Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella radicata
Abstract
:1. Introduction
2. Results
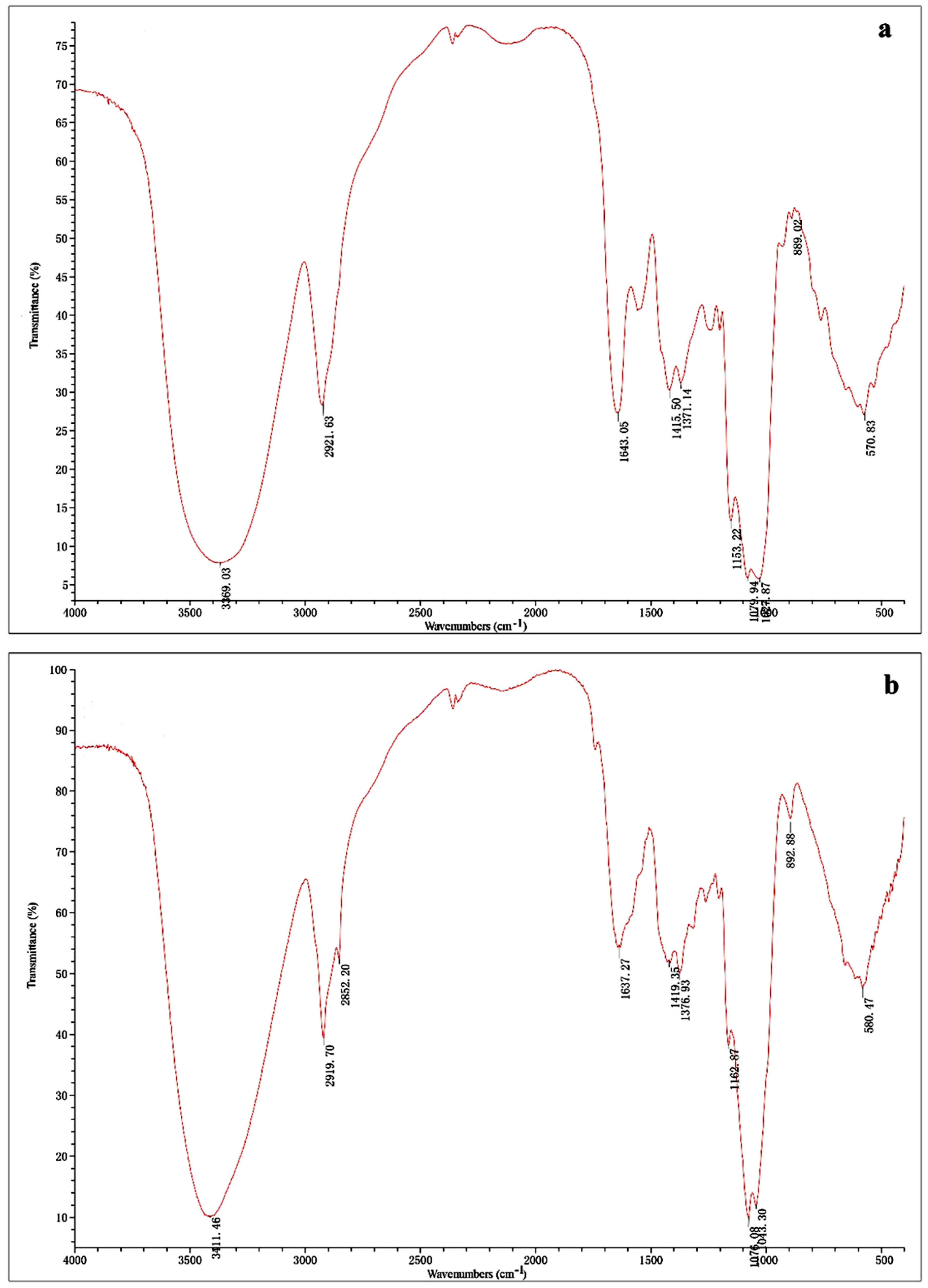
2.1. Isolation and Characterization of Polysaccharides
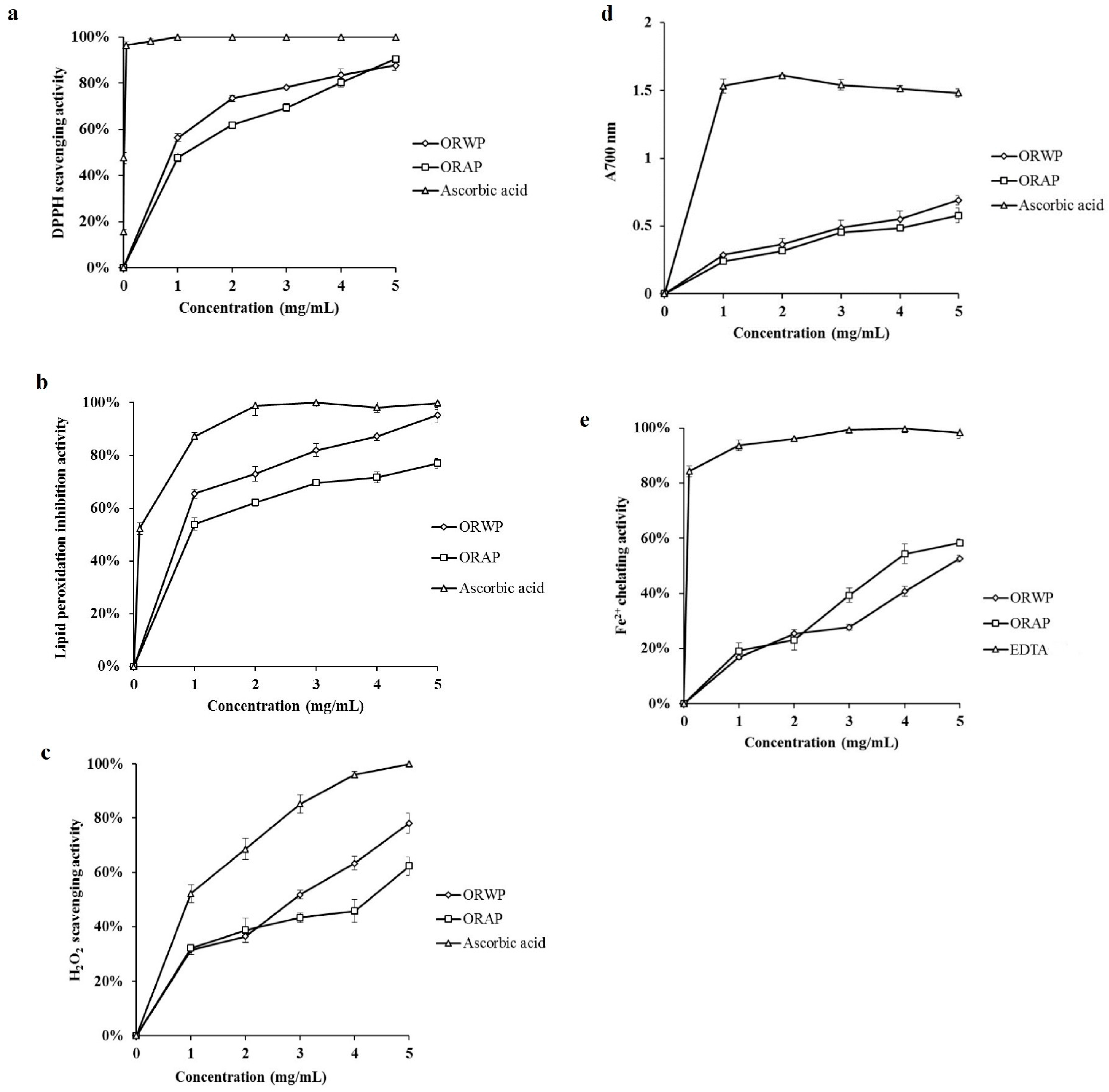
2.2. In Vitro Antioxidant Activity
2.2.1. DPPH Radical Scavenging Activity
2.2.2. Inhibitory Effect on Lipid Peroxidation
2.2.3. Hydrogen Peroxide Scavenging Activity
2.2.4. Reducing Power
2.2.5. Ferrous Ion Chelating Activity
2.3. Animal Experiments
2.3.1. Effects of Polysaccharides on Body Weight, Liver Weight and HI in Mice
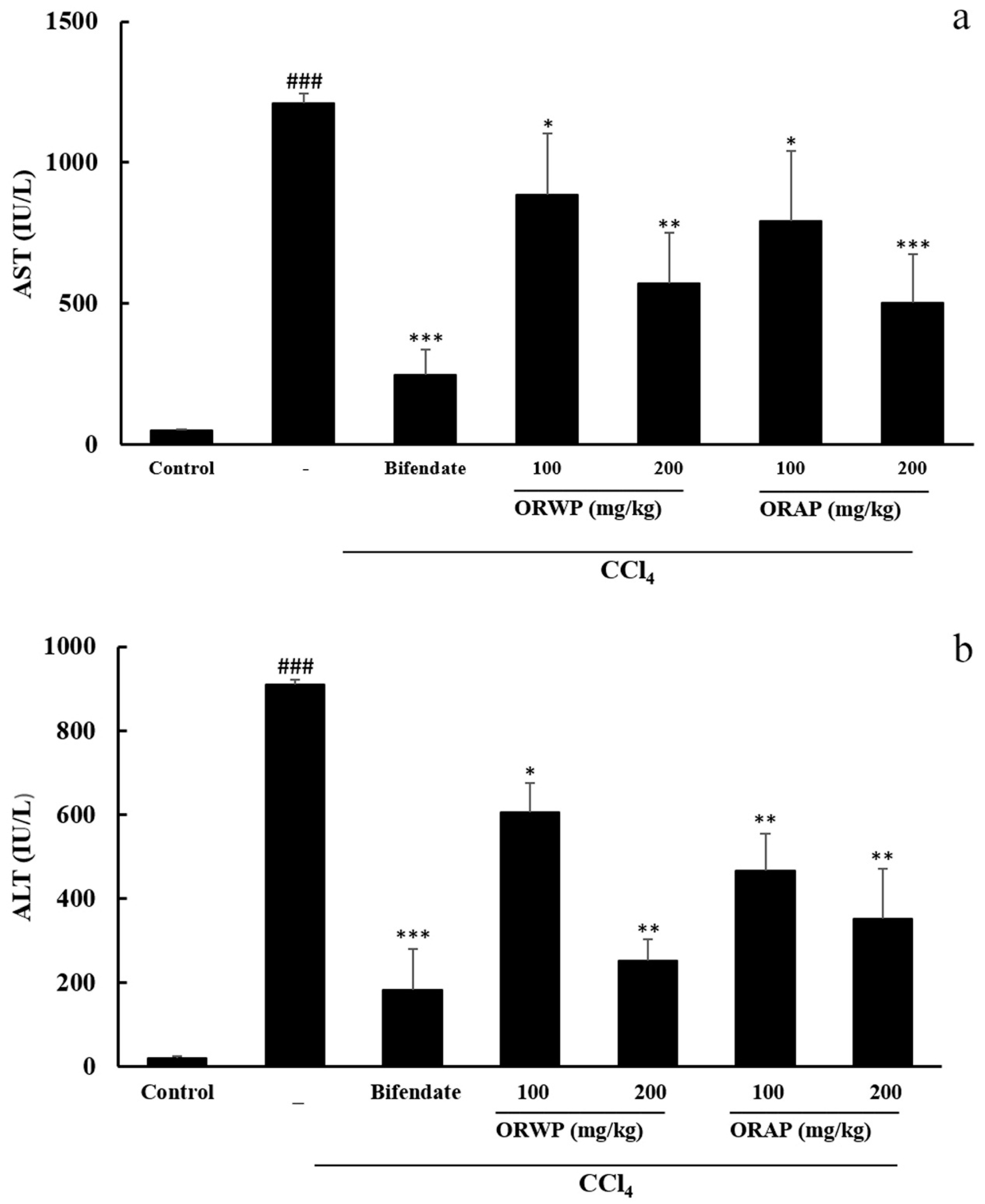
2.3.2. Effects of the Polysaccharides on AST, ALT, MDA, GSH-Px and SOD Activities
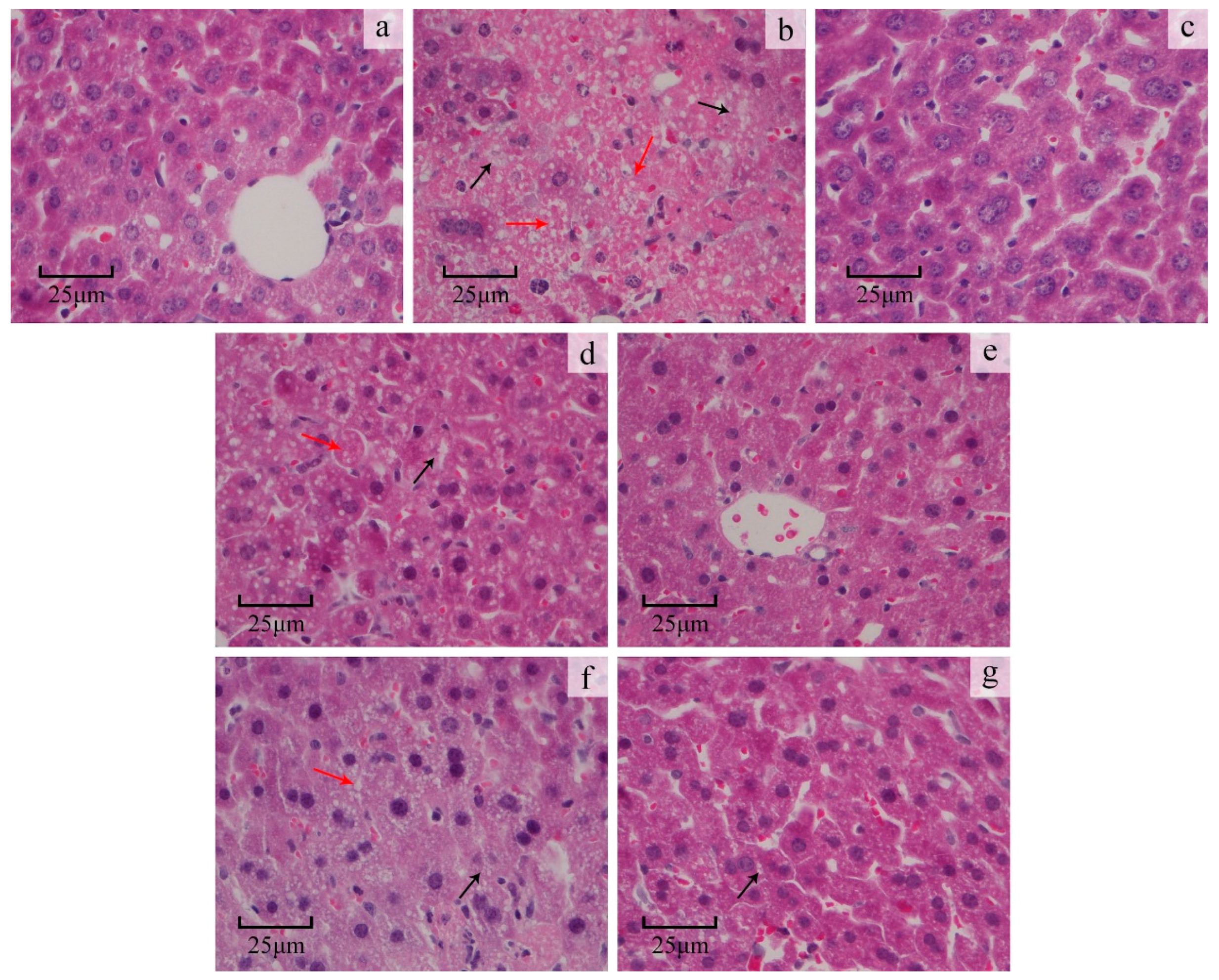
2.3.3. Liver Histopathological Study
2.3.4. Study on the Acute Toxicity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction and Isolation of Polysaccharides
4.3. Molecular Weight Determination
4.4. Preliminary Characterization of ORWP/ORAP
4.5. UV and Infrared Spectral Analysis
4.6. Analyse of the Antioxidant Activity In Vitro
4.6.1. DPPH Radical Scavenging Activity
4.6.2. Inhibitory Effect on Lipid Peroxidation
4.6.3. Hydrogen Peroxide Scavenging Activity
4.6.4. Reducing Power
4.6.5. Ferrous Ion Chelating Activity
4.7. Animal Experiments
4.7.1. Animals
4.7.2. In Vivo Hepatoprotective Activity
4.7.3. Biochemical Assays
4.7.4. Histopathological Study on the Liver
4.7.5. Study on the Acute Toxicity
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, H.; Qiu, Y.; Shu, Z.; Zhang, X.; Li, R.; Liu, S.; Chen, L.; Liu, H.; Chen, N. Protective effect of trillium tschonoskii saponin on CCl4-induced acute liver injury of rats through apoptosis inhibition. Can. J. Physiol. Pharmacol. 