Disparate Effects of Stilbenoid Polyphenols on Hypertrophic Cardiomyocytes In Vitro vs. in the Spontaneously Hypertensive Heart Failure Rat
Abstract
:1. Introduction
2. Results
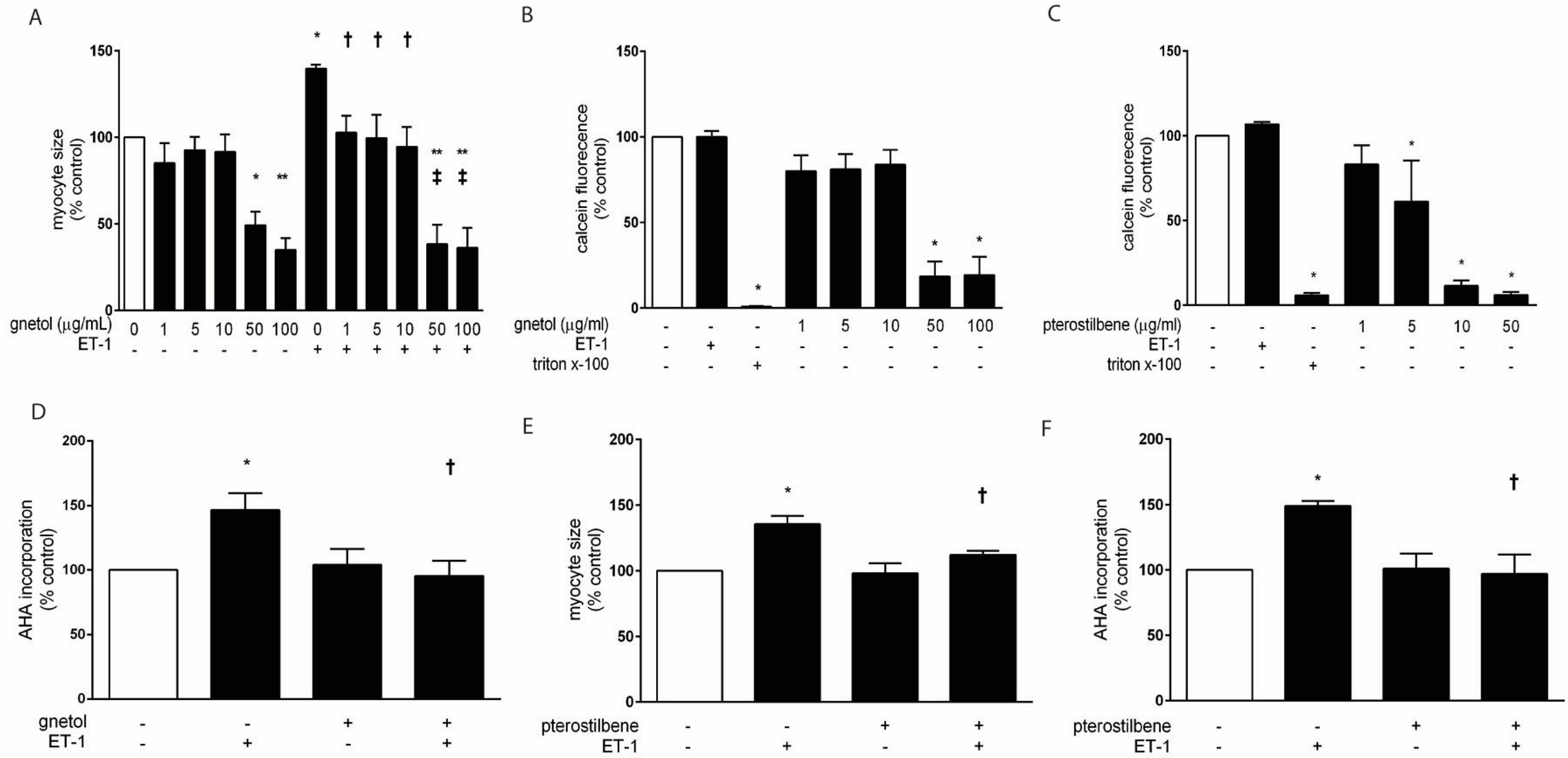
2.1. Effects of Gnetol and Pterostilbene on Cardiomyocyte Hypertrophy and Viability
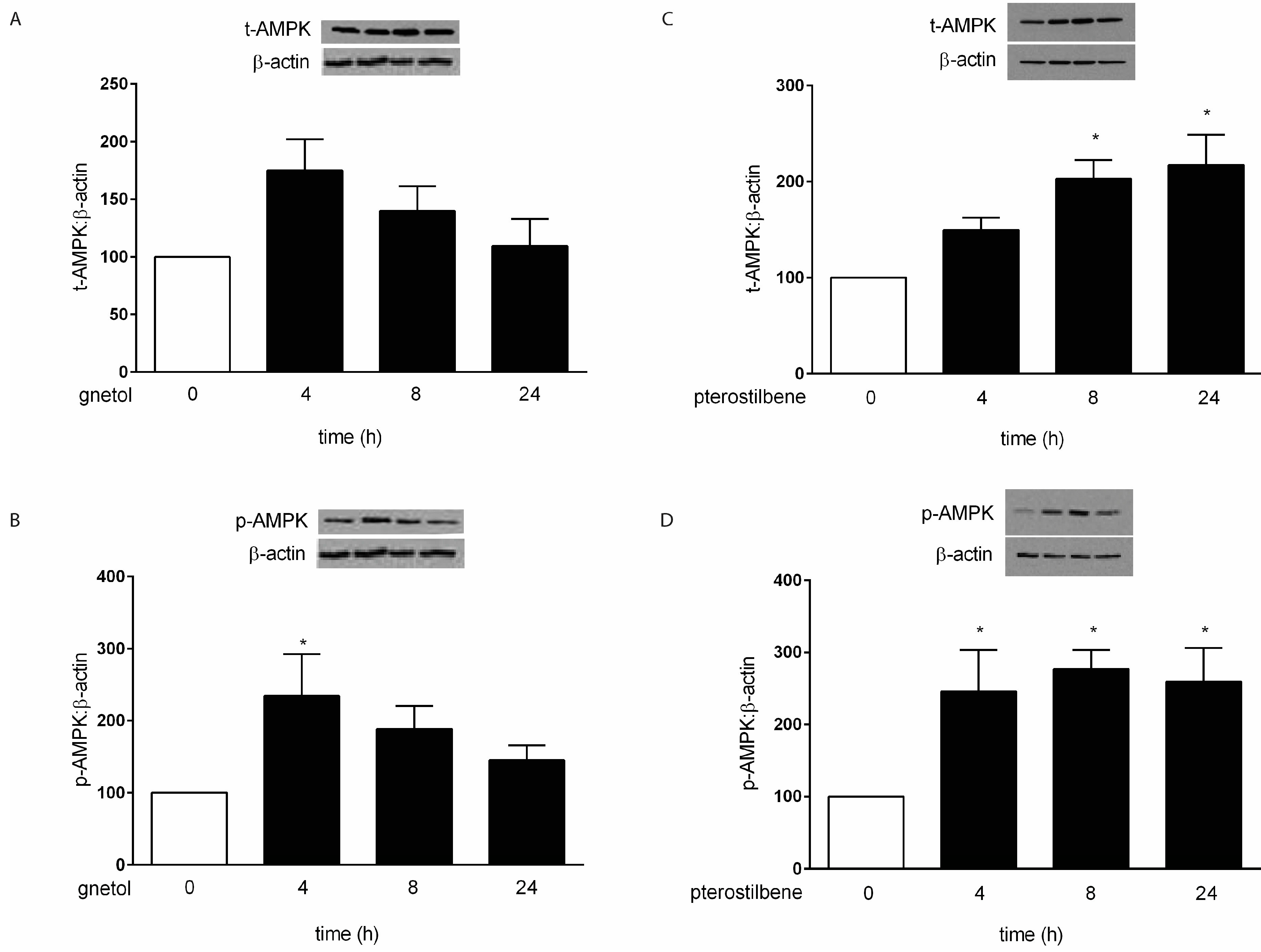
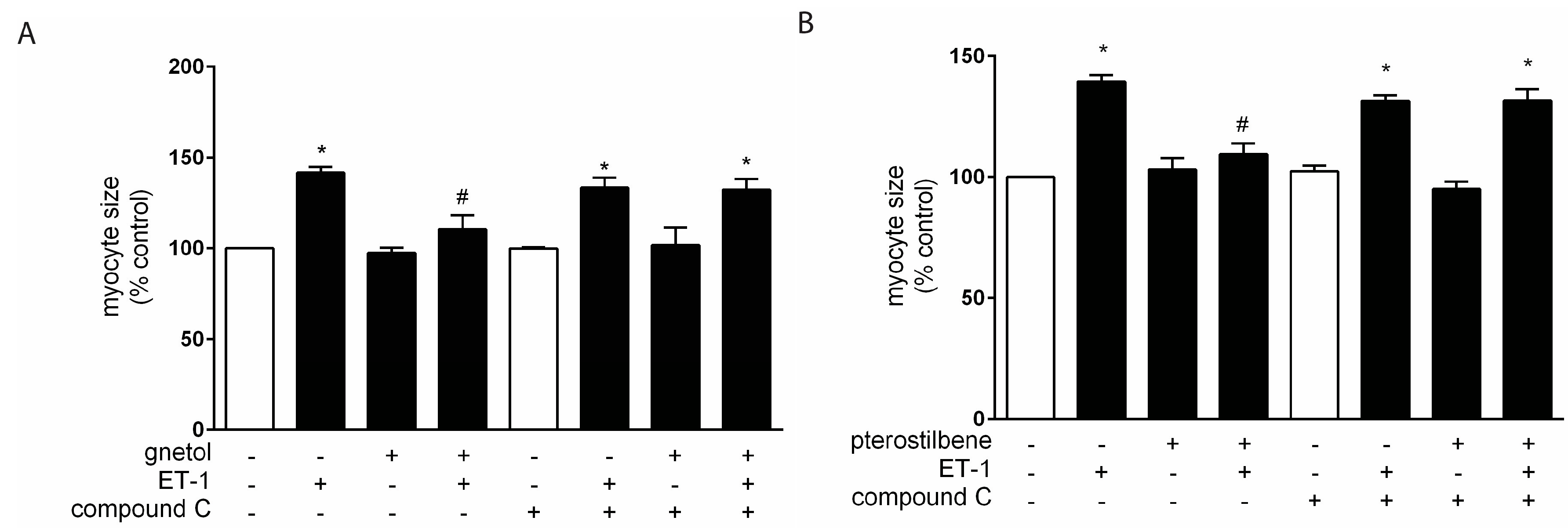
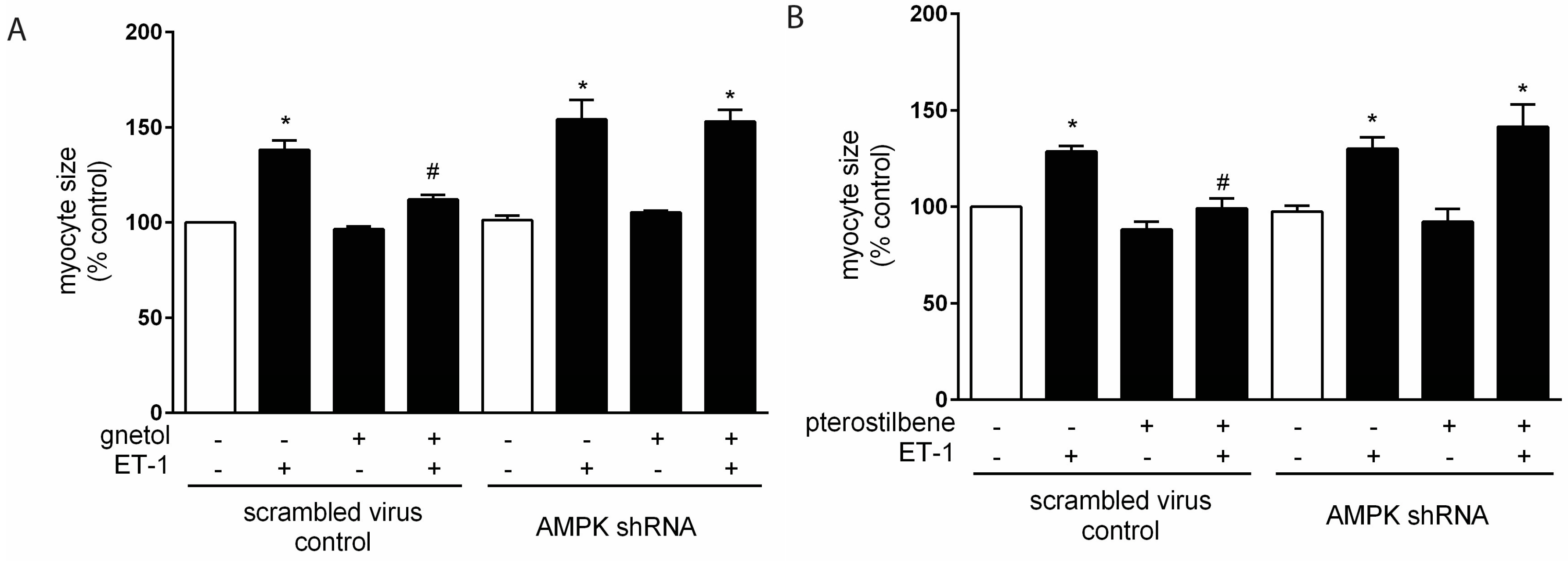
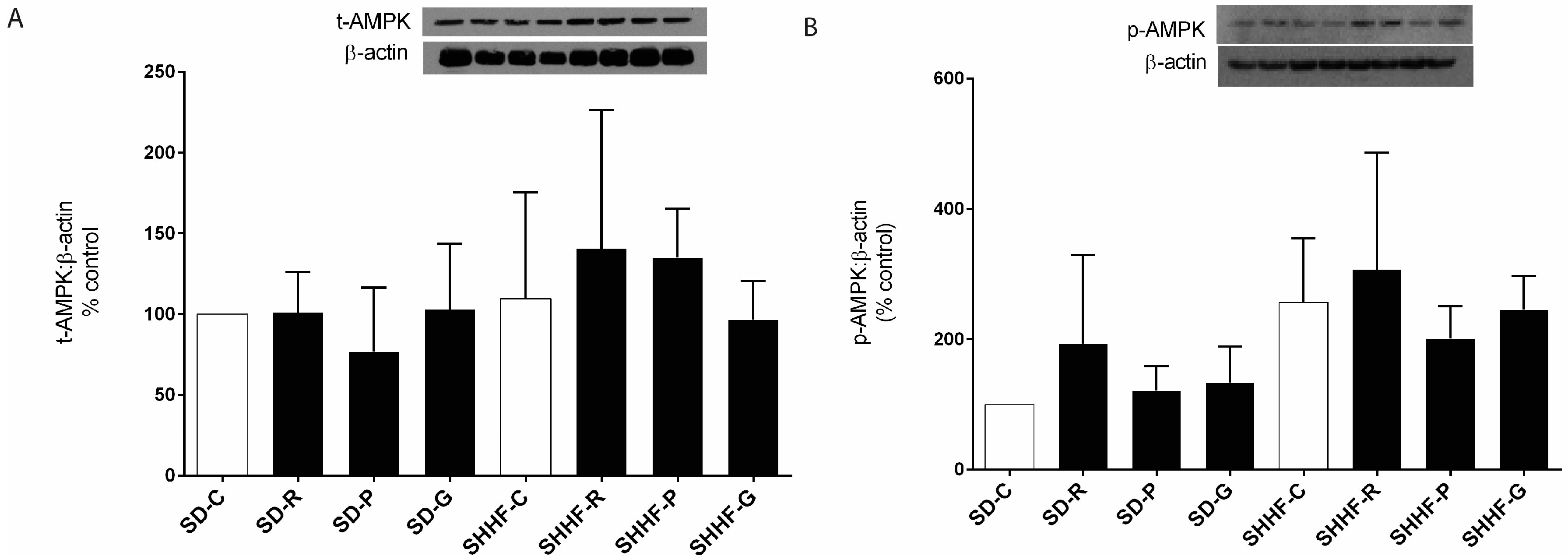
2.2. AMPK Mediates the Anti-Hypertrophic Effects of Pterostilbene and Gnetol
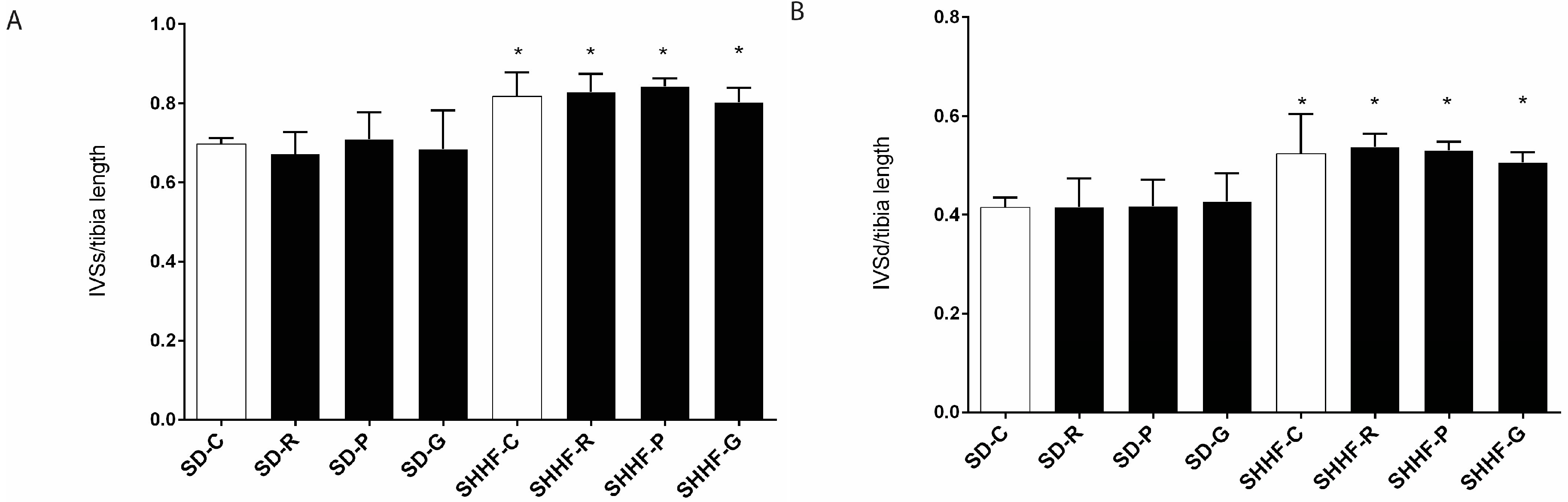
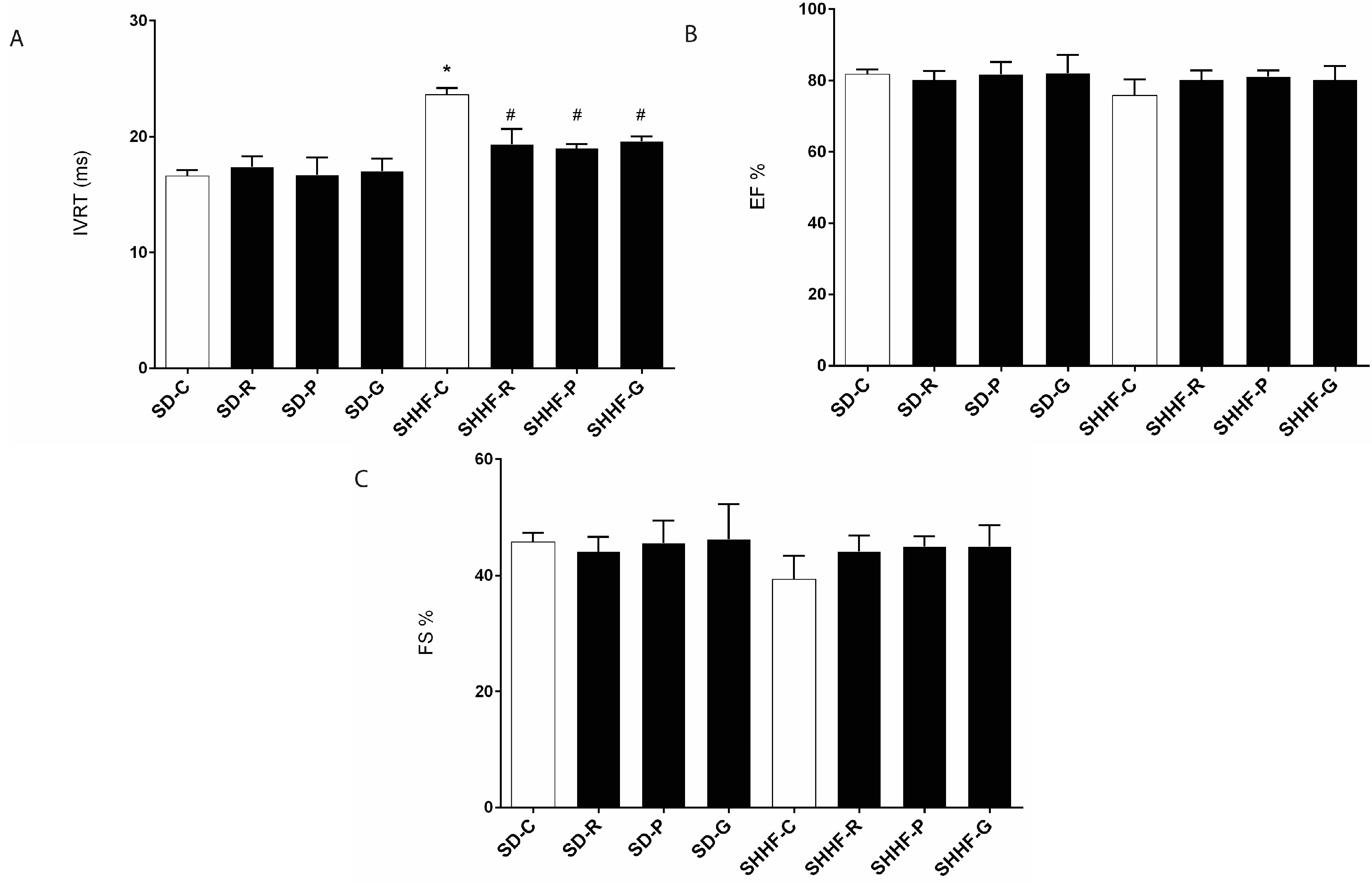
2.3. In Vivo Effects of Resveratrol, Gnetol, and Pterostilbene on Blood Pressure, Cardiac Structure and Cardiac Function
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Gnetol Synthesis Preparation of (E)-2,3’,5’,6-Tetramethoxystilbene (Scheme 1 (3))
Preparation of Gnetol (Scheme 1, (4))
4.3. Animals
4.4. In Vitro Experiments
4.4.1. Neonatal Rat Ventricular Myocytes
4.4.2. Treatments
4.4.3. Hypertrophic Indicators
4.4.4. Measurement of Cardiomyocyte Viability
4.4.5. Western Blotting
4.4.6. Lentiviral Preparation and Infection
4.5. In Vivo Experiments
4.5.1. Experimental Animals
4.5.2. Echocardiography
4.6. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parker, P.; Patterson, J.; Johnson, J. Pharmacotherapy. A Pathophysiologic Approach: Heart Failure, 6th ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; Pinsky, J.L.; Kannel, W.B.; Levy, D. The epidemiology of heart failure: The Framingham study. J. Am. Coll. Cardiol. 1993, 22, 6A–13A. [Google Scholar] [CrossRef]
