Chem. Commun. 2009, 3, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Gӧrbitz, C.H. An exceptionally stable peptide nanotube system with flexible pores. Acta Crystallogr. Sect. B 2002, 58, 849–854. [Google Scholar] [CrossRef]
- Gӧrbitz, C.H. Microporous organic materials from hydrophobic dipeptides. Chem. Eur. J. 2007, 13, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Mrackov, M.H.; Lee, C.M. 2-pyridone, 2-pyridthione, and 2-pyridselenone. Hydrogen-bonding ability as determined by moment and molecular weight determinations. J. Am. Chem. Soc. 1965, 87, 892–896. [Google Scholar] [CrossRef]
- Wash, P.L.; Maverick, E.; Chiefari, J.; Lightner, D.A. Acid-amide intermolecular hydrogen bonding. J. Am. Chem. Soc. 1997, 119, 3802–3806. [Google Scholar] [CrossRef]
- Ducharme, Y.; Wuest, J.D. Use of hydrogen bonds to control molecular aggregation. Extension, self-complementary arrays of donors and acceptors. J. Org. Chem. 1988, 53, 5787–5789. [Google Scholar] [CrossRef]
- Malek, N.; Maris, T.; Perron, M.-E.; Wuest, J.D. Molecular tectionics: Porous cleavable networks constructed from dipole-directed stacking of hydrogen-bonded sheets. Angew. Chem. Int. Ed. 2005, 44, 4021–4025. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Su, D.; Wuest, J.D. Use of hydrogen bonds to control molecular aggregation: Self-assembly of three-dimensional networks with large chambers. J. Am. Chem. Soc. 1991, 113, 4696–4698. [Google Scholar] [CrossRef]
- Yan, X.Z.; Li, S.J.; Pollock, J.B.; Cook, T.R.; Chen, J.Z.; Zhang, Y.Y.; Ji, X.F.; Yu, Y.H.; Huang, F.H.; Stang, P.J. Supramolecular polymers with tunable topologies via hierarchical coordination-driven self-assembly and hydrogen bonding interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 15585–15590. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Z.; Jiang, B.; Cook, T.R.; Zhang, Y.Y.; Li, J.Y.; Yu, Y.H.; Huang, F.H.; Yang, H.B.; Stang, P.J. Dendronized organoplatinum metallacyclic polymers constructed by hierarchical coordination-driven self-assembly and hydrogen-bonding interfaces. J. Am. Chem. Soc. 2013, 135, 16813–16816. [Google Scholar] [CrossRef] [PubMed]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Loew, J.K.L.; Meijer, E.W. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, Y.-C.; Han, B.-H. Supramolecular organic network assembled from quadruple hydrogen-bonding motifs. Chem. Commun. 2016, 52, 6597–6600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Rominger, F.; Mastalerz, M. Hydrogen-bonded chains and networks of triptycene-based triboronic acid and tripridione. Cryst. Growth Des. 2016, 16, 5542–5548. [Google Scholar] [CrossRef]
- Hirschberg, J.H.K.K.; Brunsveld, L.; Ramzi, A.; Vekemans, J.A.J.M.; Sijbesma, R.P.; Meijer, E.W. Helical self-assembled polymers from cooperative stacking of hydrogen-bonded pairs. Nature 2000, 407, 167–170. [Google Scholar] [PubMed]
- Brunsveld, L.; Vekemans, J.A.J.M.; Hirschberg, J.H.K.K.; Sijbesma, R.P.; Meijer, E.W. Hierachical formation of helical supramolecular polymers via stacking of hydrogen-bonded pairs in water. Proc. Natl. Acad. Sci. USA 2002, 99, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Wuest, J.D. Engineering crystals by the strategy of molecular tectonics. Chem. Commun. 2005, 47, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Brunet, P.; Simard, M.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 1997, 119, 2737–2738. [Google Scholar] [CrossRef]
- Li, P.; He, Y.B.; Arman, H.D.; Krishna, R.; Wang, H.L.; Weng, L.H.; Chen, B.L. A microporous six-fold interpenetrated hydrogen-bonded organic framework for highly selective separation of C2H4/C2H6. Chem. Commun. 2014, 50, 13081–13084. [Google Scholar] [CrossRef] [PubMed]