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kepekci, R.A.; Polat, S.; Celik, A.; Bayat, N.; Saygideger, S.D. Protective effect of spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem. 2013, 141, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, Y.; Sun, Y.; Yang, S.; Yang, X. Characterisation of polysaccharides from green tea of huangshan maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; Rivera-Espinoza, Y. Beneficial drugs for liver diseases. J. Appl. Toxicol. 2008, 28, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Y.; Jiao, Y.; Yu, L.; Yang, S.; Yang, X. Antioxidative and hepatoprotective effects of the polysaccharides from zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 2012, 88, 1453–1459. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, H.; Wang, H.; Ng, T.B. Isolation and characterization of a ubiquitin-like ribonuclease from the cultured deep root mushroom, Oudemansiella radicata (higher basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ren, Z.; Zhang, J.; Song, X.; Gao, Z.; Jing, H.; Li, S.; Wang, S.; Jia, L. Antioxidant and anti-hyperlipidemic effects of mycelia zinc polysaccharides by Pleurotus eryngii var. Tuoliensis. Int. J. Biol. Macromol. 2016, 95, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Wu, D.-T.; Xie, J.; Zhao, J.; Li, S.-P. Characterization and comparison of bioactive polysaccharides from the tubers of gymnadenia conopsea. Food Hydrocoll. 2015, 43, 199–206. [Google Scholar] [CrossRef]
