Recent Advances in Multinuclear NMR Spectroscopy for Chiral Recognition of Organic Compounds
Abstract
:1. Introduction
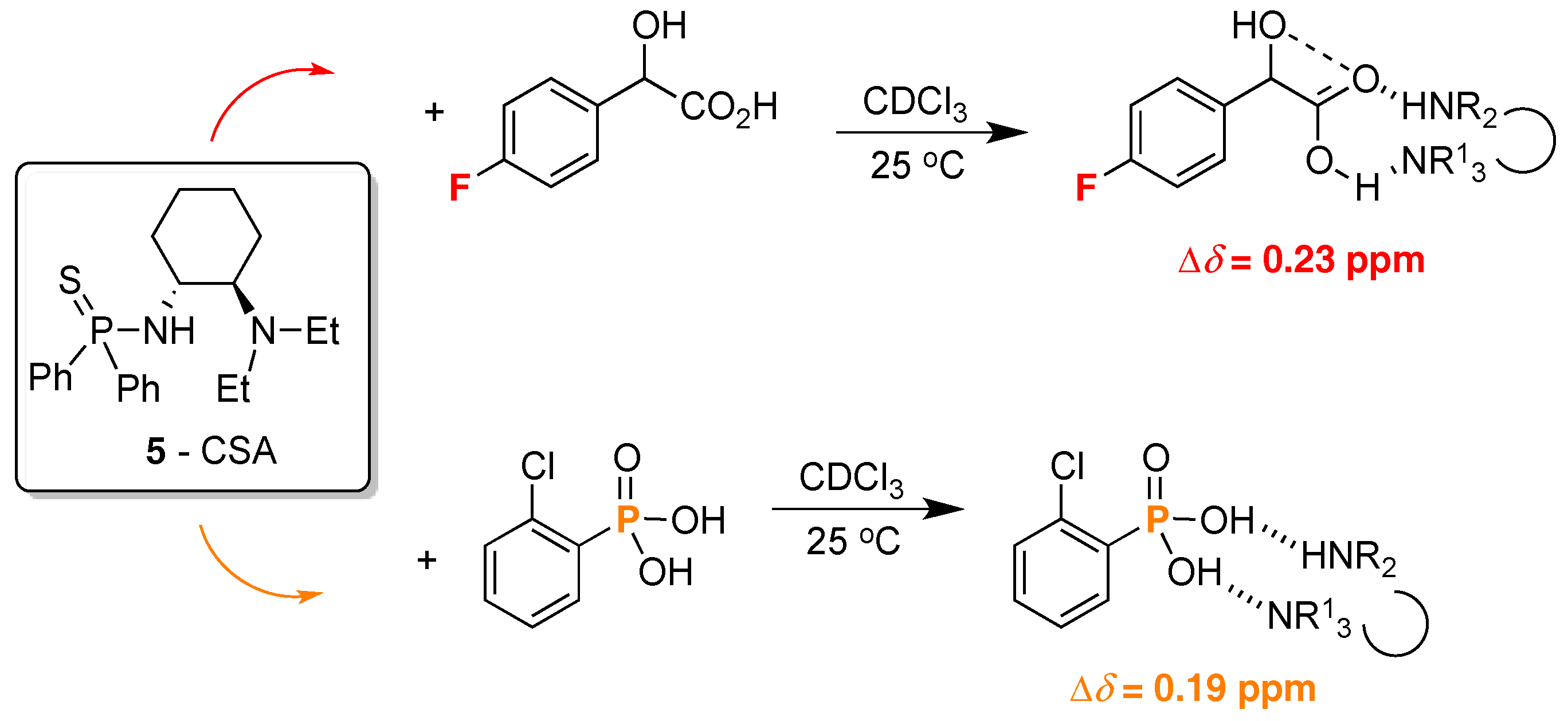
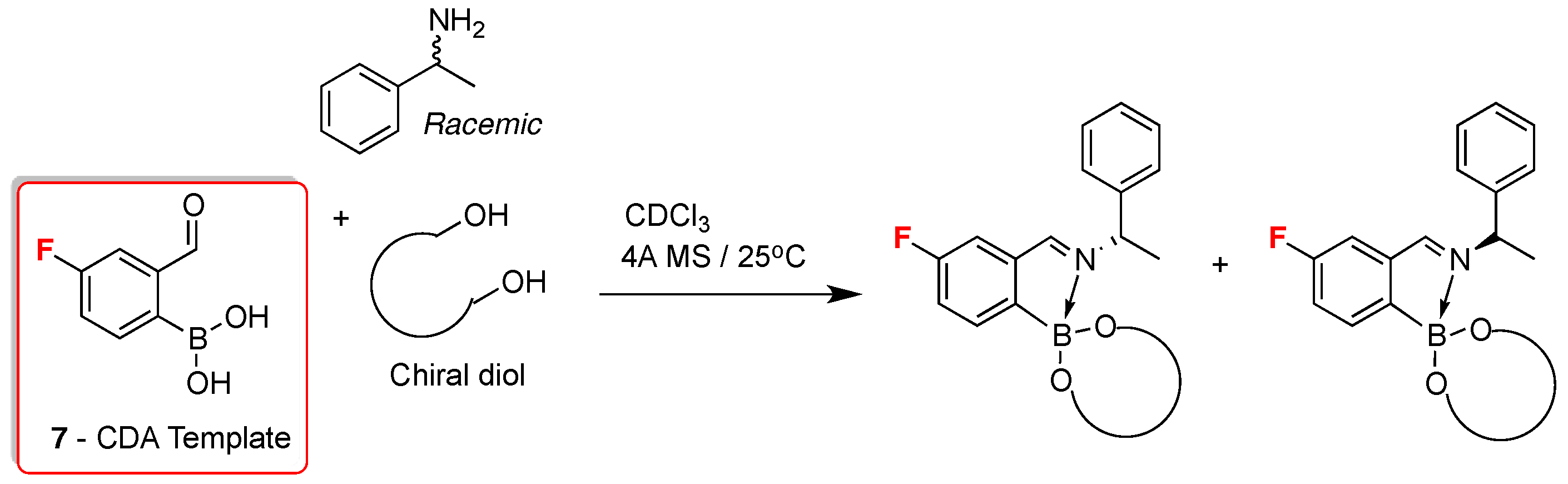
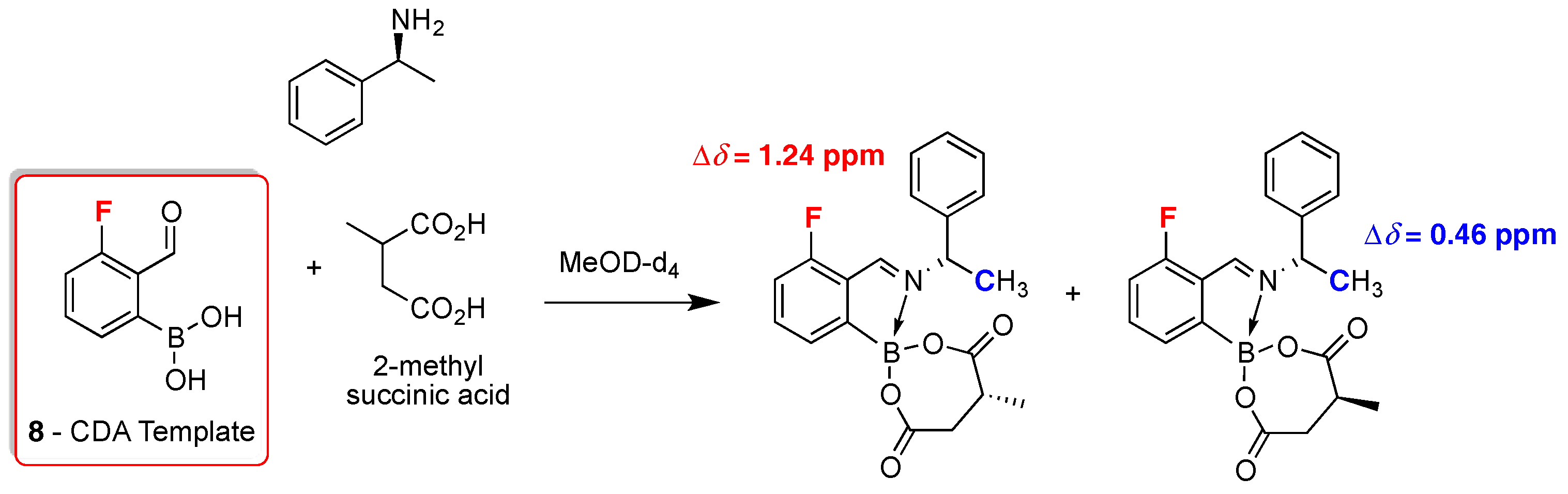
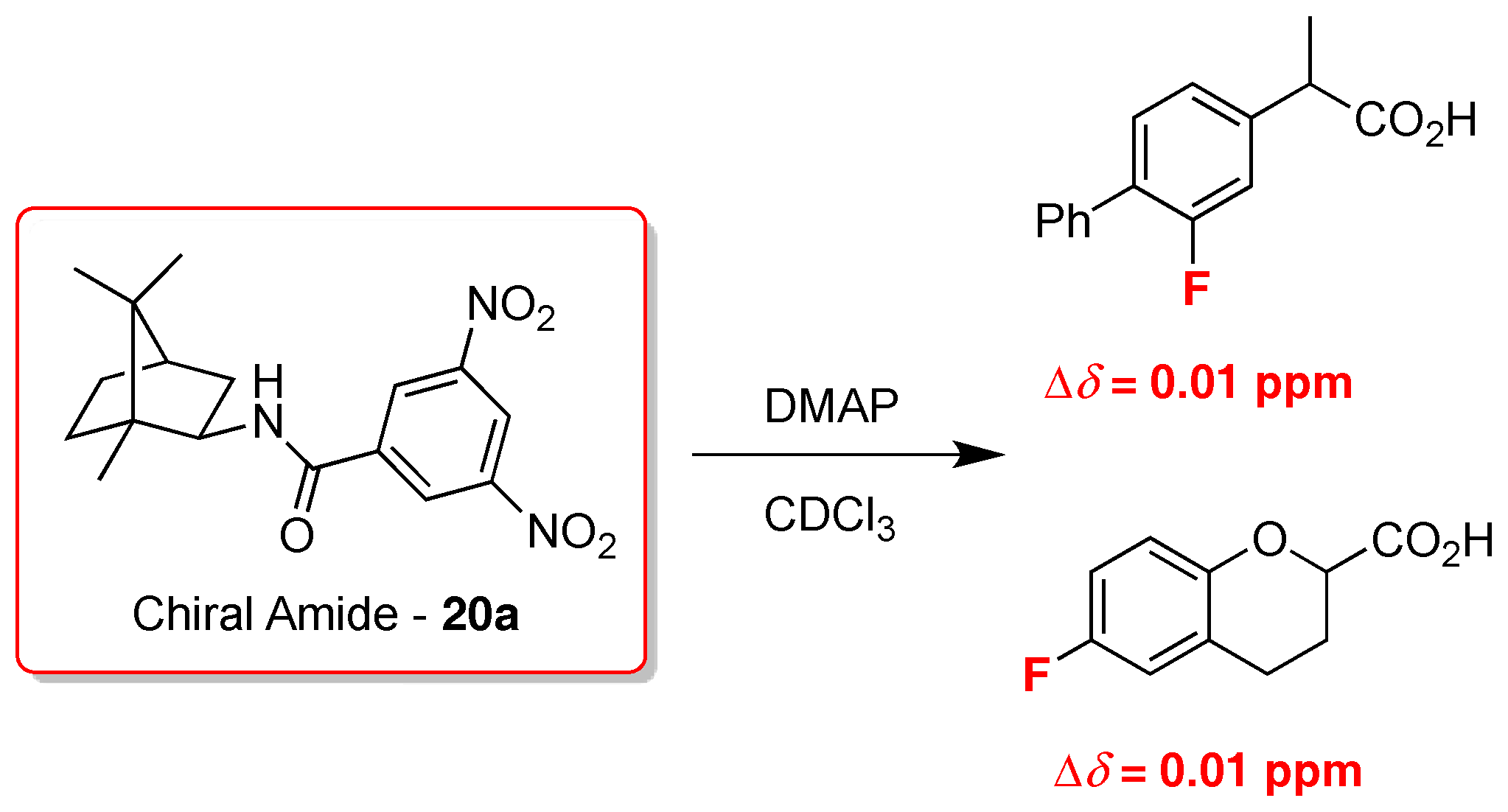
2. 19F-NMR Chiral Recognition
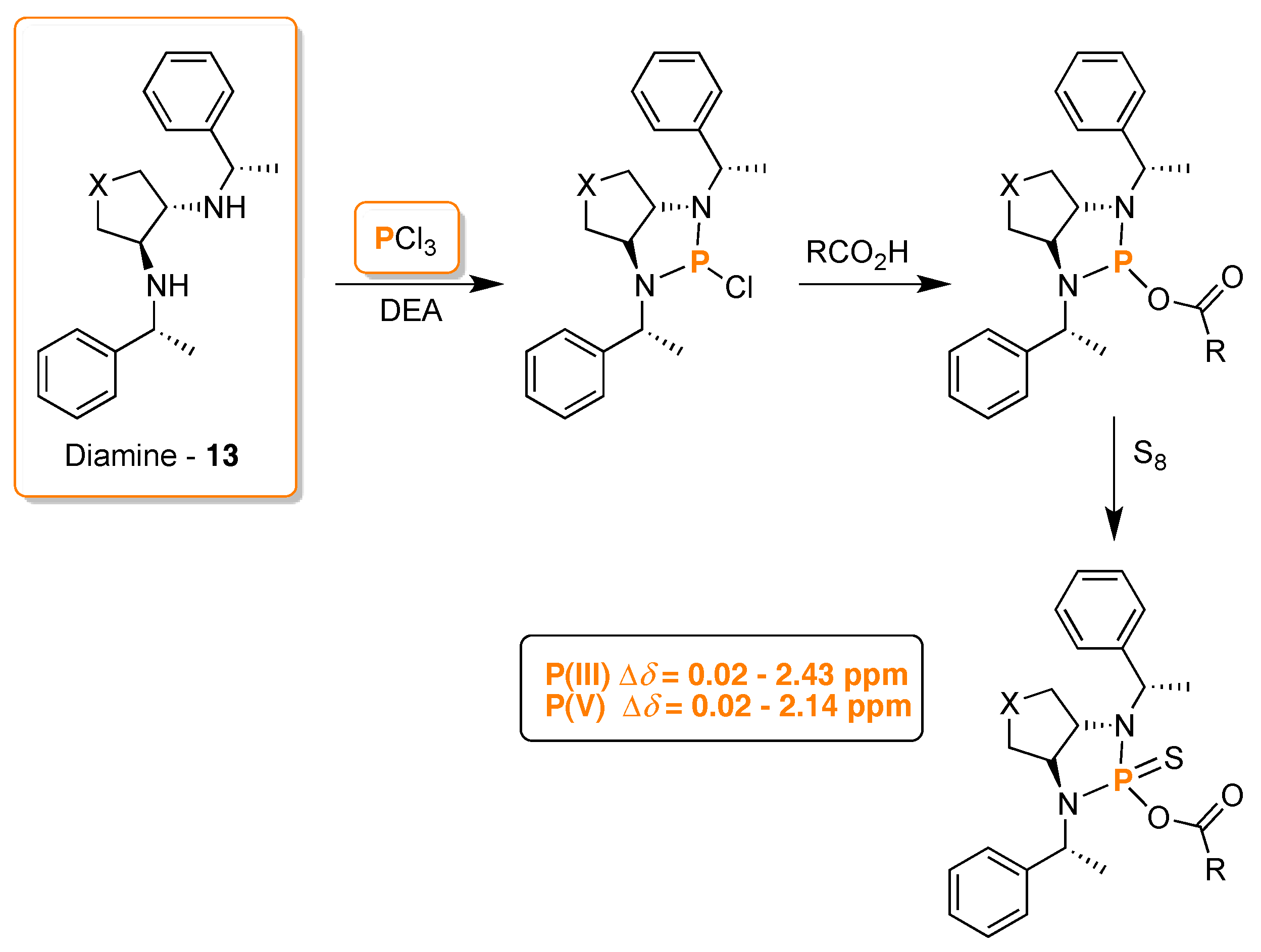
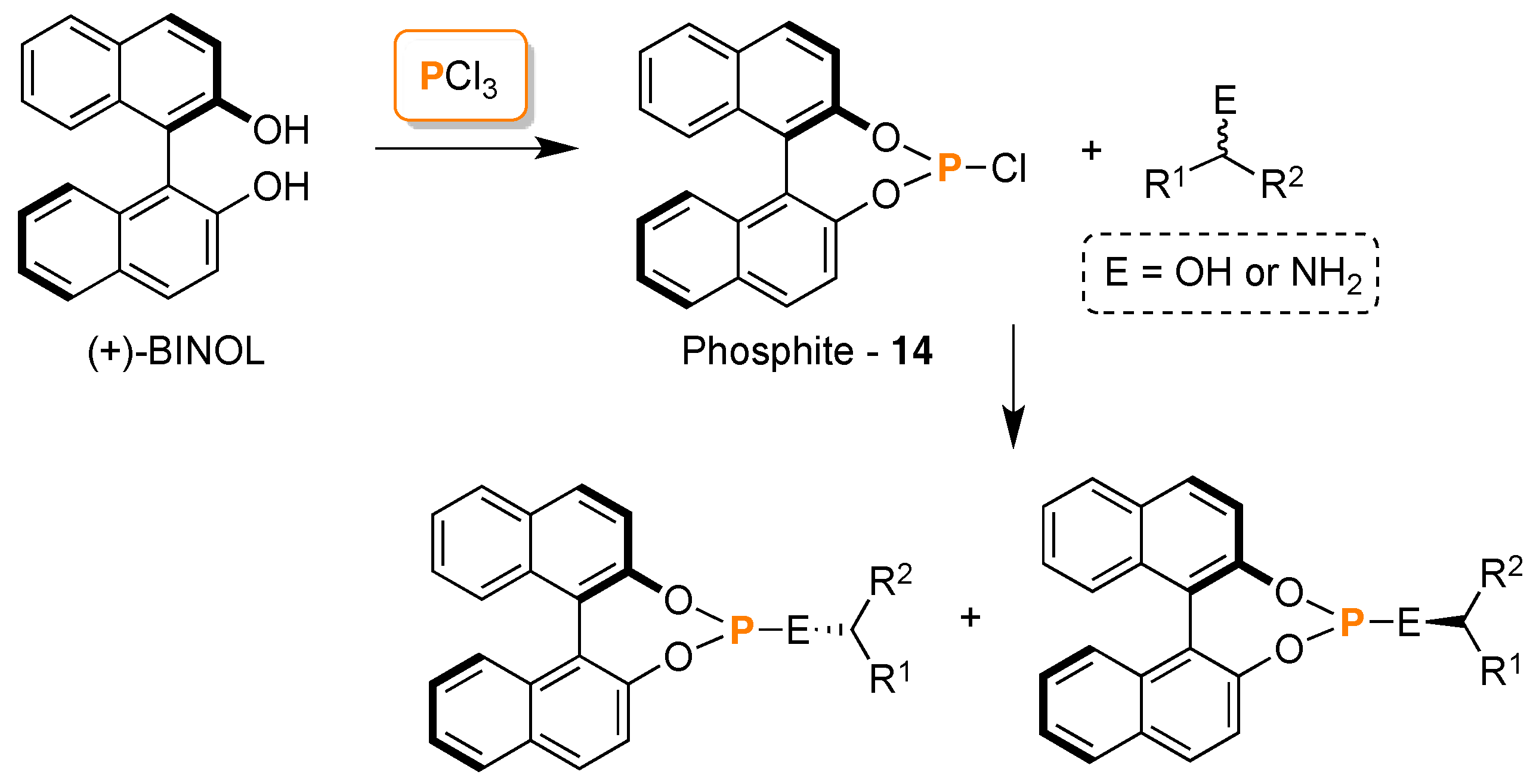
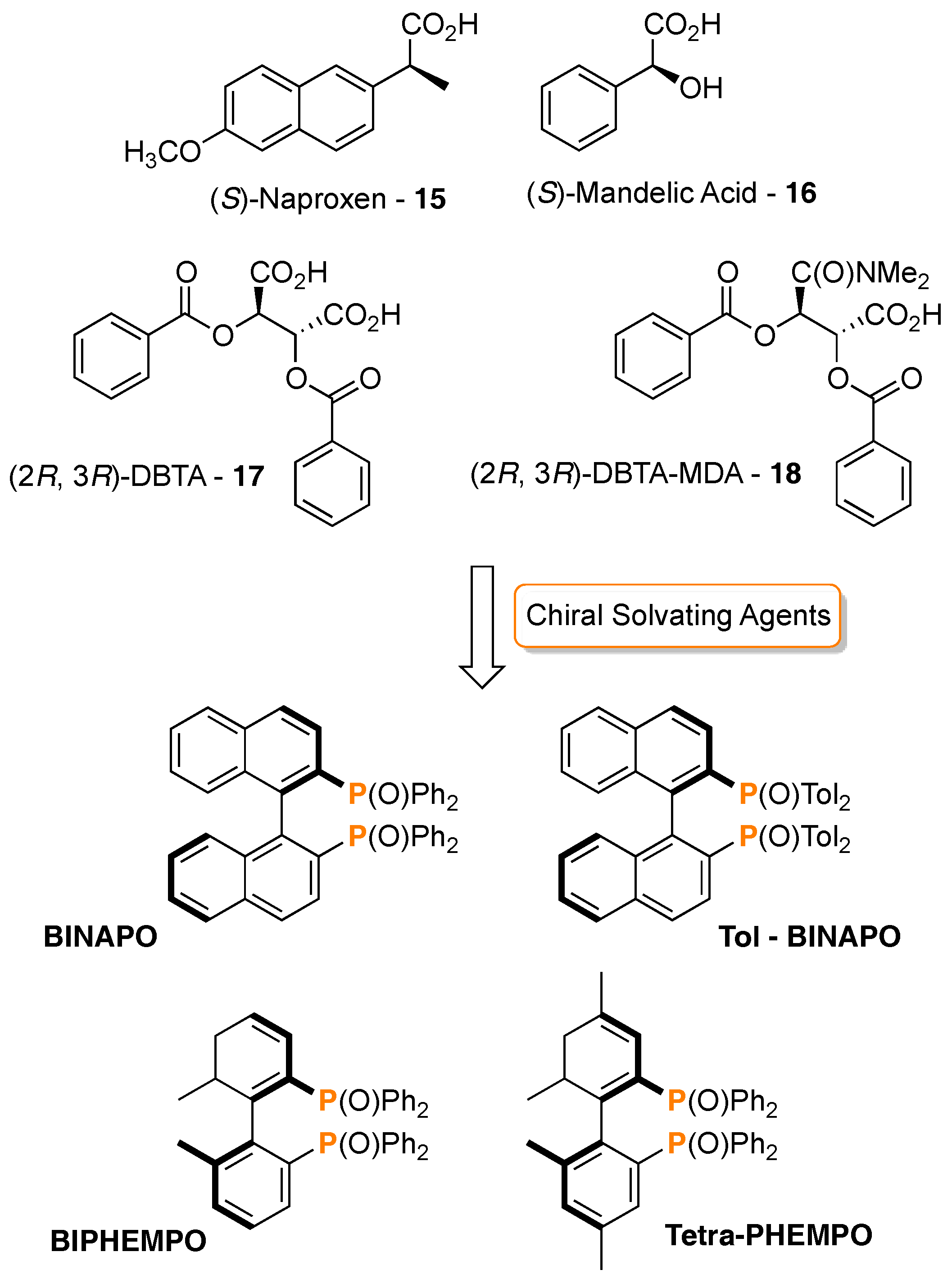
3. 31P-NMR Chiral Recognition
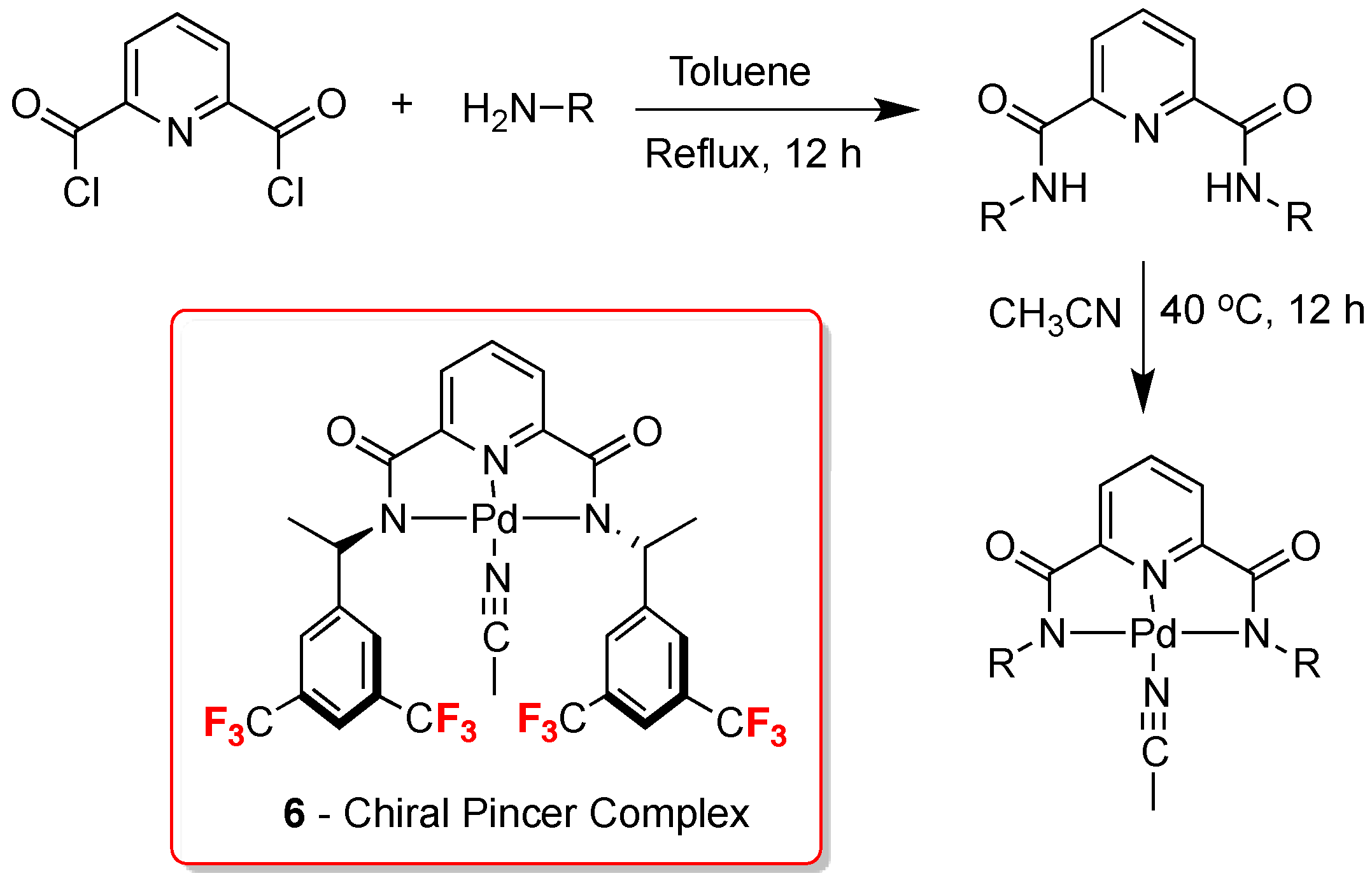
4. 13C-NMR Chiral Recognition
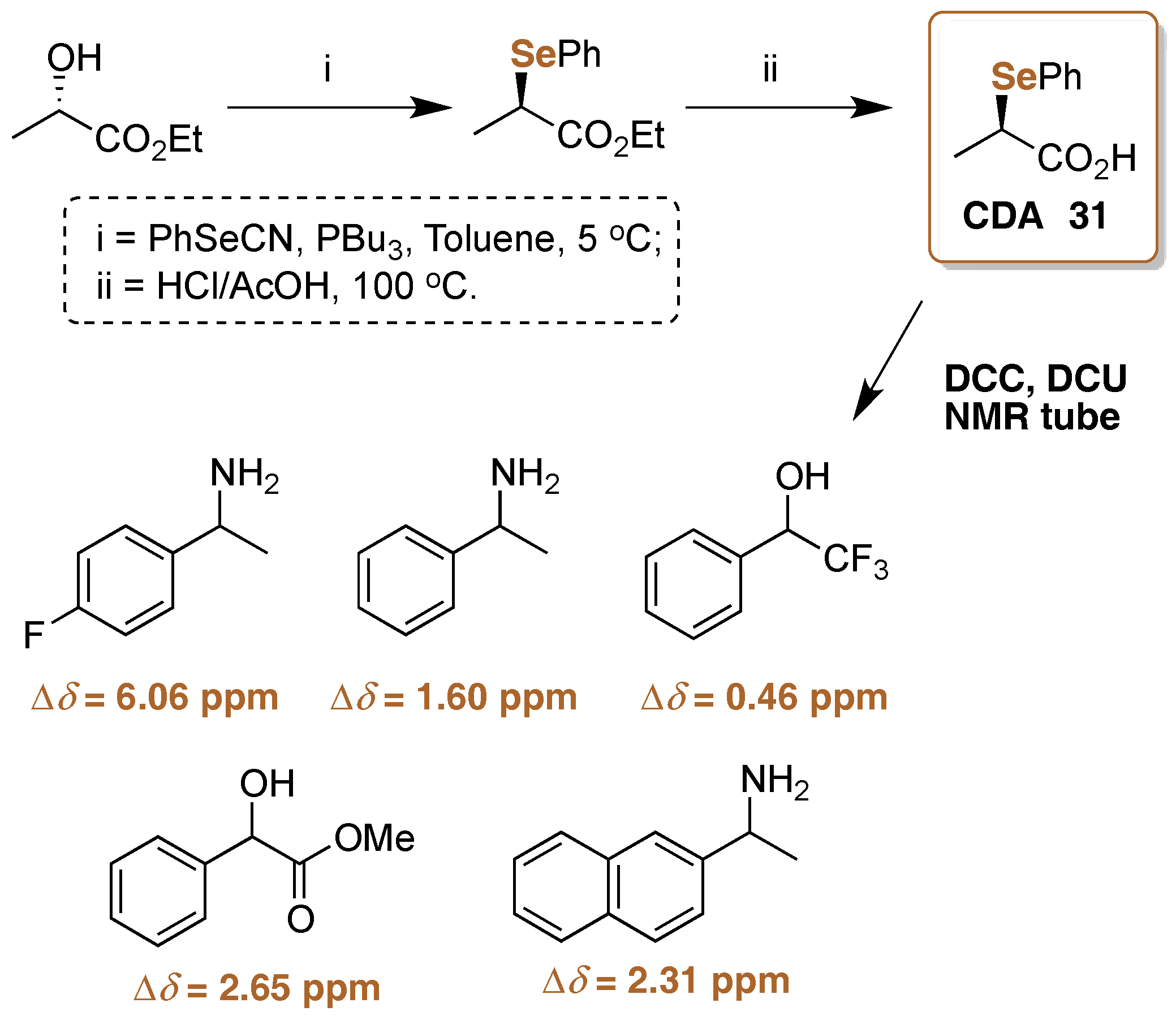
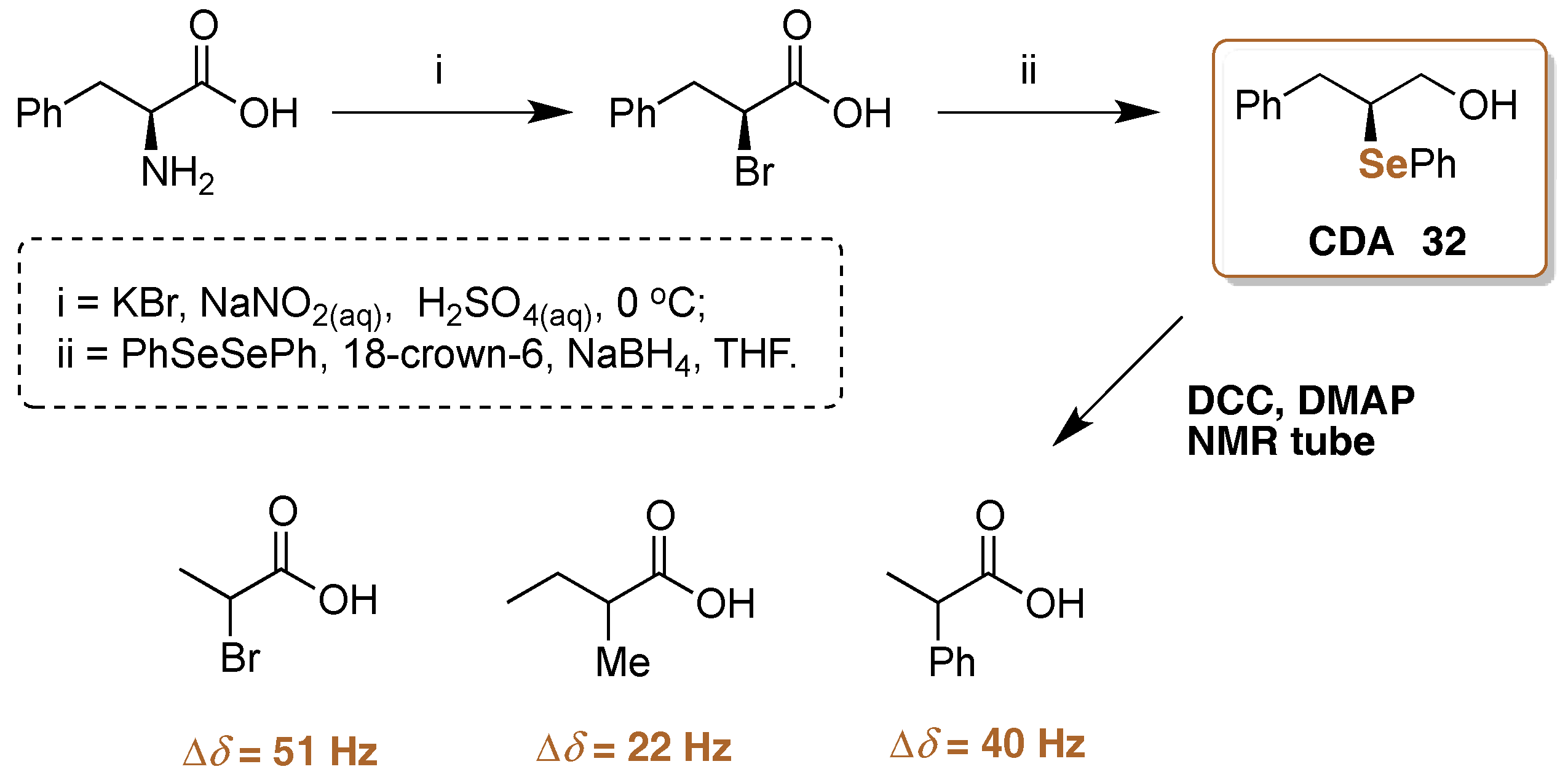
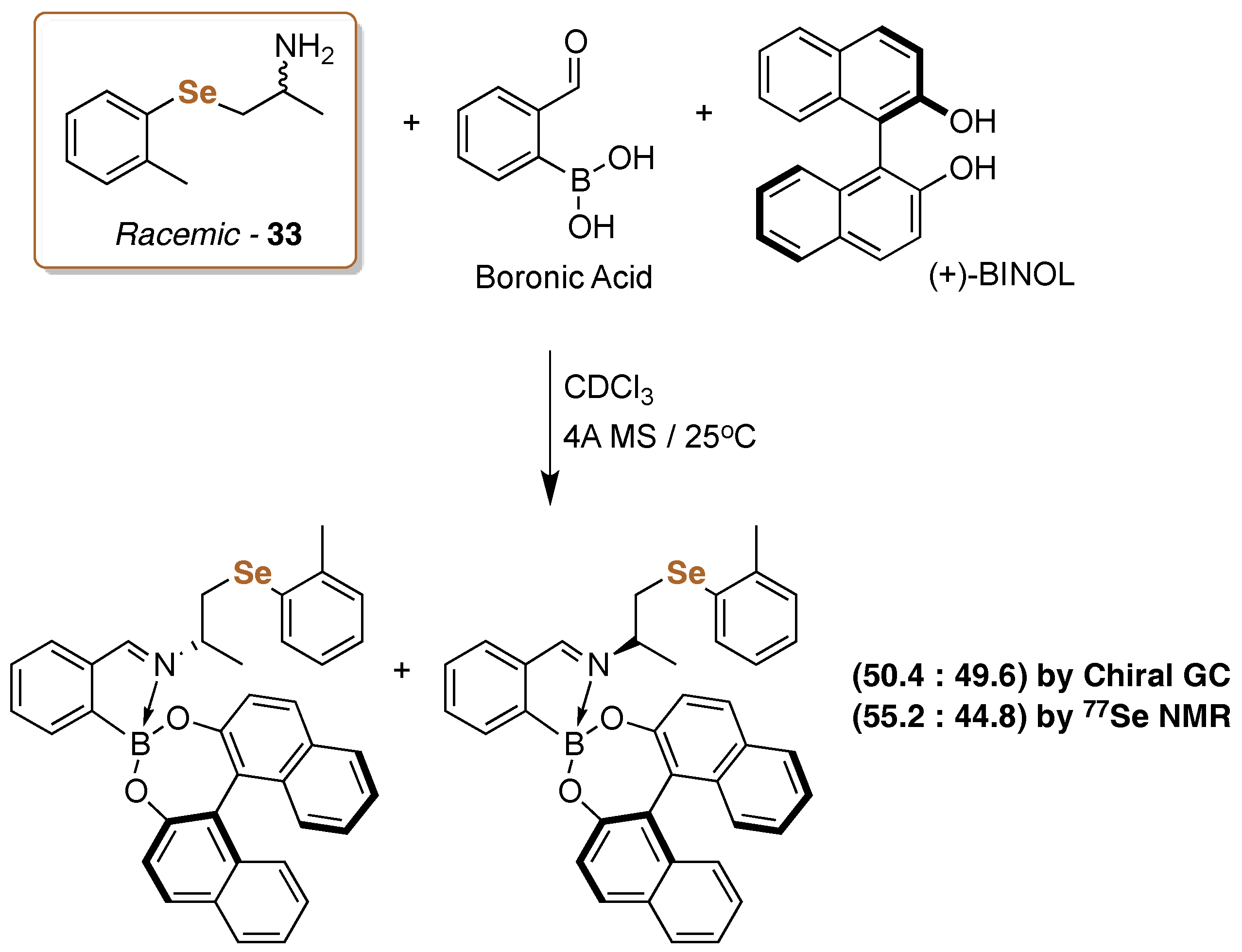
5. 77Se-NMR Chiral Recognition
6. Conclusions
Acknowledgments
Conflicts of Interests
References
- Sheldon, R.A. Chirotechnology: Industrial Synthesis of Optically Active Compounds; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Lurie-Luke, E. Product and technology innovation: What can biomimicry inspire? Biotechnol. Adv. 2014, 32, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 105, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Q.; Li, Y.M.; Chan, A.S.C. Principles and Applications of Asymmetric Synthesis; John Wiley & Sons: Oxford, UK, 2001. [Google Scholar]
- Blaser, H.U.; Schmidt, E. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions; Wiley-VCG Verlag GmbH & KgaA: Weinheim, Germany, 2004. [Google Scholar]
- Izake, E.L. Chiral Discrimination and enantioselective analysis of drugs: An overview. J. Pharm. Sci. 2007, 96, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Harada, N. HPLC Separation of Diastereomers: Chiral Molecular Tools Useful for Preparation of Enantiopure Compounds and Simultaneous Determination of Their Absolute Configurations. Molecules 2016, 21, 1328. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Berkecz, R.; Peter, A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers. J. Pharm. Biomed. Anal. 2008, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- West, C. Enantioselective Separations with Supercritical Fluids—Review. Curr. Anal. Chem. 2014, 52, 99–120. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Determination of the Absolute Configurations of Chiral Drugs Using Chiroptical Spectroscopy. Molecules 2016, 21, 1056. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J. Discrimination of Chiral Compounds Using NMR Spectroscopy; John Wiley & Sons: Weinheim, Germany, 2007. [Google Scholar]
- Wenzel, T.J.; Chisholm, C.D. Using NMR spectroscopy methods to determine enantiomeric purity and assign absolute stereochemistry. Prog. NMR Spectrosc. 2011, 59, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Yang, S.; Huang, H.; Zong, L.; Song, H.; Fan, H.; Sun, X. Chirality sensing of tertiary alcohols by a novel strong hydrogen-bonding donor—Selenourea. Chem. Sci. 2016, 7, 932–938. [Google Scholar] [CrossRef]