- Fur, E.L.; Demers, E.; Maris, T.; Wuest, J.D. Excavations in molecular crystals. Chem. Commun. 2003, 24, 2966–2967. [Google Scholar]
- Malek, N.; Maris, T.; Simard, M.; Wuest, J.D. Molecular tectonics selective exchange of cations in porous anionic hydrogen-bonded networks built from derivatives of tetraphenylborate. J. Am. Chem. Soc. 2005, 127, 5910–5916. [Google Scholar] [CrossRef] [PubMed]
- Dorizon, H.S.; Maris, T.; Wuest, J.D. Molecular tectonics construction of porous hydrogen-bonded networks from bisketals of pentaerythritol. J. Org. Chem. 2003, 68, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, D.; Maris, T.; Sirois, A.; Wuest, J.D. Molecular tectonics. Dendritic construction. Org. Lett. 2003, 5, 4787–4790. [Google Scholar] [CrossRef] [PubMed]
- Demers, E.; Maris, T.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks built from derivatives of 2,2′,7,7′-tetraphenyl-9,9′-spirobi[9H-fluorene]. Cryst. Growth Des. 2005, 5, 1227–1235. [Google Scholar] [CrossRef]
- Helzy, F.; Maris, T.; Wuest, J.D. Engineering hydrogen-bonded hexagonal networks built from flexible 1,3,5-trisubstituted derivatives of benzene. J. Org. Chem. 2016, 81, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- Helzy, F.; Maris, T.; Wuest, J.D. Engineering hydrogen-bonded molecular crystals built from 1,3,5-substituted derivatives of benzene:6,6′,6″-(1,3,5-phenylene)tris-1,3,5-triazine-2,4-diamines. Cryst. Growth Des. 2008, 8, 1547–1553. [Google Scholar] [CrossRef]