- Cagri-Mehmetoglu, A.; Kusakli, S.; van de Venter, M. Production of polysaccharide and surfactin by bacillus subtilis atcc 6633 using rehydrated whey powder as the fermentation medium. J. Dairy Sci. 2012, 95, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Sliva, D. Medicinal mushroom Phellinus linteus as an alternative cancer therapy. Exp. Ther. Med. 2010, 1, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The anti-oxidant and antitumor properties of plant polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. Reishimax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates ampk. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.M.-F.; Sit, W.-H.; Louie, J.C.-Y. Polysaccharopeptide enhances the anticancer activity of doxorubicin and etoposide on human breast cancer cells ZR-75-30. Int. J. Oncol. 2008, 32, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Ahn, H.Y.; Cho, Y.S.; Je, J.Y. Protective effect of cordycepin-enriched cordyceps militaris on alcoholic hepatotoxicity in sprague-dawley rats. Food Chem. Toxicol. 2013, 60, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.A.; de Sa-Nakanishi, A.B.; Bracht, A.; da Costa, S.M.; Koehnlein, E.A.; de Souza, C.G.; Peralta, R.M. Hepatoprotective effects of mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ng, T.; Wang, H. Isolation and characterization of a novel lectin from the wild mushroom Oudemansiella radicata (Relhan.: Fr.) sing. Biotechnol. Bioprocess Eng. 2013, 18, 465–471. [Google Scholar] [CrossRef]
- Cao, Y.R.; Zhang, X.Y.; Deng, J.Y.; Zhao, Q.Q.; Xu, H. Lead and cadmium-induced oxidative stress impacting mycelial growth of Oudemansiella radicata in liquid medium alleviated by microbial siderophores. World J. Microbiol. Biotechnol. 2012, 28, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, F.; Jiang, Q.; Chen, C.; Huang, D.; Lv, Y.; Shen, W.; Jin, Y. Purification and antitumor activity of two acidic polysaccharides from the roots of polygala tenuifolia. Carbohydr. Polym. 