- Seo, M.-S.; Kim, H. 1H-NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes. J. Am. Chem. Soc. 2015, 137, 14190–14195. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Yang, S.; Huang, H.; Zong, H.; Song, L. A bisthiourea-based 1H-NMR chiral sensor for chiral discrimination of a variety of chiral compounds. Sens. Actuators B 2016, 231, 129–134. [Google Scholar] [CrossRef]
- Lakshmipriya, A.; Chaudhari, S.R.; Suryaprakash, N. Enantio-differentiation of molecules with diverse functionalities using a single probe. Chem. Commun. 2015, 51, 13492–13495. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Fan, H.; Huang, H.; Yang, S.; Zong, H.; Song, L.; Yang, G. Highly Effective Configurational Assignment Using Bisthioureas as Chiral Solvating Agents in the Presence of DABCO. Org. Lett. 2015, 17, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Cook, A.M.; Dannatt, J.E. Enantiodifferentiation of multifunctional tertiary alcohols by NMR spectroscopy with a Whelk-O type chiral solvating agent. Tetrahedron Asymmetry 2014, 25, 163–169. [Google Scholar] [CrossRef]
- Bian, G.; Fan, H.; Yang, S.; Yue, H.; Huang, H.; Zong, H.; Song, L. A Chiral Bisthiourea as a Chiral Solvating Agent for Carboxylic Acids in the Presence of DMAP. J. Org. Chem. 2013, 78, 9137–9142. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Chisholm, C.D. Assignment of absolute configuration using chiral reagents and NMR spectroscopy. Chirality 2011, 23, 190–214. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.R.; Gayathri, C.; Tsarevsky, N.V.; Matyjaszewski, K. Stretched Poly(methyl methaacrylate) Gel Aligns Small Organic Molecules in Chloroform. Stereochemical Analysis and Diastereotopic Proton NMR Assignment in Ludartin Using Residual Couplings and 3J Coupling Constant Analysis. J. Org. Chem. 2008, 73, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Dolbier, W.R., Jr. Guide to Fluorine NMR for Organic Chemists; John Wiley & Sons: Somerset, NJ, USA, 2009. [Google Scholar]
- Cobb, S.L.; Murphy, C.D. 19F-NMR applications in chemical biology. J. Fluorine Chem. 2009, 130, 132–143. [Google Scholar] [CrossRef]
- Apparu, M.; Tiba, Y.B.; Léo, P.M.; Hamman, S.; Coulombeau, C. Determination of the enantiomeric purity and the configuration of β-aminoalcohols using (R)-2-fluorophnylacetic acid (AFPA) and fluorine-19 NMR: Application to β-blockers. Tetrahedron Asymmetry 2000, 11, 2885–2898. [Google Scholar] [CrossRef]