- Maly, K.E.; Gagnon, E.; Maris, T.; Wuest, J.D. Engineering hydrogen-bonded molecular crystals built from derivatives of hexaphenylbenzene and related compounds. J. Am. Chem. Soc. 2007, 129, 4306–4322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Li, B.; Wu, H.; Hu, T.-L.; Yao, Z.Z.; Zhou, W.; Xiang, S.C.; Chen, B.L. A flexible microporous hydrogen-bonded organic framework for gas sorption and separation. J. Am. Chem. Soc. 2015, 137, 9963–9970. [Google Scholar] [CrossRef] [PubMed]
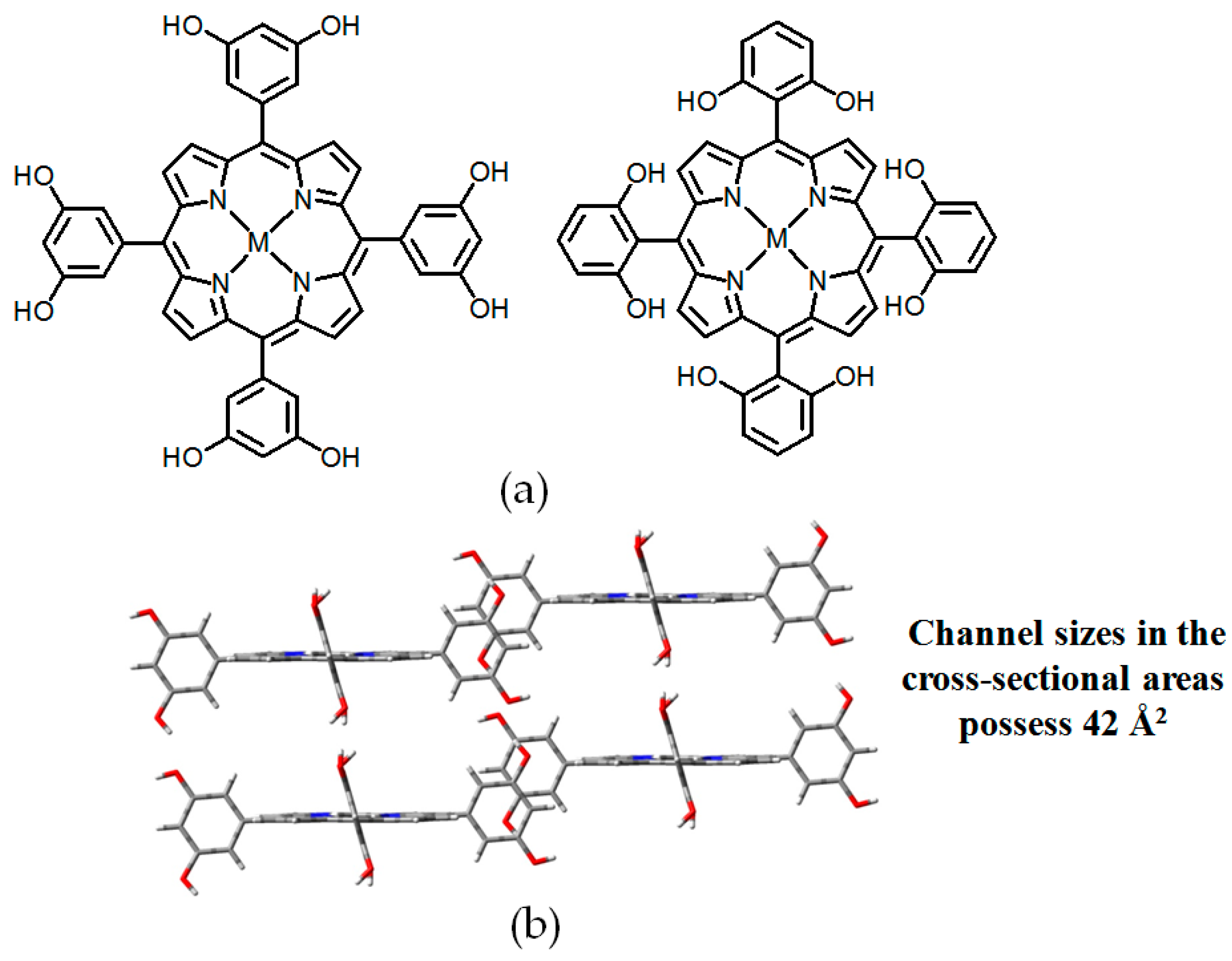
- Yang, W.; Li, B.; Wang, H.L.; Alsuhaish, O.; Alfooty, K.; Zayed, M.A.; Li, P.; Arman, H.D.; Chen, B.L. A microporous porphyrin-based hydrogen-bonded organic framework for gas separation. Cryst. Growth Des. 2015, 15, 2000–2004. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.Y.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-H.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Feng, G.; Xu, S.; Zhu, Z.; Lu, X.; Wu, J.; Liu, B. Structure-dependent cis/trans isomerization of tetraphenylethene derivatives: Consequences for aggregation-induced emission. Angew. Chem. Int. Ed. 2016, 55, 6192–6196. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Alduhaish, O.; Arman, H.D.; Wang, H.L.; Alfooty, K.; Chen, B.L. Solvent dependent structures of hydrogen-bonded organic frameworks of 2,6-diaminopurine. Cryst. Growth Des. 2014, 14, 3634–3638. [Google Scholar] [CrossRef]
- He, Y.B.; Xiang, S.C.; Chen, B.L. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 2011, 133, 14570–14573. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, Y.B.; Zhao, Y.F.; Weng, L.H.; Wang, H.L.; Krishna, R.; Wu, H.; Zhou, W.; O’Keeffe, M.; Han, Y.; et al. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. 2015, 54, 574–577. [Google Scholar]
- Yang, W.; Yang, F.; Hu, T.-L.; King, S.C.; Wang, H.L.; Wu, H.; Zhou, W.; Li, J.-R.; Arman, H.D.; Chen, B.L. Microporous diaminotriazine-decorated porphyrin-based hydrogen-bonded organic framework: Permanent porosity and proton conduction. Cryst. Growth Des. 2016, 16, 5831–5835. [Google Scholar] [CrossRef]