2012, 90, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wang, L.; Chen, C.; Teng, B.; Wang, C.; Xu, Z.; Hu, B.; Zhou, P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chem. 2012, 135, 1097–1103. [Google Scholar]
- Ganie, S.A.; Haq, E.; Masood, A.; Hamid, A.; Zargar, M.A. Antioxidant and protective effect of ethyl acetate extract of podophyllum hexandrum rhizome on carbon tetrachloride induced rat liver injury. Evid. Based Complement. Altern. Med. 2011, 2011, 238020. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; You, Y.; Yoon, H.G.; Lee, Y.H.; Kim, K.; Lee, J.; Kim, M.S.; Kim, J.C.; Jun, W. Hepatoprotective effects of fermented Curcuma longa L. On carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2014, 151, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Kang, S.C.; Baek, K.-H. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from metasequoia glyptostroboides. Asian Pac. J. Trop. Med. 2014, 7, 9–15. [Google Scholar] [CrossRef]
- Saha, D.; Paul, S. Evaluation of antioxidant and free radical scavenging activities of different fractions of pterospermum suberifolium leaf extract. Thai J. Pharm. Sci. 2014, 38, 28–35. [Google Scholar]
- Sun, Y.X.; Kennedy, J.F. Antioxidant activities of different polysaccharide conjugates (CRPs) isolated from the fruiting bodies of Chroogomphis rutilus (Schaeff.: Fr.) O. K. Miller. Carbohydr. Polym. 2010, 82, 510–514. [Google Scholar] [CrossRef]