- Pomares, M.; Sánchez-Ferrando, F.; Alvarez-Larena, A.; Piniella, J.F. Preparation and Structural Study of the Enantiomers of α,α’-Bis(trifluoromethyl)-9-10-anthracenedimethanol and Its Perdeuterated Isotopomer, Highly Effective Chiral Solvating Agents. J. Org. Chem. 2002, 67, 753–758. [Google Scholar] [CrossRef] [PubMed]
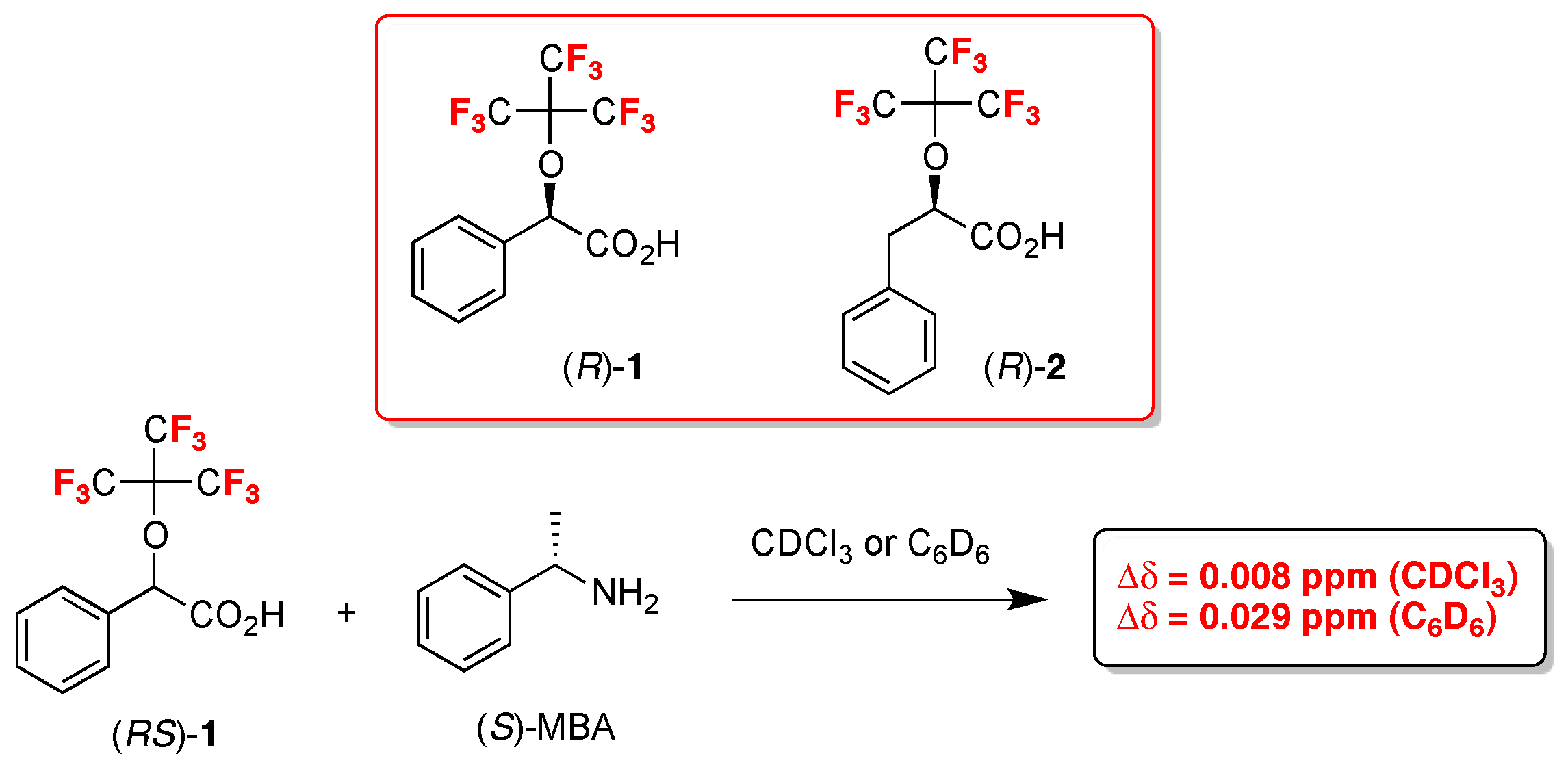
- Nemes, A.; Csóka, T.; Béni, S.; Farkas, V.; Rábai, J.; Szabó, D. Chiral Recognition Studies of α-(Nonafluoro-terc-butoxy)carboxylic Acids by NMR Spectroscopy. J. Org. Chem. 2015, 80, 6267–6274. [Google Scholar] [CrossRef] [PubMed]
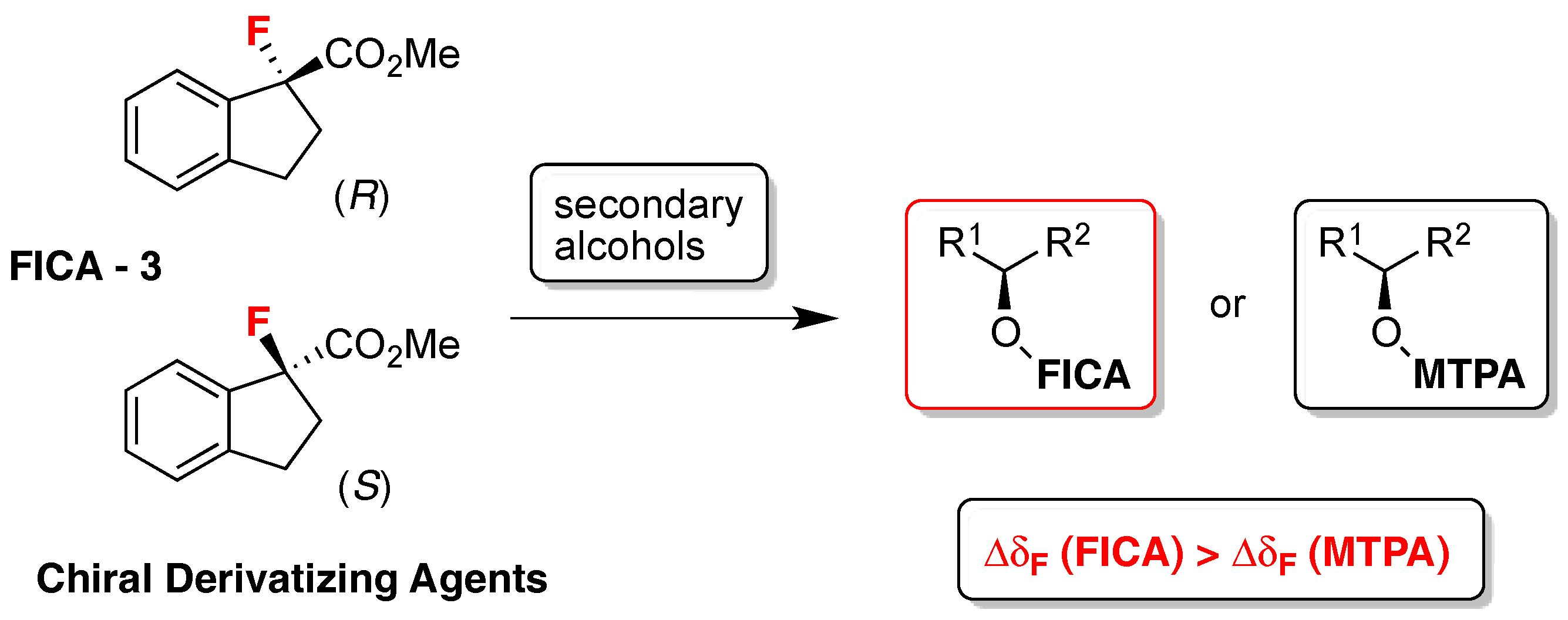
- Takahashi, T.; Kameda, H.; Kamei, T.; Koyanagi, J.; Pichierri, F.; Omata, K.; Ishizaki, M.; Nakamura, H. FICA, a new derivatizing agent for determining the absolute configuration of secondary alcohols by 19F- and 1H-NMR spectroscopies. Tetrahedron Asymmetry 2013, 24, 1001–1009. [Google Scholar] [CrossRef]
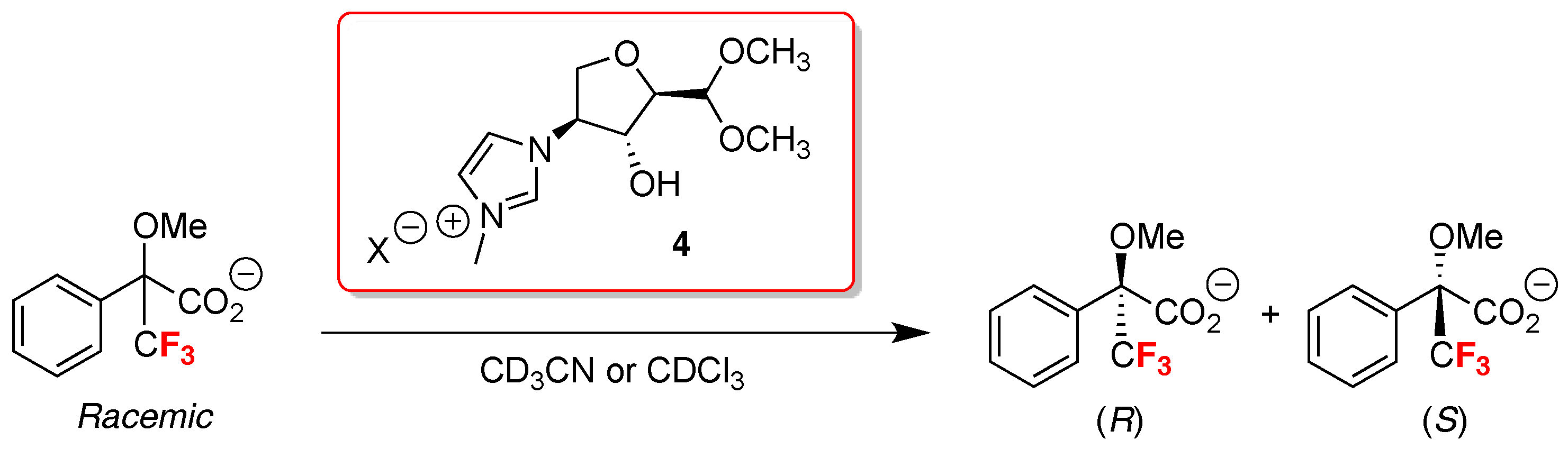
- Jayachandra, R.; Reddy, S.R. A remarkable chiral recognition of racemic Mosher’s acid salt by naturally derived chiral ionic liquids using 19F-NMR spectroscopy. RSC Adv. 2016, 6, 39758–39761. [Google Scholar] [CrossRef]
- Huang, H.; Bian, G.; Zong, H.; Wang, Y.; Yang, S.; Yue, H.; Song, L.; Fan, H. Chiral Sensor for Enantiodiscrimination of Varied Acids. Org. Lett. 2016, 18, 2524–2527. [Google Scholar] [CrossRef] [PubMed]
- Swager, T.M.; Zhao, Y. Simultaneous Chirality Sensing of Multiple Amines by 19F-NMR. J. Am. Chem. Soc. 2015, 137, 3221–3224. [Google Scholar]
- Yeste, S.L.; Powell, M.E.; Bull, S.D.; James, T.D. Simple Chiral Derivatization Protocols for 1H-NMR and 19F-NMR Spectroscopic Analysis of the Enantiopurity of Chiral Diols. J. Org. Chem. 2009, 74, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.R.; Suryaprakash, N. Simple and efficient methods for discrimination of chiral diacids and chiral alpha-methyl amines. Org. Biomol. Chem. 2012, 10, 6410–6419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wager, T.M. Detection and Differentiation of Neutral Organic Compounds by 19F-NMR with a Tungsten Calix[4]arene Imido Complex. J. Am. Chem. Soc. 2013, 135, 18770–18773. [Google Scholar] [CrossRef] [PubMed]
- Axthelm, J.; Görls, H.; Schubert, U.S.; Schiller, A. Fluorinated Boronic Acid-Appended Bipyridinium Salts for Diol Recognition and Discrimination via 19F-NMR Barcodes. J. Am. Chem. Soc. 2015, 137, 15402–15405. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Oliver, A.G.; Smith, B.D. Fluorine NMR reporter for phosphate anions. Chem. Commun. 2013, 49, 5070–5072. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Chen, X.X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective Sensing of sccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 2013, 42, 8032–8048. [Google Scholar] [CrossRef] [PubMed]