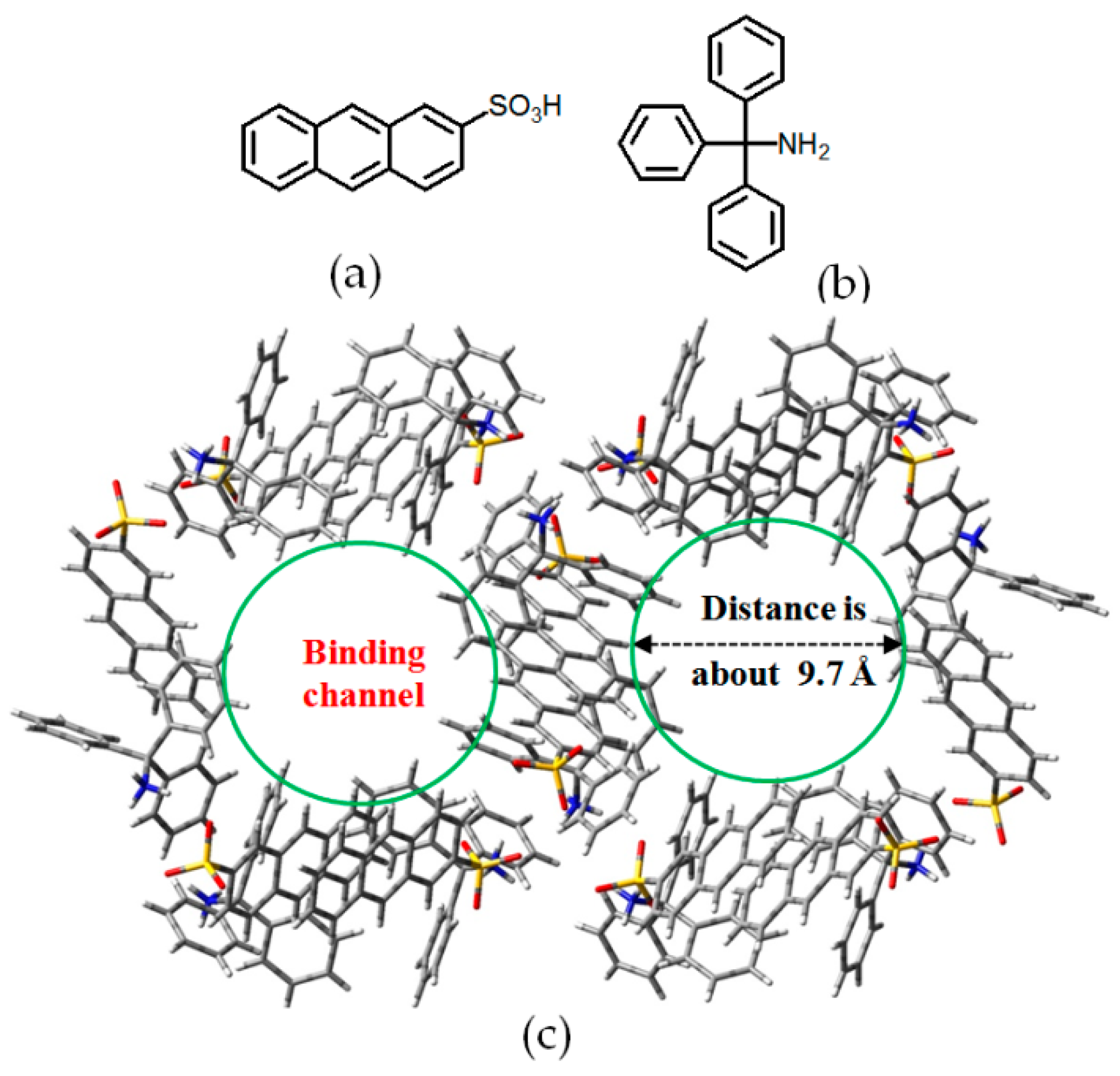
- Sun, Z.Y.; Li, Y.X.; Chen, L.; Jing, X.B.; Xie, Z.G. Fluorescent hydrogen-bonded organic framework for sensing of aromatic compounds. Cryst. Growth Des. 2015, 15, 542–545. [Google Scholar] [CrossRef]
- Li, P.; He, Y.B.; Guang, J.; Weng, L.H.; Zhao, J.C.-G.; Xiang, S.C.; Chen, B.L. A homo chiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohol. J. Am. Chem. Soc. 2014, 136, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Y.M.; Gao, F.; Zhai, X.Y.; Sun, M.G.; Liu, W.; Cao, Z.Q.; Liu, W.G. High strength multifunctional multiwalled hydrogel tubes: Ion-triggered shape memory, anti-bacterial, and anti-inflammatory efficacies. ACS Appl. Mater. Interfaces 2015, 7, 16865–16872. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, Y.Y.; Liu, W.G. Hydrogen-bonding toughened hydrogels and emerging CO2-responsive shape memory effect. Macromol. Rapid. Commun. 2015, 36, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, Y.M.; Zhang, Y.Y.; Liao, Y.; Liu, W.G. High-strength photoresponsive hydrogels enable surface-mediated gene delivery and light-induced reversible cell adhesion/detachment. Langmuir 2014, 30, 11823–11832. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Ban, T.; Gotoh, S.; Hishiya, T.; Komiyama, M. Hydrogen bonding in water by poly(vinyldiaminotriazine) for the molecular recognition of nucleic acid bases and their derivatives. Macromolecules 1998, 31, 371–377. [Google Scholar] [CrossRef]