- Berenji, K.; Drazner, M.H.; Rothermel, B.A.; Hill, J.A. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J. Physiol. 2005, 289, H8–H16. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the heart: A new therapeutic target? Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Scarpace, P.J.; Whidden, M.A.; Erdos, B.; Kirichenko, N.; Sakarya, Y.; Utkan, T.; Tumer, N. Age impaired endothelium-dependent vasodilation is improved by resveratrol in rat mesenteric arteries. J. Exerc. Nutr. Biochem. 2016, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Jugder, B.E.; Poljak, A.; Jayasena, T.; Mansour, H.; Nabavi, S.M.; Sachdev, P.; Grant, R. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer‘s disease. Curr. Top. Med. Chem. 2016, 16, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, J.; Thandapilly, S.J.; Louis, X.L.; Huang, Y.; Shao, Z.; Kopilas, M.A.; Wojciechowski, P.; Netticadan, T.; Anderson, H.D. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am. J. Hypertens. 2010, 23, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using Box-Behnken design. AAPS PharmSciTech 2015, 16, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. Pterostilbene and cancer: Current review. J. Surg. Res. 2012, 173, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Masih, I.; Deopujari, J.; Charpentier, C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an Ayurvedic medicine from India. J. Ethnopharmacol. 1999, 68, 71–76. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yanez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Tanaka, T.; Iliya, I.; Iinuma, M.; Furusawa, M.; Ito, T.; Nakaya, K.; Murata, J.; Darnaedi, D. Phenolic constituents of Gnetum klossii. J. Nat. Prod. 2003, 66, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.S.; Wang, Y.H.; Li, R.L.; Lin, M. Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry 2000, 54, 875–881. [Google Scholar] [CrossRef]