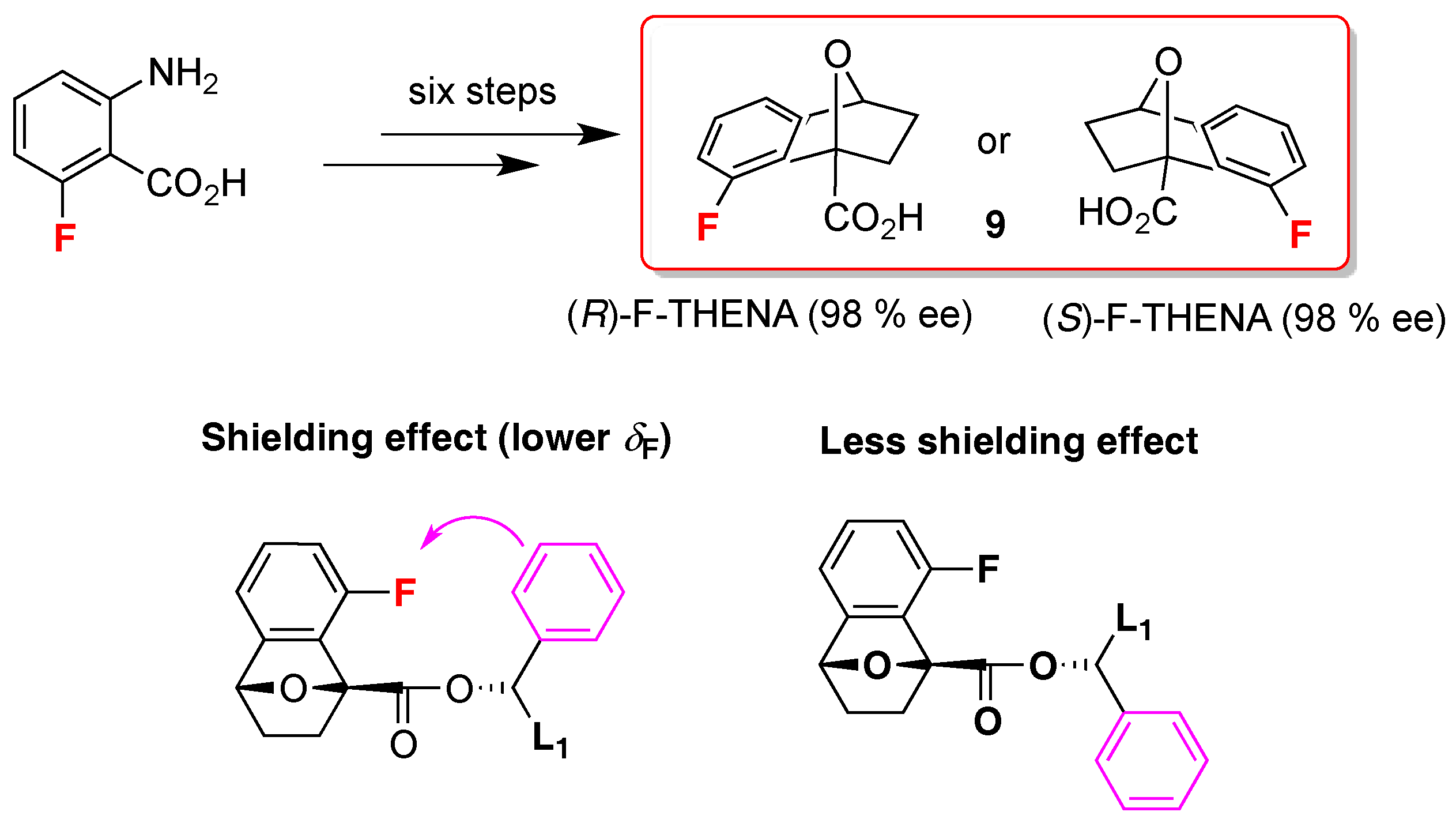
- Dolsophon, K.; Soponpong, J.; Kornsakulkarn, J.; Thongpanchang, C.; Prabpai, S.; Kongsaeree, P.; Thongpanchang, T. F-THENA: A chiral derivatizing agent for the determination of the absolute configuration of secondary aromatic alcohols with a self-validating system. Org. Biomol. Chem. 2016, 14, 11002–11012. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Mohapatra, P.P.; Fedoseyenko, D.; Duncton, M.; Steel, P.J. 1-Benzotriazol-1-yl-3,3,3-trifluoro-2-methoxy-2-phenylpropan-1-ones: Mosher-Bt Reagents. J. Org. Chem. 2007, 72, 4268–4271. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H.; Mikata, Y. Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database. Molecules 2015, 20, 12880–12900. [Google Scholar] [CrossRef] [PubMed]
- Gotor, V.; Cerrada, S.G.; Mendiola, J.; Frutos, O.; Collado, I.; Fernández, V.G.; Lavandera, I.; Busto, E.; Mata, M.R.; Paul, C.E. Transaminases Applied to the Synthesis of High Added-Value Enantiopure Amines. Org. Process. Res. Dev. 2014, 18, 788–792. [Google Scholar]
- Nestl, B.M.; Hammer, S.C.; Nebel, B.A.; Hauer, B. New generation of biocatalysts for organic synthesis. Angew. Chem. Int. Ed. 2014, 53, 3070–3095. [Google Scholar] [CrossRef] [PubMed]
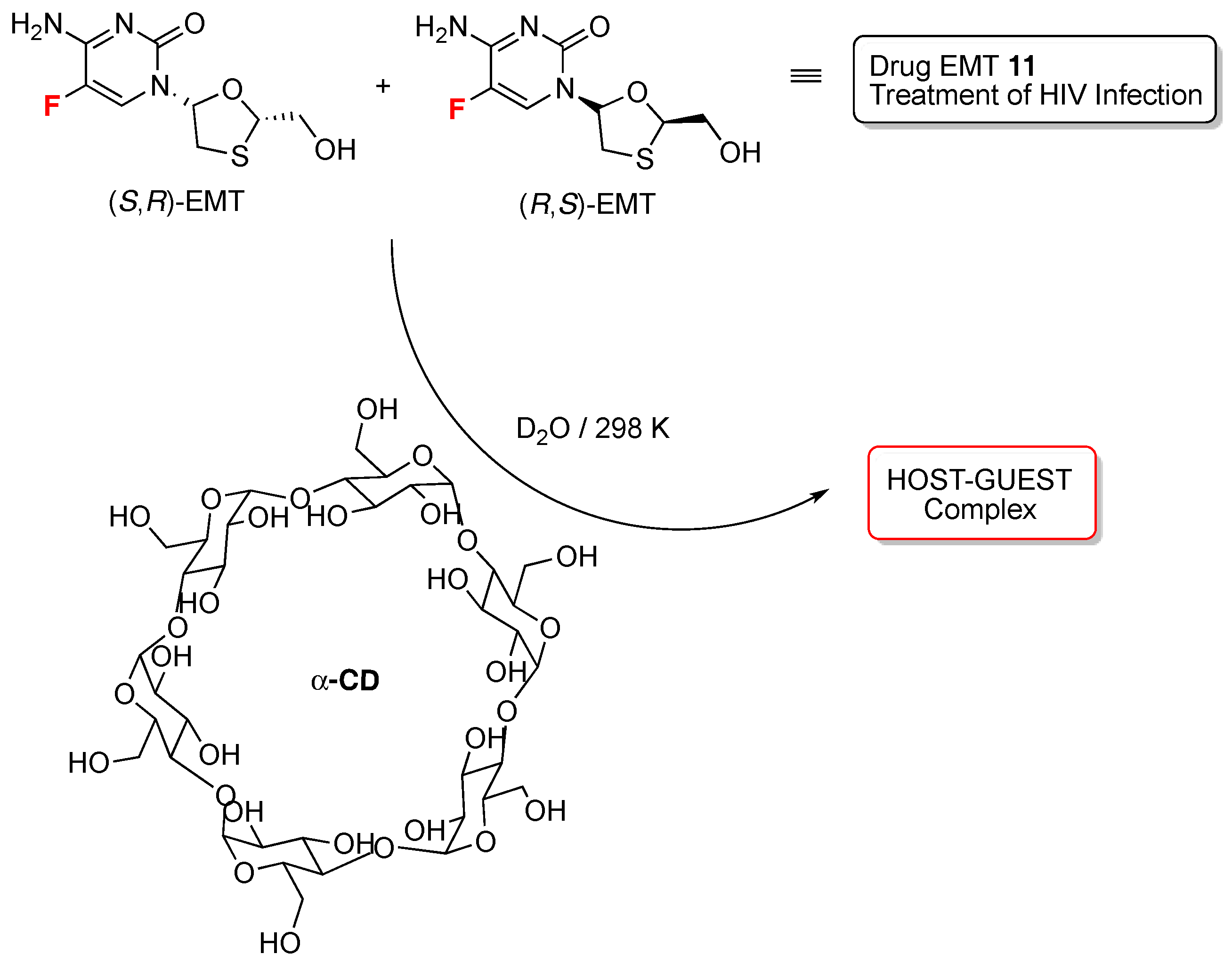
- Rao, R.N.; Santhakumar, K. Cyclodextrin assisted enantiomeric recognition of emtricitabine by 19F-NMR spectroscopy. New J. Chem. 2016, 40, 8408–8417. [Google Scholar]
- Karl, D.M. Aquatic ecology. Phophorus, the staff of life. Nature 2000, 406, 31–33. [Google Scholar]
- Kochian, L.V. Plant nutrition: Rooting for more phosphorus. Nature 2012, 488, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J. The 13th Element: The Sordid Tale of Murder, Fire and Phosphorus; Wiley-VCH: New York, NY, USA, 2000; pp. 65–101. [Google Scholar]
- Kühl, O. Phosphorus-31 NMR Spectroscopy; Springer: Berlin, Germany, 2008. [Google Scholar]
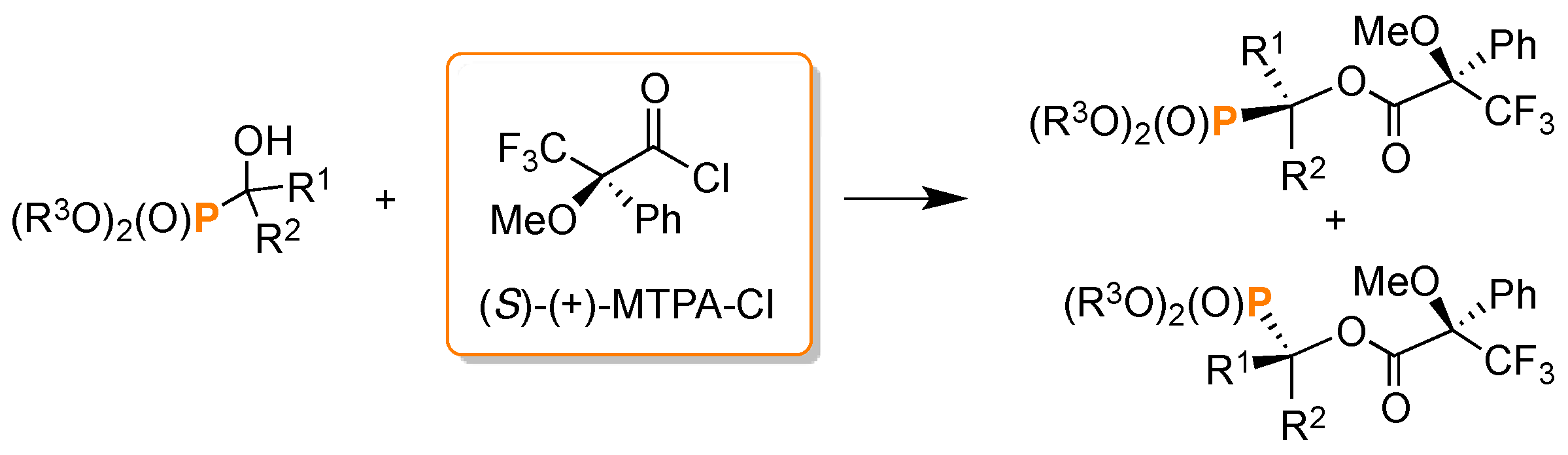
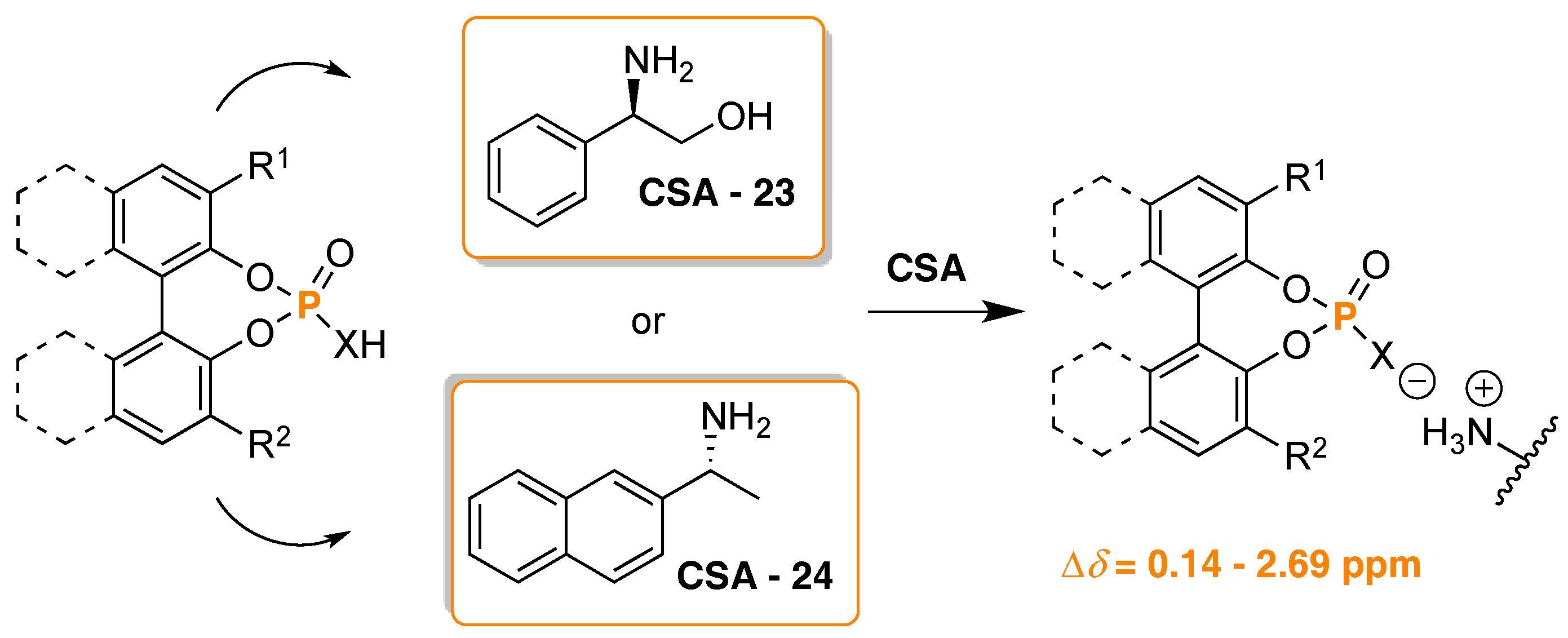
- Szyszkowiak, J.; Majewska, P. Determination of absolute configuration by 31P-NMR. Tetrahedron Asymmetry 2014, 25, 103–112. [Google Scholar] [CrossRef]
- Hammerschmidt, F.; Li, Y.F. Determination of absolute configuration of α-hydroxyphosphonates by 31P-NMR spectroscopy. Tetrahedron 1994, 50, 10253–10264. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. Assignment of the Absolute Configuration of Polyfunctional Compounds by NMR Using Chiral Derivatizing Agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Raushel, F.M. Differentiation of chiral phosphorus enantiomers by 31P- and 1H-NMR spectroscopy using amino acid derivatives as chemical solvating agents. Tetrahedron Asymmetry 2007, 18, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Mastranzo, V.M.; Quintero, L.; Parrodi, C.A. Determination of the enantiomeric excess of chiral carboxylic acids by 31P-NMR with phosphorylated derivatizing agents from C2-symmetrical diamines containing the (S)-α-phenylethyl group. Chirality 2007, 19, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; Naraschewski, F.N.; Eppinger, J. 31P-NMR assays for rapid determination of enantiomeric excess in catalytic hydrosilylations and transfer hydrogenations. Tetrahedron Asymmetry 2009, 20, 362–367. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Swierczynska, W.; Pietrusiewicz, K.M.; Woznica, M.; Wojcik, D.; Frelek, J. A convenient application of the NMR and CD methodologies for determination of enantiomeric ratio and absolute configuration of chiral atropoisomeric phosphine oxides. Tetrahedron Asymmetry 2008, 19, 2339–2345. [Google Scholar] [CrossRef]
- Fauconnot, L.; Nugier-Chauvin, C.; Noiret, N. Enantiomeric excess determination of some chiral sulfoxides by NMR: Use of (S)-Ibuprofen® and (S)-Naproxen® as shift reagents. Tetrahedron Lett. 1997, 38, 7875–7878. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Polavarapu, P.L.; Li, T.; Drabowicz, J.; Pietrosiewicz, K.M.; Zygo, K. Absolute Configuration of tert-Butyl-1-(2-methylnaphthyl)phosphine Oxide. J. Org. Chem. 2002, 67, 6539–6541. [Google Scholar] [CrossRef] [PubMed]
- Kannapan, J.; Jain, N.; Bedekar, A.V. Synthesis and applications of exo N-((1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl)benzamides as NMR solvating agents for the chiral discrimination of 1,1’-binaphthyl-2,2’-diylhydrogenphosphates and α-substituted acids. Tetrahedron Asymmetry 2015, 26, 1102–1107. [Google Scholar] [CrossRef]
- Pitchen, P.; Duñach, E.; Juge, S.; Kagan, H.B. An efficient asymmetric oxidation of sulfides to sulfoxides. J. Am. Chem. Soc. 1984, 106, 8188–8193. [Google Scholar] [CrossRef]
- Jain, N.; Patel, R.B.; Bedekar, A.V. Modified Kagan’s amide: Synthesis and applications as a chiral solvating agent for hydrogen-bonding based chiral discrimination in NMR. RSC Adv. 2015, 5, 45943–45955. [Google Scholar] [CrossRef]
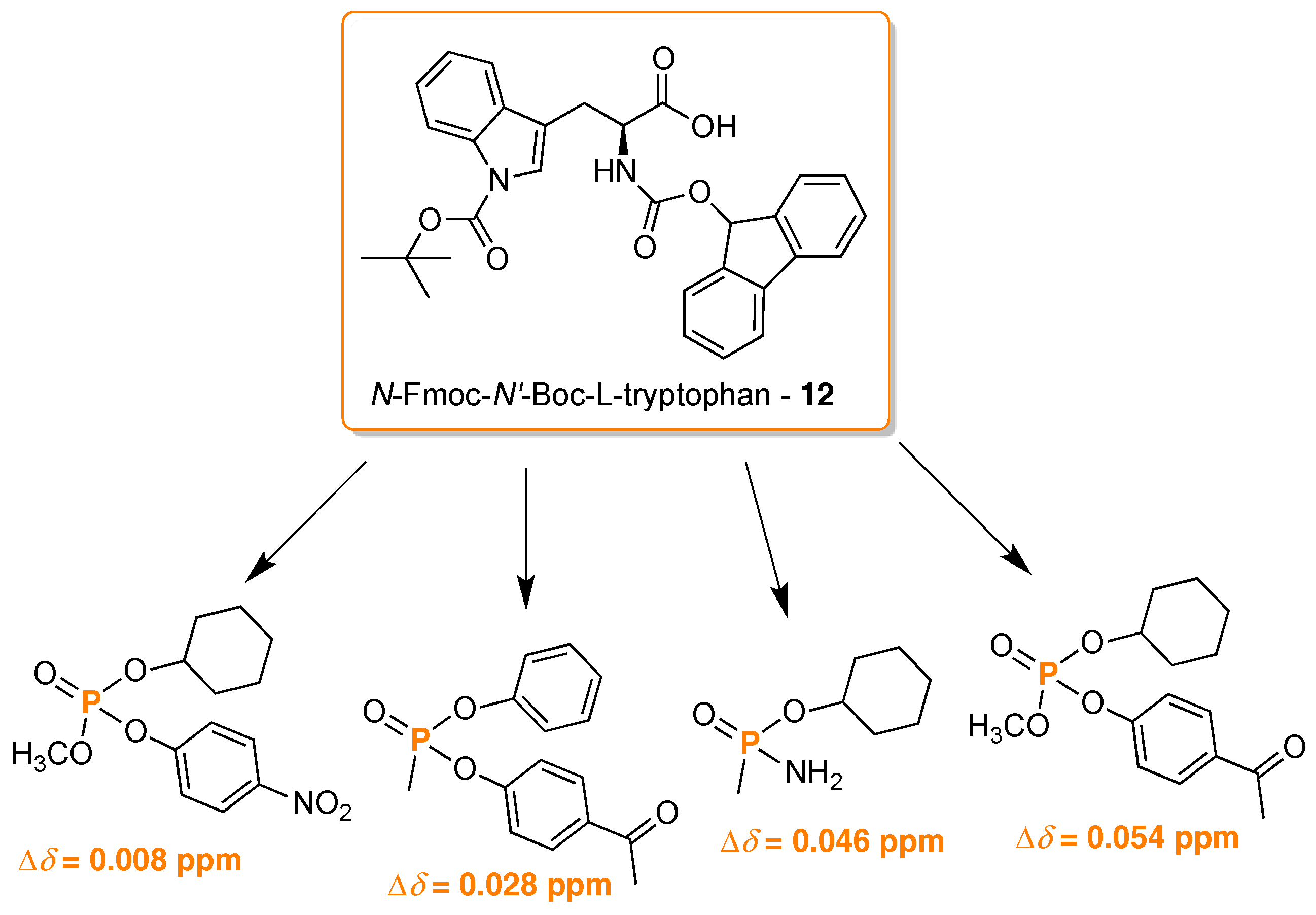
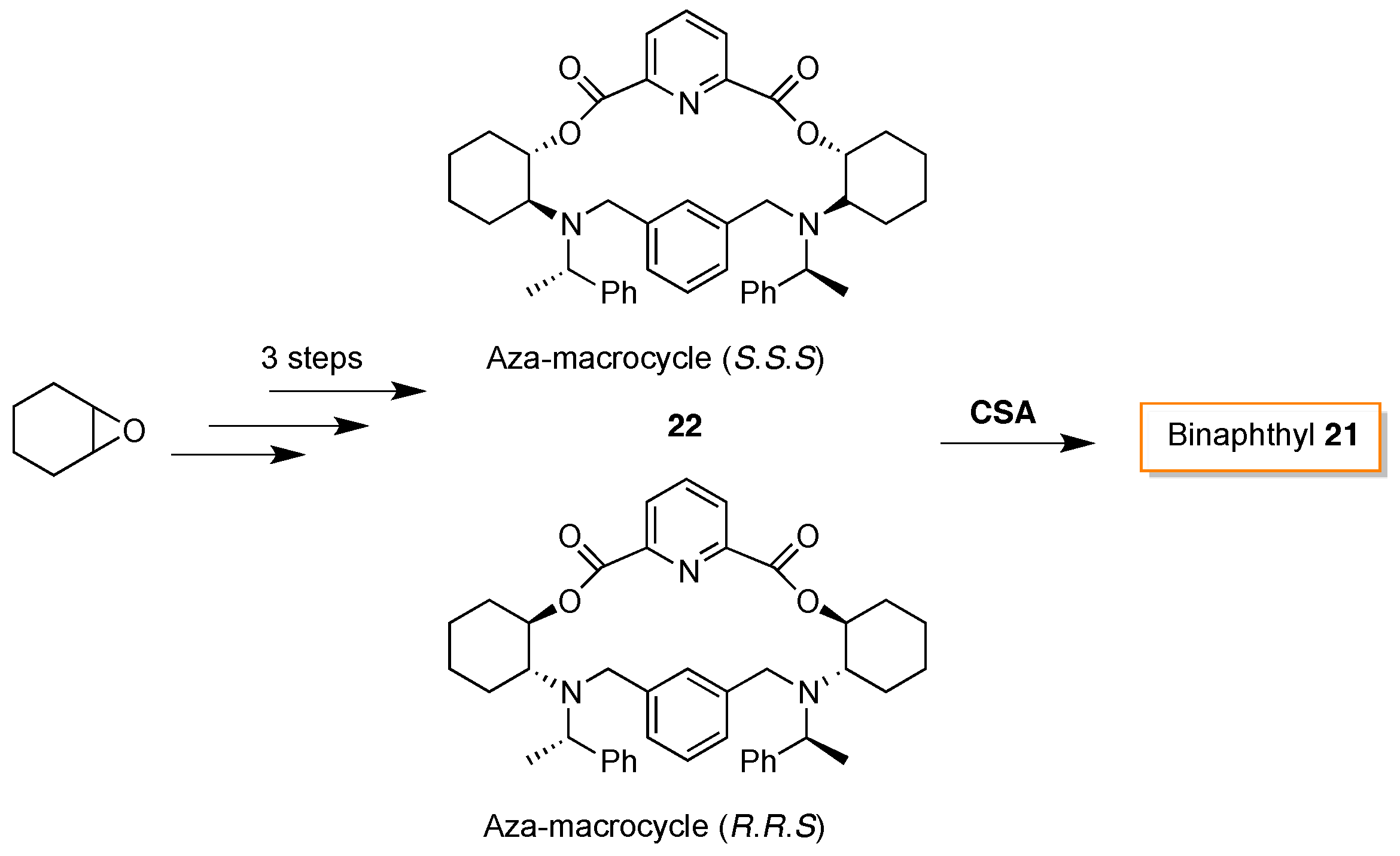
- Khanvilkar, A.N.; Bedekar, A.V. Synthesis and characterization of chiral aza-macrocycles and study of their enantiomer recognition ability for organo-phosphoric acid and phosphoric acid derivatives by 31P-NMR and fluorescence spectroscopy. Org. Biomol. Chem. 2016, 14, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Nagorny, P.; Tay, J.H. 31P-NMR Based Method for Determining Enantiopurity of Chiral Phosphoric Acids and Its Application to the BINOL- and H8-BINOL-Based Chiral Phosphoric Acid Thermal Racemization Studies. Synlett 2016, 27, 551–554. [Google Scholar] [CrossRef]