- Nie, L.H.; Ma, H.M.; Li, X.H.; Sun, M.; Xiong, S.X. Recognition of thymine by triazine fluorescent probe through intermolecular multiple hydrogen bonding. Biopolymers 2003, 72, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.M.; Nie, L.H.; Xiong, S.X. Recognition of guanine by a designed triazine-based fluorescent probe through intermolecular multiple hydrogen bonding. Supramol. Chem. 2004, 16, 311–317. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hirukawa, T.; Hisaki, I.; Miyata, M.; Tohnai, N. Multifunctionalised porosity in zeolitic diamondoid porous organic salt: Selective adsorption and guest-responsive fluorescent properties. Tetrahedron Lett. 2013, 54, 1268–1273. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hamada, T.; Hisaki, I.; Miyata, M.; Tohnai, N. Dynamically deformable cube-like hydrogen-bonding networks in water-responsive diamondoid porous organic salts. Angew. Chem. Int. Ed. 2013, 52, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Lie, S.; Maris, T.; Wuest, J.D. Molecular networks created by charged–assisted hydrogen-bonding in phosphonate, phosphate, and sulfonate salts of bsi(amidines). Cryst. Growth Des. 2014, 14, 3658–3666. [Google Scholar] [CrossRef]
- Lie, S.; Maris, T.; Malveau, C.; Beaudoin, D.; Helzy, F.; Wuest, J.D. Molecular networks created by charged–assisted hydrogen-bonding in carboxylate salts of a bis(amidine). Cryst. Growth Des. 2013, 13, 1872–1877. [Google Scholar] [CrossRef]
- Zhang, L.; Kucera, L.R.; Ummadisetty, S.; Nykaza, J.R.; Elabd, Y.A.; Storey, R.F.; Cavicchi, K.A.; Weiss, R.A. Supramolecular Multiblock polystyrene-polyisobutylene copolymers via ionic interactions. Macromolecules 2014, 47, 4387–4396. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Zhang, L.H.; Caicch, K.A.; Singba, N.K. Tailor-made fluorinated copolymer/clay nanocomposite by cationic RAFT assisted pickering miniemulsion polymerization. Langmuir 2015, 31, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Trolliet, C.; Poulet, G.; Tuel, A.; Wuest, J.D.; Sautet, P. A