- Ohguchi, K.; Tanaka, T.; Kido, T.; Baba, K.; Iinuma, M.; Matsumoto, K.; Akao, Y.; Nozawa, Y. Effects of hydroxystilbene derivatives on tyrosinase activity. Biochem. Biophys. Res. Commun. 2003, 307, 861–863. [Google Scholar] [CrossRef]
- Xiang, W.; Jiang, B.; Li, X.M.; Zhang, H.J.; Zhao, Q.S.; Li, S.H.; Sun, H.D. Constituents of Gnetum montanum. Fitoterapia 2002, 73, 40–42. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids isolated from the seeds of melinjo (Gnetum gnemon L.) and their biological activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Kunimasa, K.; Yamori, Y.; Mori, M.; Mori, H.; Nakamura, K.; Miller, G.; Manne, U.; Tiwari, A.K.; Narayanan, B. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med 2015, 4, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Wojciechowski, P.; Das, D.K.; Netticadan, T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am. J. Physiol. 2007, 292, H2138–H2143. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, P.; Juric, D.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Taylor, C.; Netticadan, T. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J. Nutr. 2010, 140, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chan, A.Y.; Robillard Frayne, I.; Light, P.E.; Des Rosiers, C.; Dyck, J.R. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 2009, 119, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.H.; Liu, J.C.; Lin, H.; Shih, N.L.; Chen, Y.L.; Huang, M.T.; Chan, P.; Cheng, C.F.; Chen, J.J. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004, 369, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Dolinsky, V.W.; Soltys, C.L.; Viollet, B.; Baksh, S.; Light, P.E.; Dyck, J.R. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and akt. J. Biol. Chem. 2008, 283, 24194–24201. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with ppar{alpha} to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2010. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Louis, X.L.; Yang, T.; Stringer, D.M.; Yu, L.; Zhang, S.; Wigle, J.; Kardami, E.; Zahradka, P.; Taylor, C.; et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating no-AMPK pathway. Eur. J. Pharmacol. 2011, 668, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Dyck, J.R. Role of AMP-activated protein kinase in healthy and diseased hearts. Am. J. Physiol. 2006, 291, H2557–H2569. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Soltys, C.L.; Young, M.E.; Proud, C.G.; Dyck, J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004, 279, 32771–32779. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Ma, Y.D.; Meng, R.S.; Xiong, Z.J.; Wang, H.N.; Zeng, J.Y.; Liu, C.; Dong, Y.G. Activation of AMPK inhibits cardiomyocyte hypertrophy by modulating of the Foxo1/Murf1 signaling pathway in vitro. Acta Pharmacol. Sin. 2010, 31, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.J.; Lenski, M.; Bohm, M.; Laufs, U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 32562–32569. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yin, R.; Chen, D.; Liu, D.; Wang, D.; Yang, Q.; Dong, Y.G. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J. Cell. Biochem 2007, 100, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Witters, L.A.; Kemp, B.E.; Means, A.R. Chutes and ladders: The search for protein kinases that act on AMPK. Trends Biochem. Sci. 2006, 31, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, C.; Bertrand, L.; Horman, S.; Hue, L. Ampk activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc. Res. 2011, 90, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J. Contemporary treatment of hypertrophic cardiomyopathy. Tex. Heart Inst. J. 2009, 36, 194–204. [Google Scholar] [PubMed]