- Pregosin, P.S. NMR in Organometallic Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
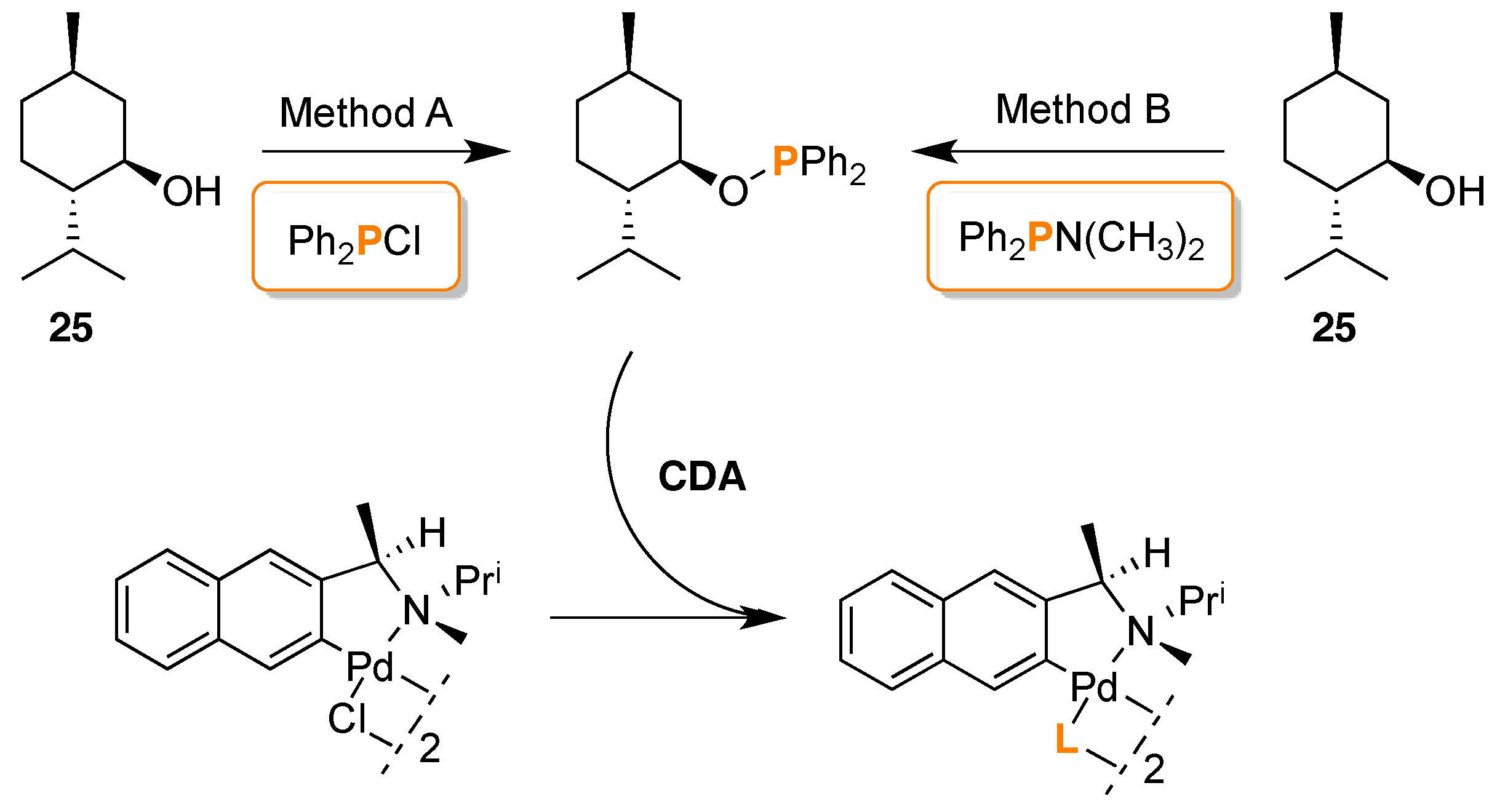
- Gorunova, O.N.; Novitskiy, I.M.; Livantsov, M.V.; Grishin, Y.K.; Kochetkov, K.A.; Dunina, V.V. Enantiopurity determination of CN-palladacycles using 31P-NMR spectroscopy with (1R,2S,5R)-menthyloxydiphenylphosphine as chiral derivatizing agent. J. Organometal. Chem. 2015, 783, 96–104. [Google Scholar] [CrossRef]
- Gorunova, O.N.; Novitskiy, I.M.; Gloriozov, I.P.; Grishin, Y.K.; Roznyatovsky, V.A.; Khrustalev, V.N.; Kochetkov, K.A.; Dunina, V.V. Determination of the Absolute Configuration of CN-Pallacycles by 31P{1H} NMR Spectroscopy Using (1R,2S,5R)-Menthyloxydiphenylphosphine as the Chiral Derivatizing Agent: Efficient Chirality Transfer in Phosphinite Adducts. Organometallics 2016, 35, 75–90. [Google Scholar] [CrossRef]
- Martin, G.E.; Hilton, B.D.; Blinov, K.A. HSQC-1,1-ADEQUATE and HSQC-1,n-ADEQUATE: Enhanced Methods for Establishing Adjacent and Long-Range 13C-13C Connectivity Networks. J. Nat. Prod. 2011, 74, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Capanema, E.A. Comprehensive structural analysis of biorefinery lignins with a quantitative 13C-NMR approach. RSC Adv. 2015, 5, 87187–87199. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Use of 13C-NMR Chemical Shift as QSAR/QSPR Descriptor. Chem. Rev. 2011, 111, 2865–2899. [Google Scholar] [CrossRef] [PubMed]
- Wiitala, K.W.; Cramer, C.J.; Hoye, T.R. Comparison of various density functional methods for distinguishing stereoisomers based on computed 1H- or 13C-NMR chemical shifts using diastereomeric penam β-lactams as a test set. Magn. Reson. Chem. 2007, 45, 819–829. [Google Scholar] [CrossRef] [PubMed]
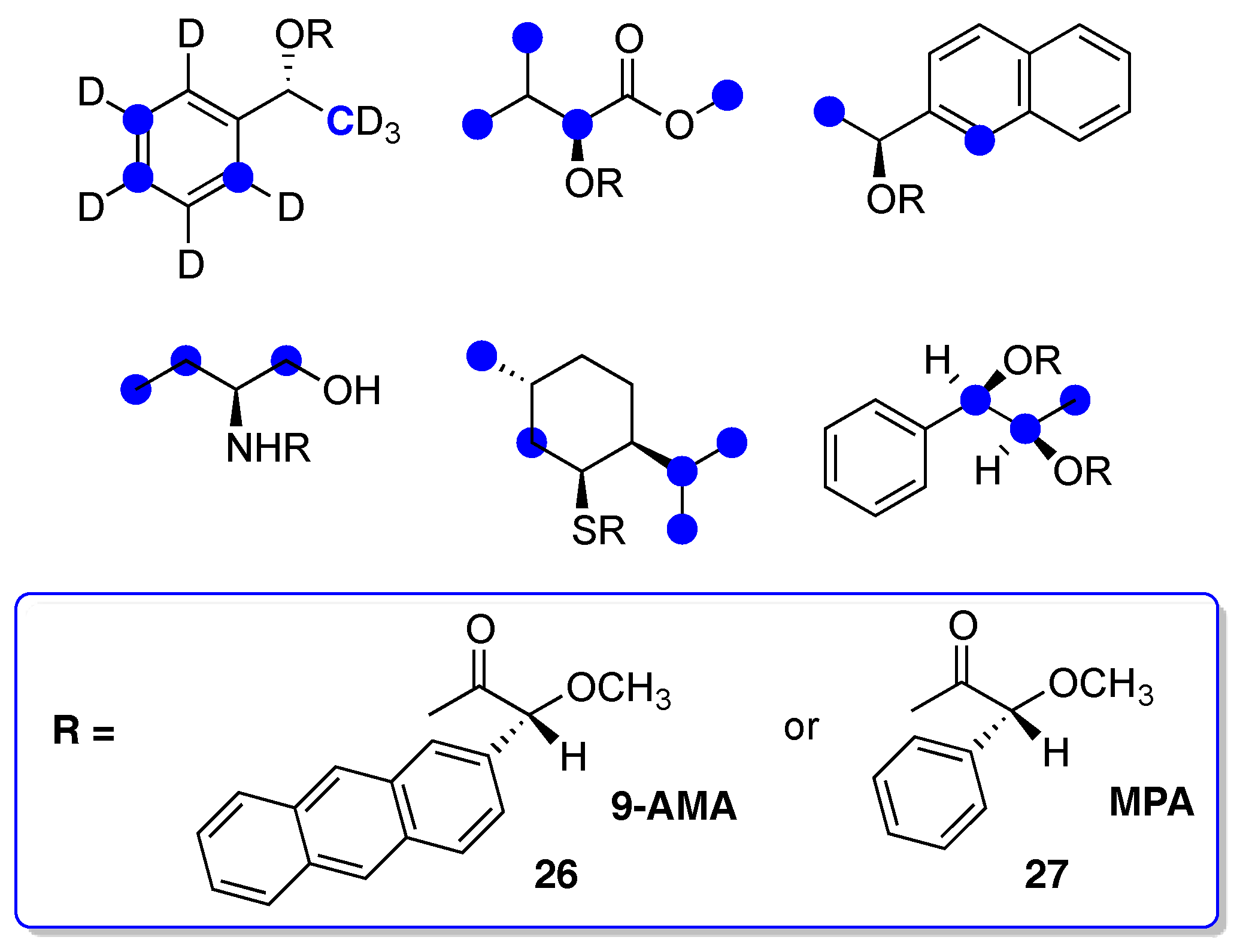
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar] [CrossRef]
- Louzao, I.; Seco, J.M.; Riguera, R. 13C-NMR as a general tool for the assignment of absolute configuration. Chem. Commun. 2010, 46, 7903–7905. [Google Scholar] [CrossRef] [PubMed]