theoretical study of cohesion, structural deformation, inclusion, and dynamics in porous hydrogen-bonded molecular networks. J. Am. Chem. Soc. 2007, 129, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.A.; Samori, P.; Cechini, M. Atomistic simulations of 2D bicomponent self-assembly: From molecular recognition to self-healing. J. Am. Chem. Soc. 2010, 132, 17880–17885. [Google Scholar] [CrossRef] [PubMed]

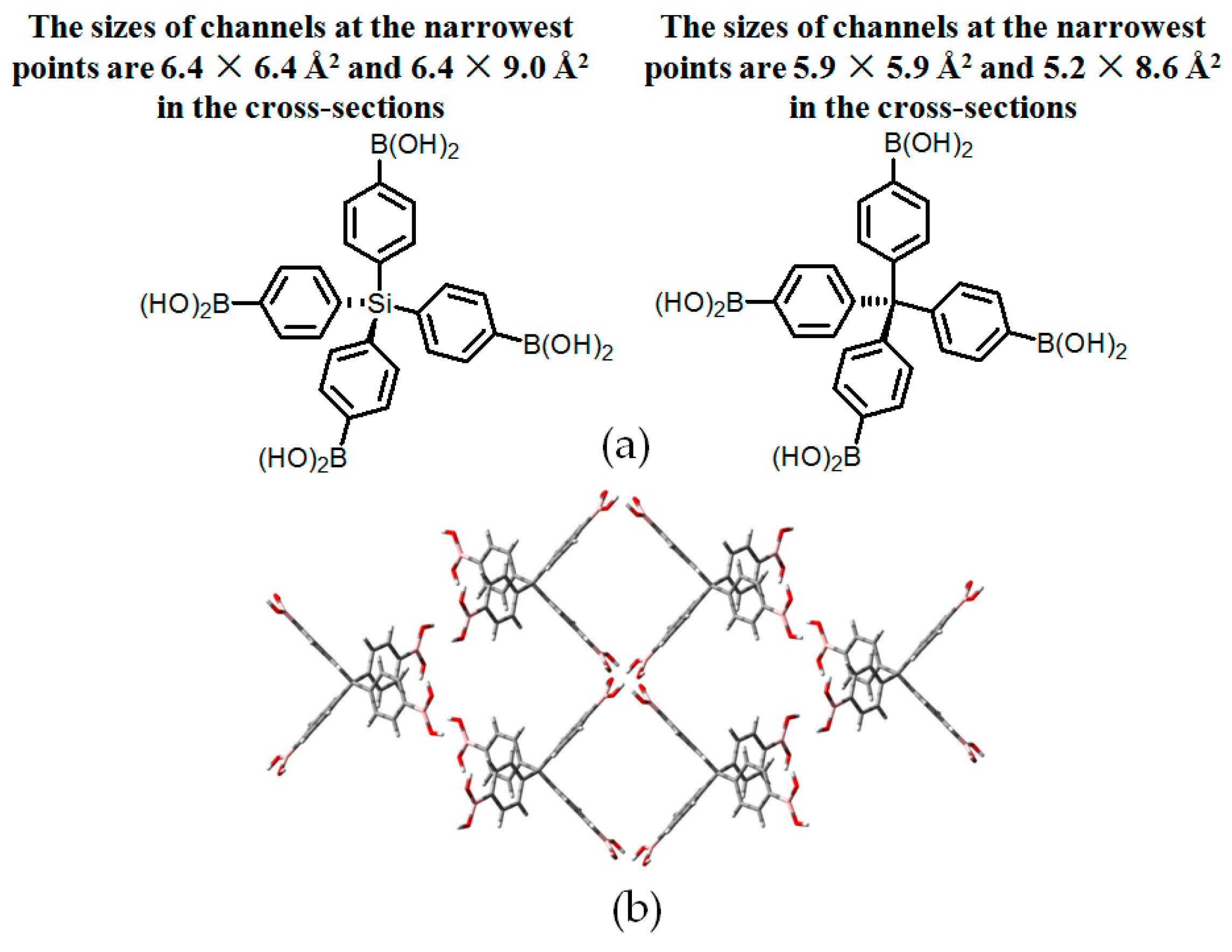


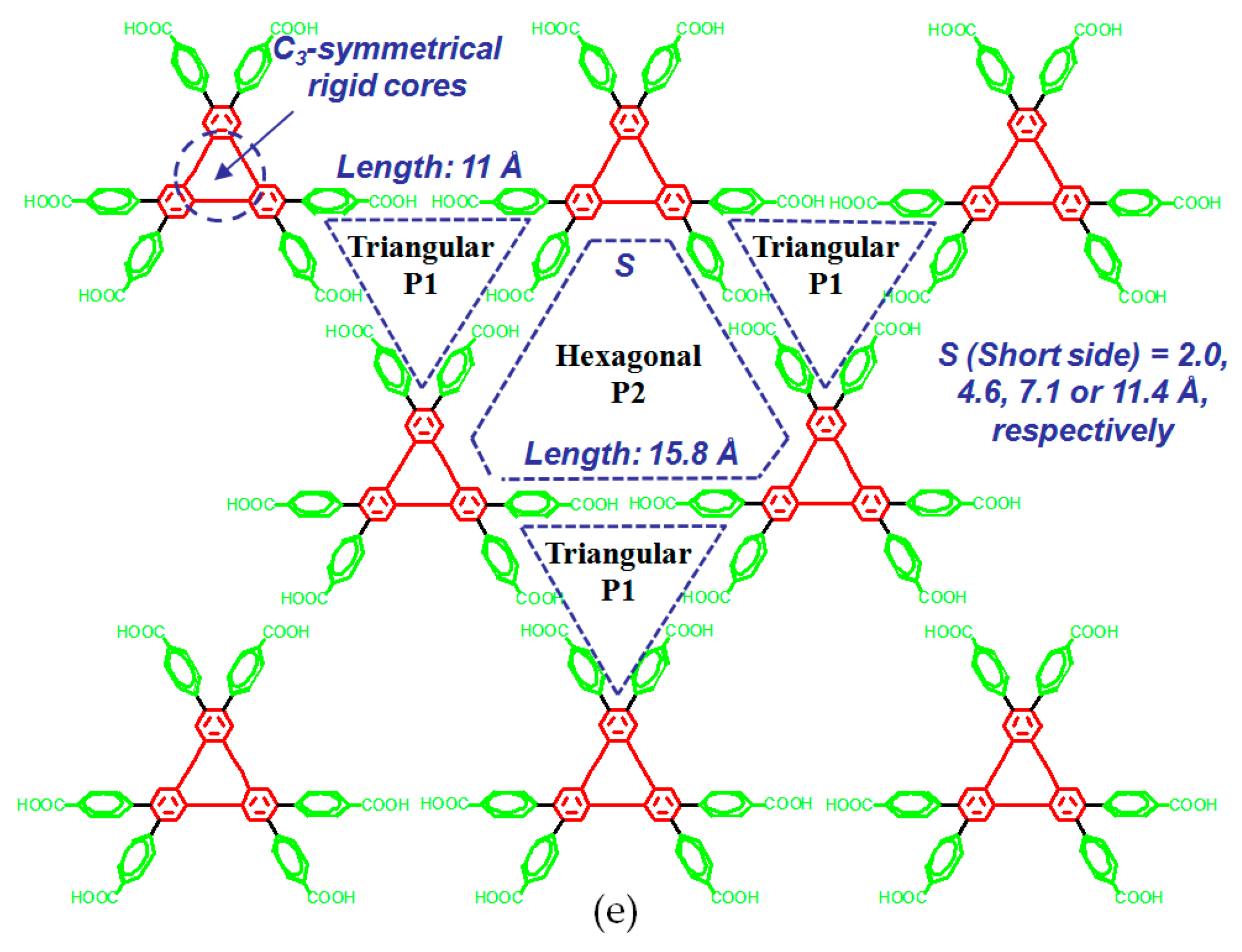

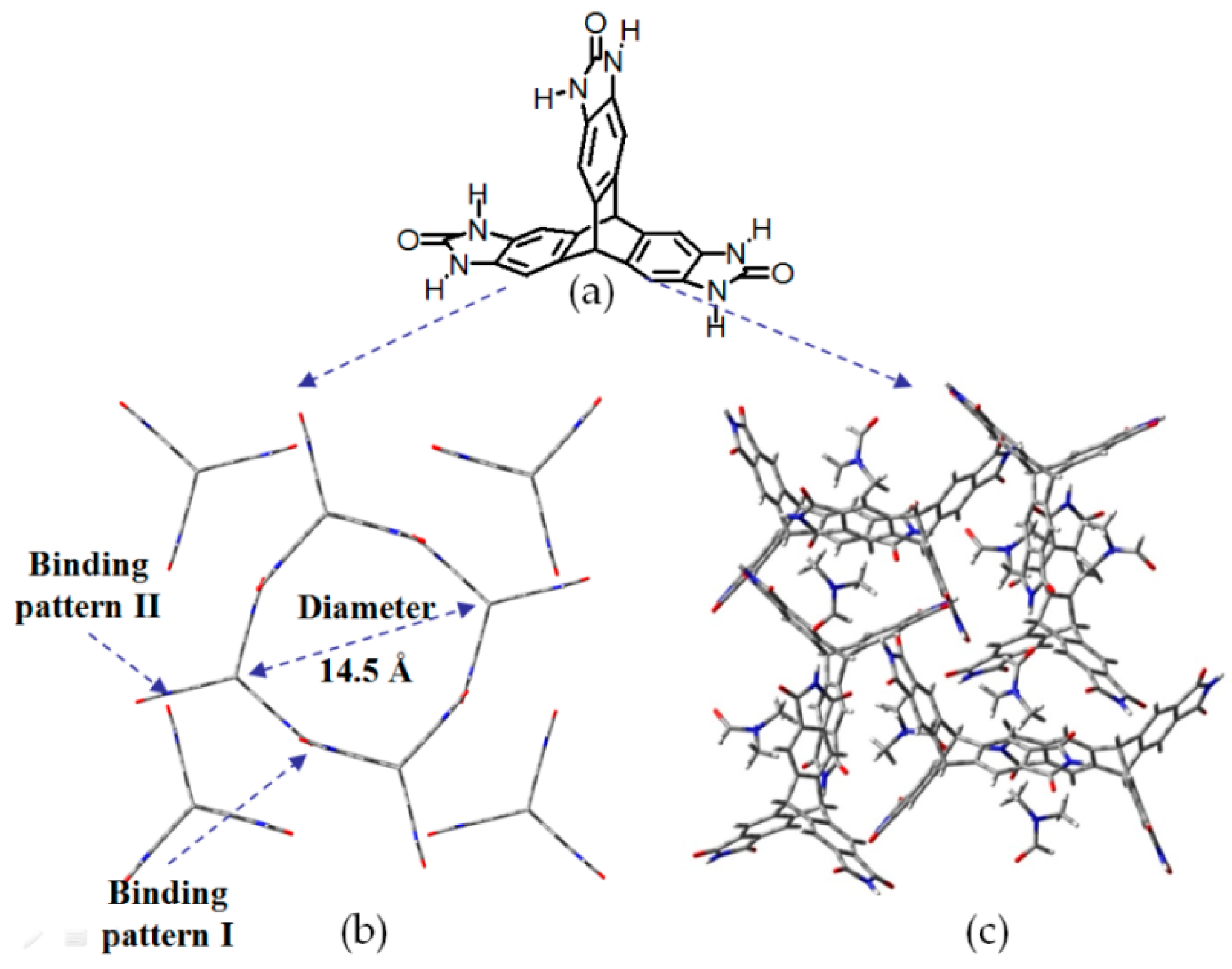
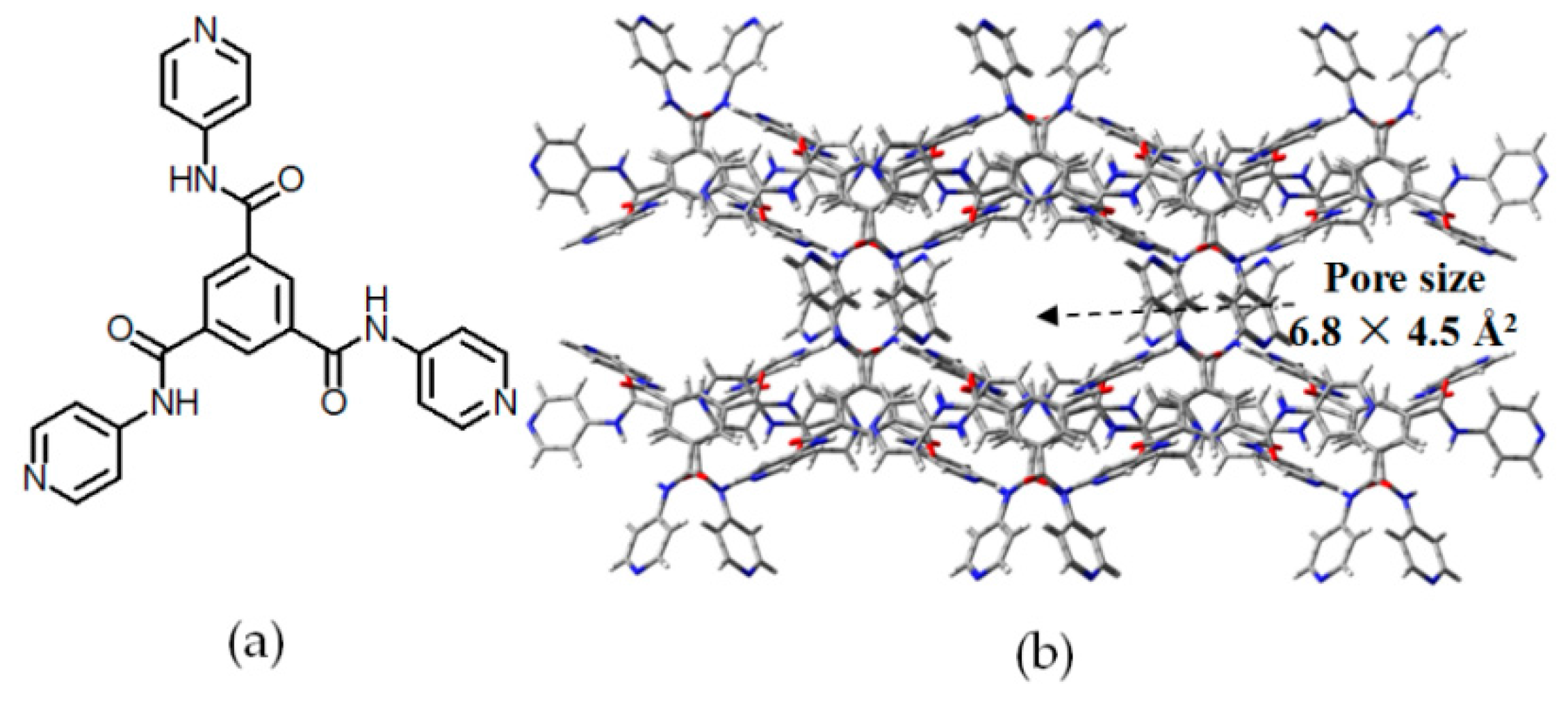




| Scaffold | Sticky Side = X | Porosity (Solvent System) |
|---|---|---|
| RB1 | OH | No significant volume for guests (EtOAc) |
| RB2 | OH NH2 | Close packed (DMF/CHCl3) About 28% (CH3COOC2H5) |
| RB3 | OH | No significant volume for guests (EtOAc) |
| RB4 | NH2 | <18% (DMF/water) |
| RB5 | NH2 | Close packed (benzene/CH3OH/hexane) |
| RB6 | NH2 | Close packed (THF) |
| RB7 | NH2 | Close packed (DMF/water) |
| RB8 | CN | 16% (EtOH), 40% (EtOAc), 57% (DMF), 58% (DMSO), 50% (1,4-dioxane), 50% (CH3CN) |
| RB8 | OH [23] | 48% (diethyl ether) |
| RB8 | CONH2 [24] | 48% (n-C3H7OH/water), 59% (DMSO), 7% (water) |
| RB8 | COOH [25] | 46% (MeOH) |
| Entry | Average Channel Diameter | Porosity |
|---|---|---|
| AV (H14a) | 5.36 Å | 12.5% |
| VA (H14b) | 5.08 Å | 11.2% |
| AI (H14c) | 4.3 Å | 8.3% |
| VV (H14d) | 4.0 Å | 6.9% |
| IA (H14e) | 3.6 Å | 5.7% |
| IV (H14f) | 3.4 Å | 4.8% |
| VI (H14g) | 3.0 Å | 3.7% |
| LS (H14h) | 4.3 Å | 5.1% |
| Entry | Secondary Alcohols | e.e.Value |
|---|---|---|
| 1 | 1-phenylethanol | 92% |
| 2 | 1-(4-chloropheny)ethanol | 79% |
| 3 | 2-butanol | 77% |
| 4 | 1-(3-chlorophenyl)ethanol | 66% |
| 5 | 2-pentanol | 48% |
| 6 | 2-hexanol | <10% |
| 7 | 2-heptanol | <4% |