- Shimizu, M.; Sugihara, N.; Shimizu, K.; Yoshio, H.; Ino, H.; Nakajima, K.; Takeda, R. Asymmetrical septal hypertrophy in patients with hypertension: A type of hypertensive left ventricular hypertrophy or hypertrophic cardiomyopathy combined with hypertension? Clin. Cardiol. 1993, 16, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Heyen, J.R.; Blasi, E.R.; Nikula, K.; Rocha, R.; Daust, H.A.; Frierdich, G.; Van Vleet, J.F.; De Ciechi, P.; McMahon, E.G.; Rudolph, A.E. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am. J. Physiol. 2002, 283, H1775–H1784. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M.; Onodera, T.; Wang, X.; McCune, S.A. Myocyte remodeling during the progression to failure in rats with hypertension. Hypertension 1996, 28, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.C.; Radin, M.J.; Heller, L.; Rogers, L.K.; Tobias, A.; Matise, I.; Wang, Q.; Apple, F.S.; McCune, S.A. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol. Appl. Pharmacol. 2013, 273, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Napoli, R.; Monti, M.G.; Rea, D.; Longobardi, S.; Netti, P.A.; Walser, M.; Sama, M.; Aimaretti, G.; Isgaard, J.; et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes 2012, 61, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Remsberg, C.M.; Martinez, S.E.; Akinwumi, B.C.; Anderson, H.D.; Takemoto, J.K.; Sayre, C.L.; Davies, N.M. Preclinical pharmacokinetics and pharmacodynamics and content analysis of gnetol in foodstuffs. Phytother. Res. 2015, 29, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Akinwumi, B.C.; Shao, Z.; Anderson, H.D. Ligand activation of cannabinoid receptors attenuates hypertrophy of neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Loirand, G.; Guerin, P.; Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Gan, X.T.; Thomas, A.; Karmazyn, M. Prevention of rhoa activation and cofilin-mediated actin polymerization mediates the antihypertrophic effect of adenosine receptor agonists in angiotensin II- and endothelin-1-treated cardiomyocytes. Mol. Cell. Biochem. 