- Dang, Z.; Feng, D.; Liu, X.; Yang, T.; Guo, L.; Liang, J.; Liang, J.; Hu, F.; Cui, F.; Feng, S. Structure and antioxidant activity study of sulfated acetamido-polysaccharide from radix hedysari. Fitoterapia 2013, 89, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Kan, Y.; Chen, T.; Wu, Y.; Wu, J.; Wu, J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015, 72, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011, 51, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Guo, L. Lipid peroxidation generates biologically active phospholipids including oxidatively n-modified phospholipids. Chem. Phys. Lipids 2014, 181, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Y.; Mu, H.; Lv, Z.; Yang, Y.; Zhang, J. Structural elucidation and antioxidant activity of a novel polysaccharide (tapb1) from tremella aurantialba. Food Hydrocoll. 2015, 43, 459–464. [Google Scholar] [CrossRef]
- Zeng, B.; Su, M.; Chen, Q.; Chang, Q.; Wang, W.; Li, H. Antioxidant and hepatoprotective activities of polysaccharides from anoectochilus roxburghii. Carbohydr. Polym. 2016, 153, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Ke, C.; Hu, B.; Luo, J.; Ye, H.; Sun, Y.; Yan, X.; Zeng, X. Antioxidant activities of polysaccharides from hyriopsis cumingii. Carbohydr. Polym. 2009, 78, 199–204. [Google Scholar] [CrossRef]
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ker, Y.-B.; Chen, K.-C.; Chyau, C.-C.; Chen, C.-C.; Guo, J.-H.; Hsieh, C.-L.; Wang, H.-E.; Peng, C.-C.; Chang, C.-H.; Peng, R.Y. Antioxidant capability of polysaccharides fractionated from submerge-culturedagaricus blazeimycelia. J. Agric. Food Chem. 2005, 53, 7052–7058. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms agaricus bisporus, agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lee, C.Y.