| Category | HOF | Porosity |
|---|---|---|
| 2.1. Hydroxyl or amide groups as hydrogen-bonded motifs | H1 H2 H3 H4 H5 | >60% 52% 60% 64% not reported |
| 2.2. Carboxylic groups or pyrazole moieties as hydrogen-bonded motifs | H6 H7 | 38%–59% for H6a–6d 51% |
| 2.3. Amide or urea groups as hydrogen-bonded motifs | H8 H9 H10 | not reported 24% 13.7% |
| 2.4. Macrocyclic receptors as hydrogen-bonded motifs | H11 H12 H13 | not reproted 24.7% not reproted |
| 2.5. Linear dipeptide as hydrogen-bonded motifs | H14 H15 | 3.7%–12.5% not reproted |
| 2.6. Pyridone or UPy moieties as hydrogen-bonded motifs | H16 H17 H18 H19 | 60% 50% 24% not determined (powder) |
| 2.7. DAT or DAP moieties as hydrogen-bonded motifs | H20 H21 H22 H23 H24 H25 H26 H27 H28 H29 H30 H31 H32 H33 H34 H35 H36 | 45% 42.5% 40%–50% 74% 60% for H24a, no crystals for H24b–d 66% (H25a) and 75% (H25b) 66% 53% (H27a), 44% (H27b), 60% (H27c), and 75% (H27d) 32% (H28a), 44% (H28b), and 60% (H28c) 56% (H29a) and 54% (H29b) [a] 72% (H30a) [a], 75% (H30b) [a] 55.3% (the value substantially decreased to 41.1% due to severely crystal contraction upon removal of guests) not reported not reported not reported 63.4% 54.3% |
| 2.8. Charge-assisted hydrogen-bonded motifs | H37 H38 | 31.5% not reported |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.-F.; Yuan, Y.-X.; Wang, H.-B. Porous Hydrogen-Bonded Organic Frameworks. Molecules 2017, 22, 266. https://doi.org/10.3390/molecules22020266
Han Y-F, Yuan Y-X, Wang H-B. Porous Hydrogen-Bonded Organic Frameworks. Molecules. 2017; 22(2):266. https://doi.org/10.3390/molecules22020266
Chicago/Turabian StyleHan, Yi-Fei, Ying-Xue Yuan, and Hong-Bo Wang. 2017. "Porous Hydrogen-Bonded Organic Frameworks" Molecules 22, no. 2: 266. https://doi.org/10.3390/molecules22020266