2014, 385, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Del Re, D.P.; Sussman, M.A. The Rac and Rho hall of fame: A decade of hypertrophic signaling hits. Circ. Res. 2006, 98, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Pan, S.N.; Meng, R.S.; Peng, C.Q.; Xiong, Z.J.; Chen, B.L.; Chen, G.Q.; Yao, F.J.; Chen, Y.L.; Ma, Y.D.; et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Morton, J.S.; Oka, T.; Robillard-Frayne, I.; Bagdan, M.; Lopaschuk, G.D.; Des Rosiers, C.; Walsh, K.; Davidge, S.T.; Dyck, J.R. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension 2010, 56, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.C.; Zeidan, A.; Javadov, S.; Kilic, A.; Rajapurohitam, V.; Karmazyn, M. Nitric oxide inhibits endothelin-1-induced neonatal cardiomyocyte hypertrophy via a rhoa-rock-dependent pathway. J. Mol. Cell. Cardiol. 2009, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.R.; Naugle, J.E.; Zhang, X.; Bomser, J.A.; Meszaros, J.G. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am. J. Physiol. 2005, 288, H1131–H1138. [Google Scholar] [CrossRef] [PubMed]
- Sutra, T.; Oiry, C.; Azay-Milhau, J.; Youl, E.; Magous, R.; Teissedre, P.L.; Cristol, J.P.; Cros, G. Preventive effects of nutritional doses of polyphenolic molecules on cardiac fibrosis associated with metabolic syndrome: Involvement of osteopontin and oxidative stress. J. Agric. Food Chem. 2008, 56, 11683–11687. [Google Scholar] [CrossRef] [PubMed]