- Louzao, I.; Seco, J.M.; Quiñoá, E.; Riguera, R. The assignment of absolute configuration of cyanohydrins by NMR. Chem. Commun. 2006, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Hyun, M.H.; Cho, Y.J.; Ryoo, J.J. Determination of optical purity of 3,5-dimethoxybenzoyl-leucine diethylamide by chiral chromatography and 1H- and 13C-NMR spectroscopy. Chirality 2011, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.E.; Górecki, L.; Berlicki, L.; Slepokura, K.; Mucha, A. Zwitterionic Phosphorylated Quinines as Chiral Solvating Agents for NMR Spectroscopy. Chirality 2015, 27, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, P.P.; Netscher, T.; Duchateau, A.L.L. A Simple 13C-NMR Method for the Discrimination of Complex Mixtures of Stereoisomers: All Eight Stereoisomers of α-Tocopherol Resolved. Chirality 2015, 27, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Trujillo, M.; Castãnar, L.; Monteagudo, E.; Kuhn, L.T.; Nolis, P.; Virgili, A.; Williamson, R.T.; Parella, T. Simultaneous 1H- and 13C-NMR enantiodifferentiation from highly-resolved pure shift HSQC spectra. Chem. Commun. 2014, 50, 10214–10217. [Google Scholar] [CrossRef] [PubMed]
- Lesot, P.; Lofan, O.; Aroulanda, C.; Dong, R.Y. 2H-NMR Studies on Two-Homopolypeptide Lyotropic Enantiodiscriminating Mesophases: Experimental Quantification of Solute-Fiber Affinites. Chem. Eur. J. 2008, 14, 4082–4092. [Google Scholar] [CrossRef] [PubMed]
- Kobzar, K.; Kessler, H.; Luy, B. Stretched Gelatin Gels as Chiral Alignment Media for the Discrimination of Enantiomers by NMR Spectroscopy. Angew. Chem. Int. Ed. 2005, 44, 3145–3147. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.R. Constitutional, Configurational, and Conformational Analysis of Small Organic Molecules on the Basis of NMR Residual Dipolar Couplings. Angew. Chem. Int. Ed. 2011, 50, 7222–7224. [Google Scholar] [CrossRef] [PubMed]
- Lesot, P.; Lafon, O.; Berdagué, P. Correlation 2D-NMR experiments involving both 13C and 2H isotopes in oriented media: Methodological developments and analytical applications. Magn. Reson. Chem. 2014, 52, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.R.; Sharman, G.J. The measurement of high enantiomeric excess in chiral liquid crystals using 19F-NMR and exchangeable protons in 2H-NMR. Chem. Commun. 2004, 11, 1330–1331. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G. Organoselenium Chemistry: A Practical Approach; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Santi, C.; Jacob, R.G.; Monti, B.; Bagnoli, L.; Sancineto, L.; Lenardão, E.J. Water and Aqueous Mixtures as Convenient Alternative Media for Organoselenium Chemistry. Molecules 2016, 21, 1482. [Google Scholar] [CrossRef] [PubMed]
- Araie, H.; Shiraiwa, Y. Selenium Utilization Strategy by Microalgae. Molecules 2009, 14, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Brumaghim, J.L. Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium and Tellurium. In Proceedings of the ACS Symposium Series 1152, Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium, Clemson, SC, USA, 1 January 2013.