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, Y.H. Hepatoprotective effects of eburicoic acid and dehydroeburicoic acid from antrodia camphorata in a mouse model of acute hepatic injury. Food Chem. 2013, 141, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Yang, R.; Dong, H.; Yu, Z.L.; Ko, K.M. Bifendate treatment attenuates hepatic steatosis in cholesterol/bile salt- and high-fat diet-induced hypercholesterolemia in mice. Eur. J. Pharmacol. 2006, 552, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-J.; Deng, J.-S.; Huang, S.-S.; Shao, Y.-Y.; Chen, C.-C.; Kuo, Y.-H. Protective effect of antrosterol from antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2012, 132, 709–716. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of schisandra chinensis pollen extract on ccl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Zhu, F.; Gao, S. Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem. 2012, 131, 677–684. [Google Scholar] [CrossRef]
- Miao, S.; Mao, X.; Pei, R.; Miao, S.; Xiang, C.; Lv, Y.; Yang, X.; Sun, J.; Jia, S.; Liu, Y. Antitumor activity of polysaccharides from lepista sordida against laryngocarcinoma in vitro and in vivo. Int. J. Biol. Macromol. 2013, 60, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, H.; Wang, C.; Liu, J.; Lan, M. Antitumor and immunomodulatory activities of a polysaccharide from artemisia argyi. Carbohydr. Polym. 2013, 98, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tang, G.; Zeng, G.; Yang, Y.; Cai, X.; Li, D.; Liu, H.; Zhou, N. Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydr. Polym. 2013, 93, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Chan, B.C.; Yu, H.; Yang, Y.H.; Hu, S.Q.; Ko, C.H.; Dong, C.X.; Wong, C.K.; Shaw, P.C.; Fung, K.P.; et al. Structural characterization and immuno-modulating activities of a polysaccharide from Ganoderma sinense. Int. J. Biol. Macromol. 2012, 51, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of northeast portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