- Inanaga, K.; Ichiki, T.; Matsuura, H.; Miyazaki, R.; Hashimoto, T.; Takeda, K.; Sunagawa, K. Resveratrol attenuates angiotensin II-induced interleukin-6 expression and perivascular fibrosis. Hypertens. Res. 2009, 32, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Fenning, A.; Iyer, A.; Hoey, A.; Brown, L. Resveratrol improves cardiovascular function in doca-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Louis, X.L.; Behbahani, J.; Movahed, A.; Yu, L.; Fandrich, R.; Zhang, S.; Kardami, E.; Anderson, H.D.; Netticadan, T. Reduced hemodynamic load aids low-dose resveratrol in reversing cardiovascular defects in hypertensive rats. Hypertens. Res. 2013, 36, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Haight, A.R.; Bailey, A.E.; Baker, W.S.; Cain, M.H.; Copp, R.R.; DeMattei, J.A.; Ford, K.L.; Henry, R.F.; Hsu, M.C.; Keys, R.F.; et al. A scaleable synthesis of fiduxosin. Org. Proc. Res. Dev. 2004, 8, 897–902. [Google Scholar] [CrossRef]
- Majeed, M.; Thomas, S.M.; Nagabhushanam, K.; Balakrishnan, S.K.; Prakash, S. Process for the Synthesis of Biologically Active Oxygenated Compounds by Dealkylation of the Corresponding Alkylethers. Patent U.S. 7253324 B1, 7 August 2007. [Google Scholar]
- Wu, J.; LaPointe, M.C.; West, B.L.; Gardner, D.G. Tissue-specific determinants of human atrial natriuretic factor gene expression in cardiac tissue. J. Biol. Chem. 1989, 264, 6472–6479. [Google Scholar] [PubMed]
- Alibin, C.P.; Kopilas, M.A.; Anderson, H.D. Suppression of cardiac myocyte hypertrophy by conjugated linoleic acid: Role of peroxisome proliferator-activated receptors alpha and gamma. J. Biol. Chem. 2008, 283, 10707–10715. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, H.; Shao, Z.; O′Hara, K.A.; Kopilas, M.A.; Yu, L.; Netticadan, T.; Anderson, H.D. Suppression of endothelin-1-induced cardiac myocyte hypertrophy by ppar agonists: Role of diacylglycerol kinase zeta. Cardiovasc. Res. 2011, 90, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds gnetol and pterostilbene are available from the authors.