- Orlov, N.V.; Ananikov, V.P. First principles design of derivatizing agent for direct determination of enantiomeric purity of chiral alcohols and amines by NMR spectroscopy. Chem. Commun. 2010, 46, 3212–3214. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Gonçalves, S.M.C. Enantiomeric Excess Detection with (S)-3-Phenyl-2-(selenophenyl)propan-1-ol Derivatizing Agent via Mix and Shake 77Se-NMR. J. Braz. Chem. Soc. 2010, 21, 2023–2026. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Cunha, R.L.O.R.; Silva, M.S. 77Se- and 125Te-NMR spectroscopy for enantiopurity determination of chalcogen amines. Tetrahedron Lett. 2016, 57, 4556–4559. [Google Scholar] [CrossRef]
- Murai, T. Phosphoroselenoic Acid Derivatives Bearing a Binaphthyl Group as a Chiral Molecular Tool. Phosphorus Sulfur Silicon 2008, 889–896. [Google Scholar] [CrossRef]
- Murai, T.; Itoh, H. Discrimination of remote chirality of primary alcohols using 1,1′-binaphthyl-2,2′-DIYL phosphoroselenoyl chlorides as a chiral molecular tool. Phosphorus Sulfur Silicon 2016, 191, 163–173. [Google Scholar] [CrossRef]


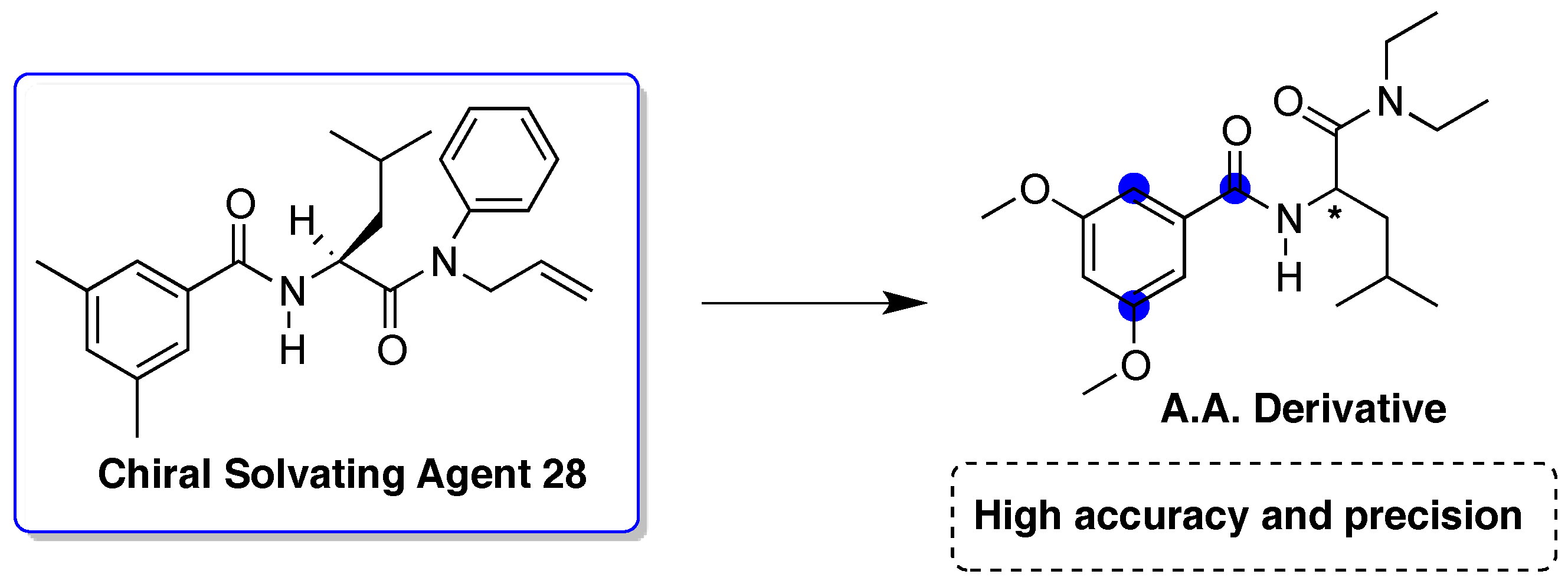
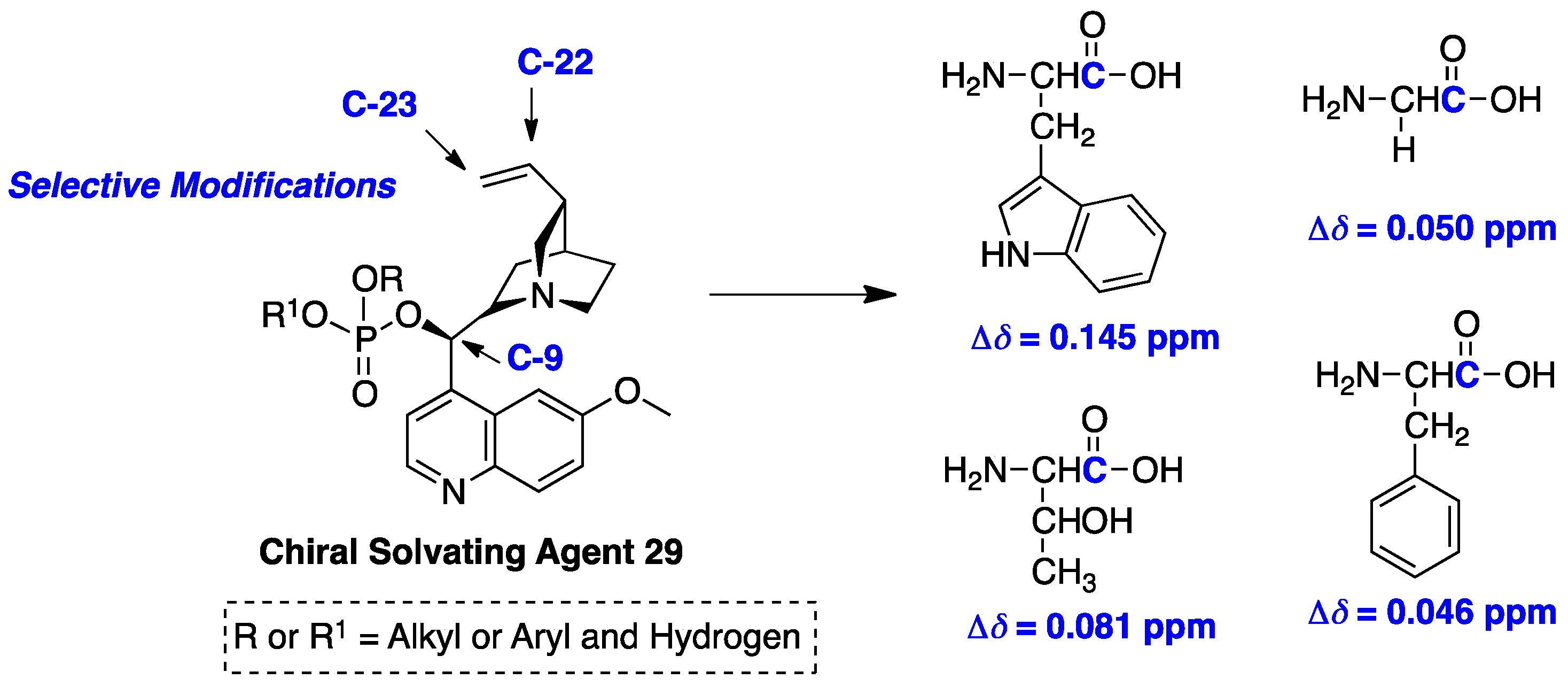
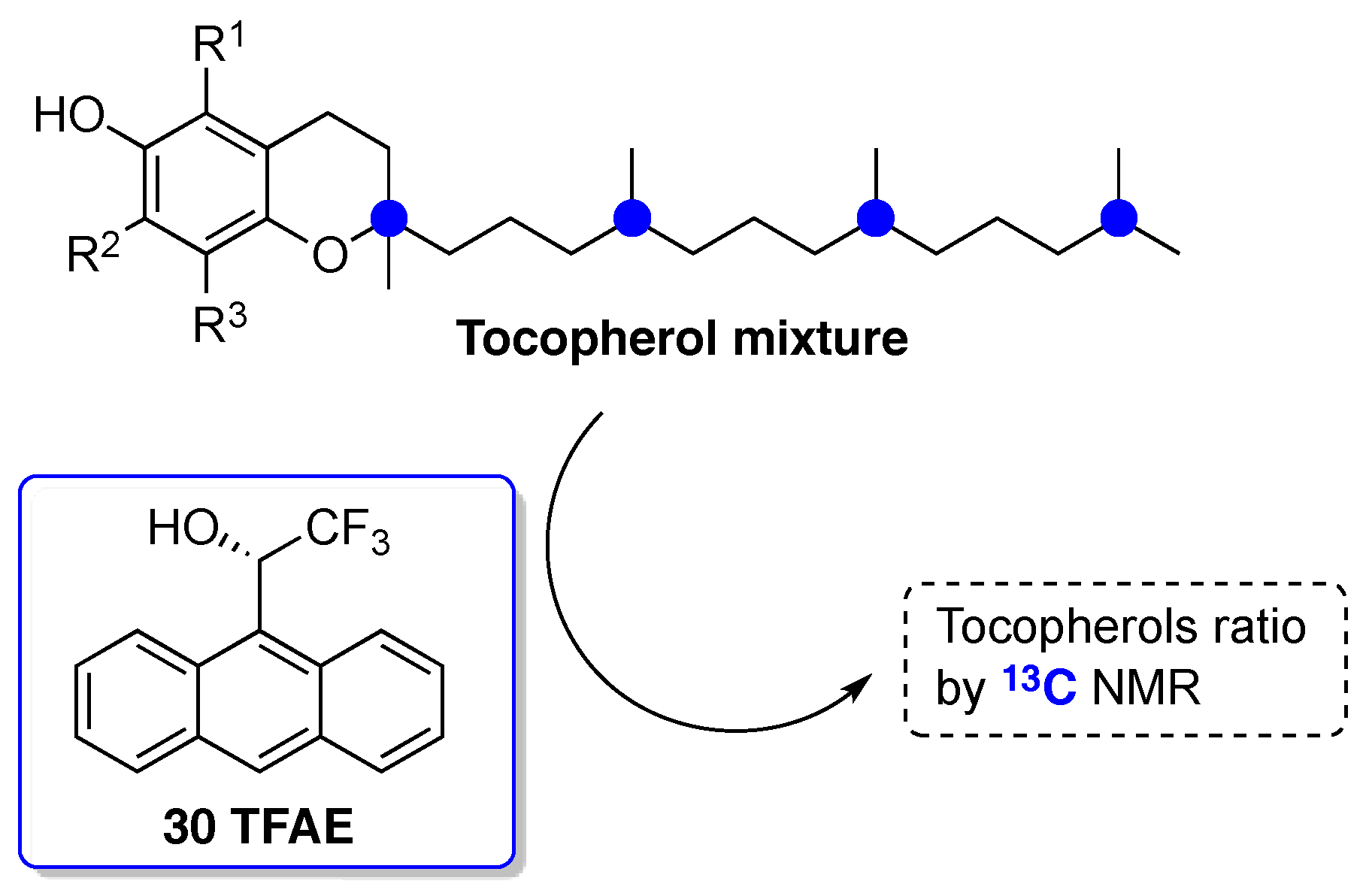
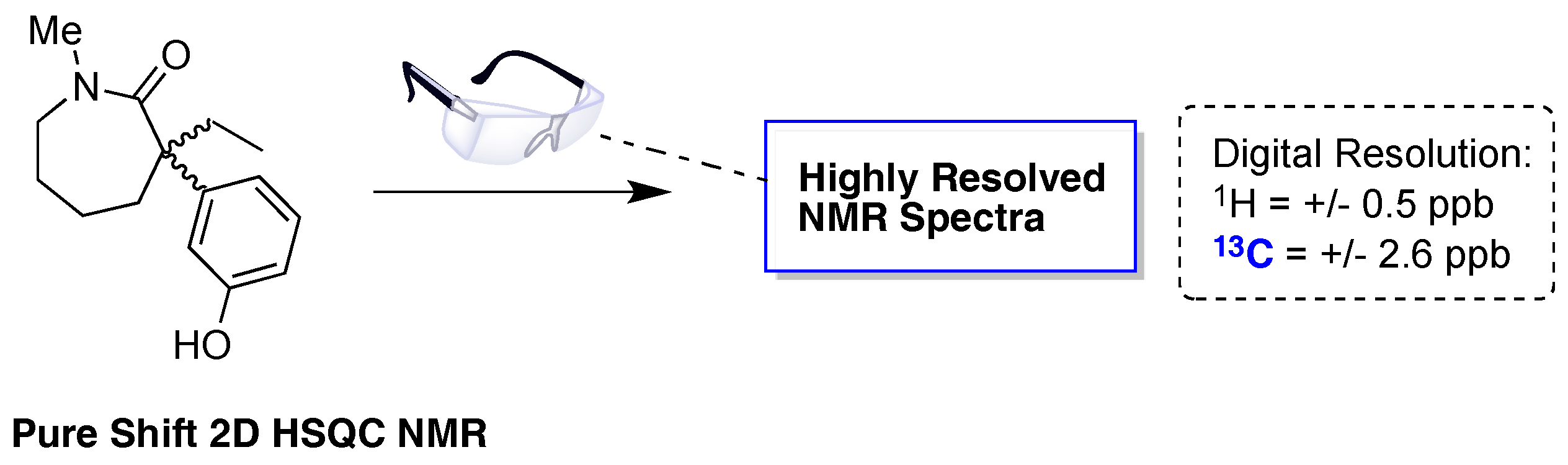

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.S. Recent Advances in Multinuclear NMR Spectroscopy for Chiral Recognition of Organic Compounds. Molecules 2017, 22, 247. https://doi.org/10.3390/molecules22020247
Silva MS. Recent Advances in Multinuclear NMR Spectroscopy for Chiral Recognition of Organic Compounds. Molecules. 2017; 22(2):247. https://doi.org/10.3390/molecules22020247
Chicago/Turabian StyleSilva, Márcio S. 2017. "Recent Advances in Multinuclear NMR Spectroscopy for Chiral Recognition of Organic Compounds" Molecules 22, no. 2: 247. https://doi.org/10.3390/molecules22020247