| Property/Component | ORWP | ORAP |
|---|---|---|
| Molecular weight (Da) | 1.73 × 105 | 1.15 × 104 |
| Carbohydrate (wt %) | 95.3 | 97.4 |
| Protein (wt %) | ND | ND |
| Components of monosaccharide (mol %) | ||
| Ribose | 0.15 | 0.16 |
| Rhamnose | 0.32 | 0.51 |
| Arabinose | 1.02 | 1.02 |
| Xylose | 2.45 | 3.31 |
| Mannose | 15.74 | 18.41 |
| Glucose | 65.85 | 72.35 |
| Galactose | 14.47 | 4.24 |
| Treatment | Dose (ORWP/ORAP) | Body wt. (g) | Liver wt. (g) | HI (%) |
|---|---|---|---|---|
| Normal | 29.13 ± 3.22 | 1.23 ± 0.20 | 42.24 ± 2.87 | |
| CCl4 alone | 29.07 ± 1.50 | 1.54 ± 0.09 ## | 53.25 ± 3.48 ### | |
| CCl4 + bifendate | 200 mg/kg | 29.86 ± 1.10 | 1.39 ± 0.12 ** | 46.66 ± 4.12 *** |
| CCl4 + ORWP | 100 mg/kg | 29.29 ± 2.71 | 1.46 ± 0.16 | 49.87 ± 3.36 * |
| CCl4 + ORWP | 200 mg/kg | 29.41 ± 1.61 | 1.43 ± 0.08 * | 48.50 ± 1.92 ** |
| CCl4 + ORAP | 100 mg/kg | 29.23 ± 1.21 | 1.42 ± 0.16 * | 48.44 ± 4.53 ** |
| CCl4 + ORAP | 200 mg/kg | 29.18 ± 2.22 | 1.39 ± 0.17 ** | 47.46 ± 2.98 *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Ng, T.B. Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella radicata. Molecules 2017, 22, 234. https://doi.org/10.3390/molecules22020234
Liu Q, Zhu M, Geng X, Wang H, Ng TB. Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella radicata. Molecules. 2017; 22(2):234. https://doi.org/10.3390/molecules22020234
Chicago/Turabian StyleLiu, Qin, Mengjuan Zhu, Xueran Geng, Hexiang Wang, and Tzi Bun Ng. 2017. "Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella radicata" Molecules 22, no. 2: 234. https://doi.org/10.3390/molecules22020234