| Parameter (mm Hg) | SD | SHHF | ||||||
|---|---|---|---|---|---|---|---|---|
| C | R | P | G | C | R | P | G | |
| Systolic BP | 140 ± 18 | 130± 18 | 140 ± 22 | 136 ± 16 | 195± 10 a | 187 ± 14 a | 192 ± 14 a | 207 ± 10 a |
| Diastolic BP | 98 ± 14 | 85 ± 20 | 100 ± 21 | 94 ± 14 | 143 ± 7 a | 137 ± 16 a | 135 ± 20 a | 152 ± 7 a |
| Mean BP | 112 ± 16 | 100 ± 19 | 113 ± 21 | 108 ± 14 | 160 ± 8 a | 153 ± 15 a | 154 ± 18 a | 170 ± 8 a |
| Pulse Pressure | 43 ± 5 | 46 ± 5 | 41 ± 5 | 42 ± 4 | 52 ± 6 | 50 ± 7 | 56 ± 9 a | 54 ± 4 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinwumi, B.C.; Raj, P.; Lee, D.I.; Acosta, C.; Yu, L.; Thomas, S.M.; Nagabhushanam, K.; Majeed, M.; Davies, N.M.; Netticadan, T.; et al. Disparate Effects of Stilbenoid Polyphenols on Hypertrophic Cardiomyocytes In Vitro vs. in the Spontaneously Hypertensive Heart Failure Rat. Molecules 2017, 22, 204. https://doi.org/10.3390/molecules22020204
Akinwumi BC, Raj P, Lee DI, Acosta C, Yu L, Thomas SM, Nagabhushanam K, Majeed M, Davies NM, Netticadan T, et al. Disparate Effects of Stilbenoid Polyphenols on Hypertrophic Cardiomyocytes In Vitro vs. in the Spontaneously Hypertensive Heart Failure Rat. Molecules. 2017; 22(2):204. https://doi.org/10.3390/molecules22020204
Chicago/Turabian StyleAkinwumi, Bolanle C., Pema Raj, Danielle I. Lee, Crystal Acosta, Liping Yu, Samuel M. Thomas, Kalyanam Nagabhushanam, Muhammed Majeed, Neal M. Davies, Thomas Netticadan, and et al. 2017. "Disparate Effects of Stilbenoid Polyphenols on Hypertrophic Cardiomyocytes In Vitro vs. in the Spontaneously Hypertensive Heart Failure Rat" Molecules 22, no. 2: 204. https://doi.org/10.3390/molecules22020204





