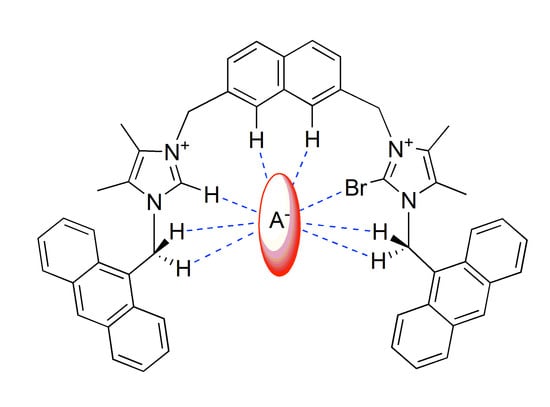
Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions
Abstract
:1. Introduction
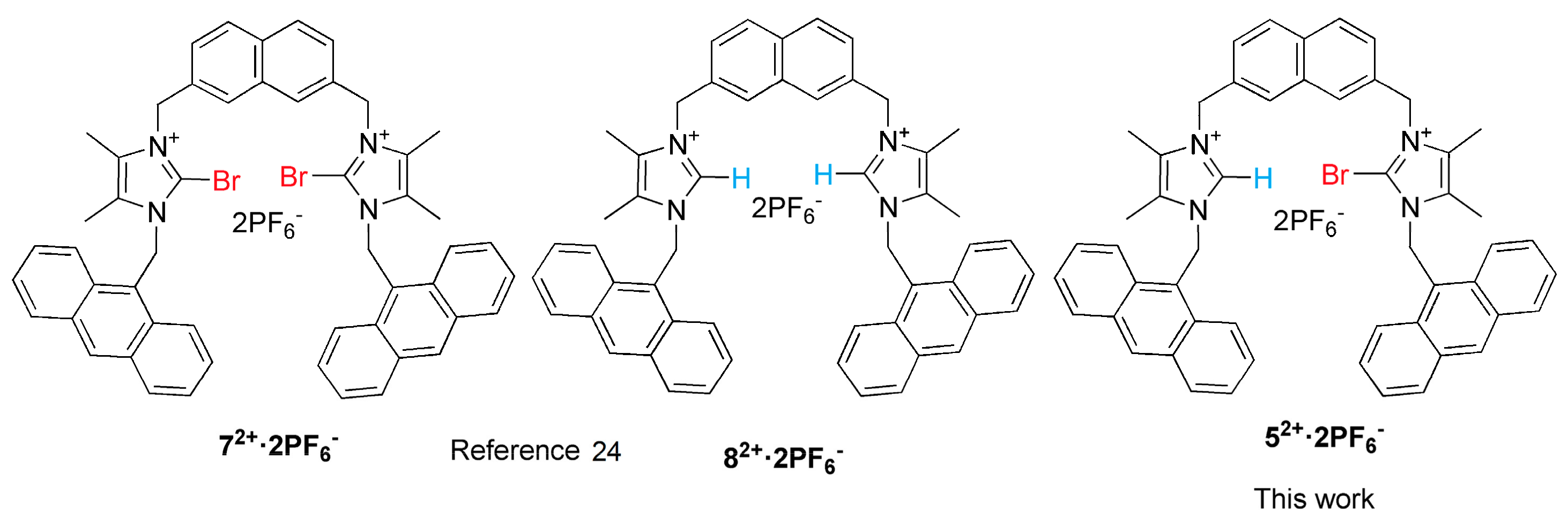
2. Results and Discussion
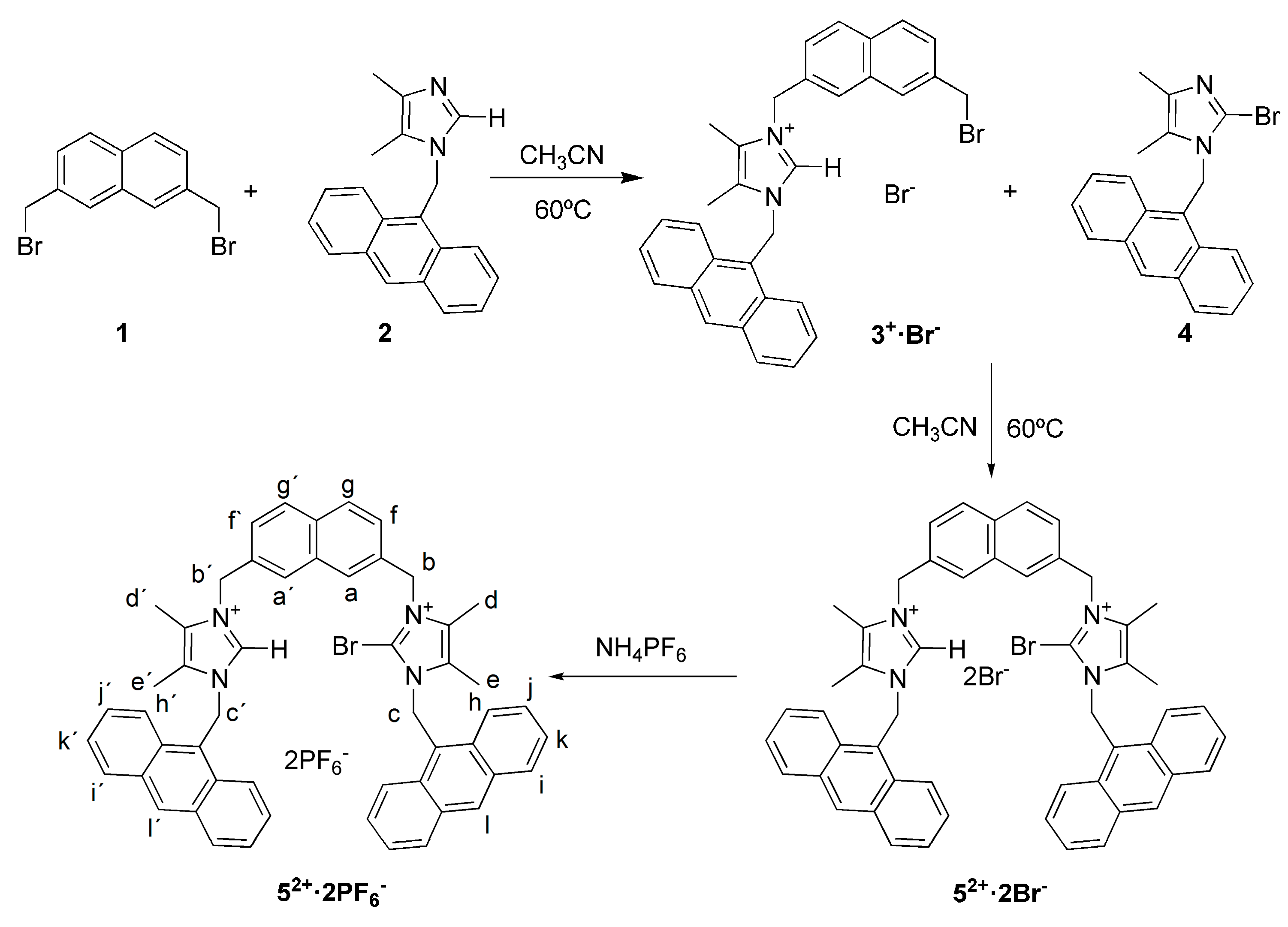
2.1. Synthesis
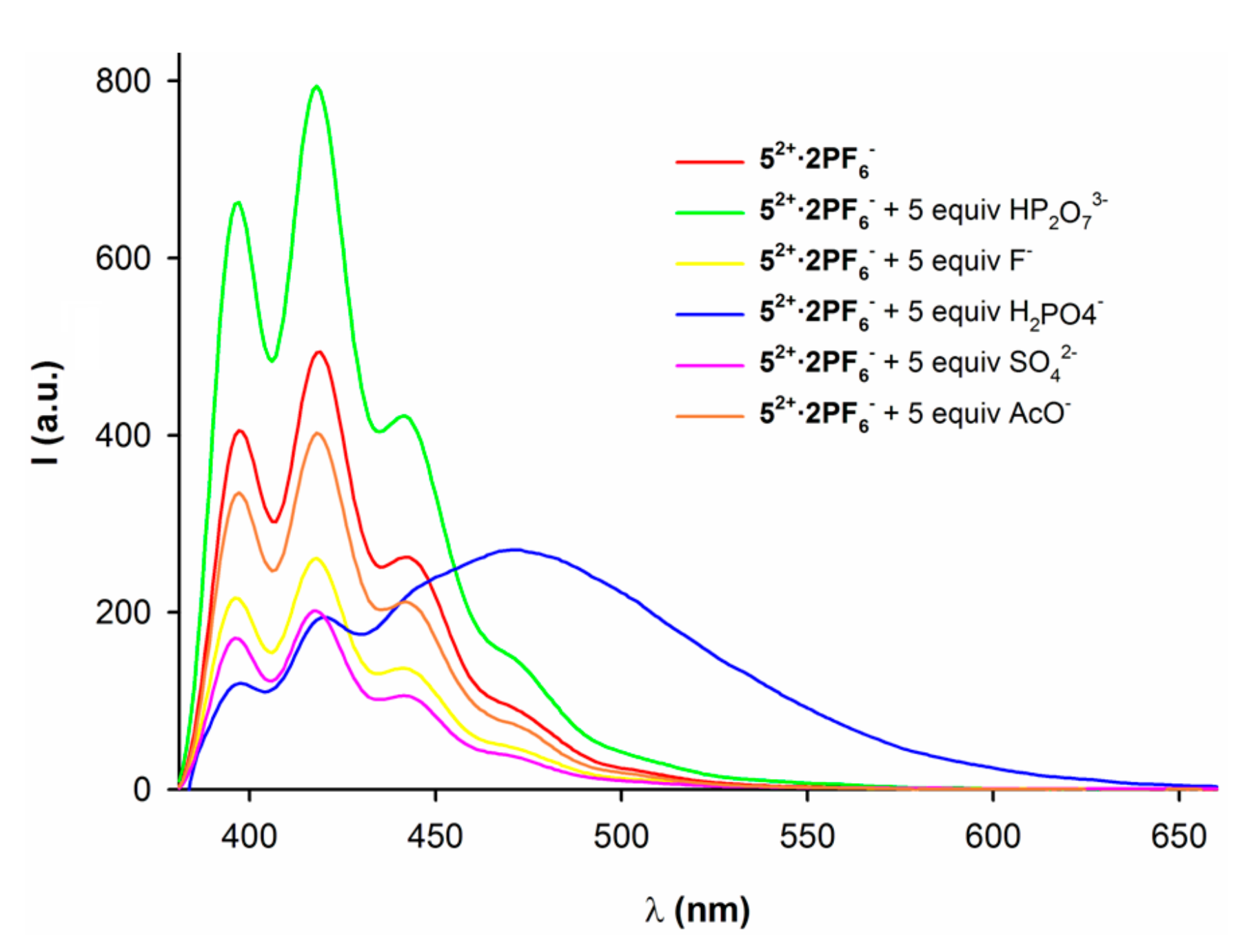
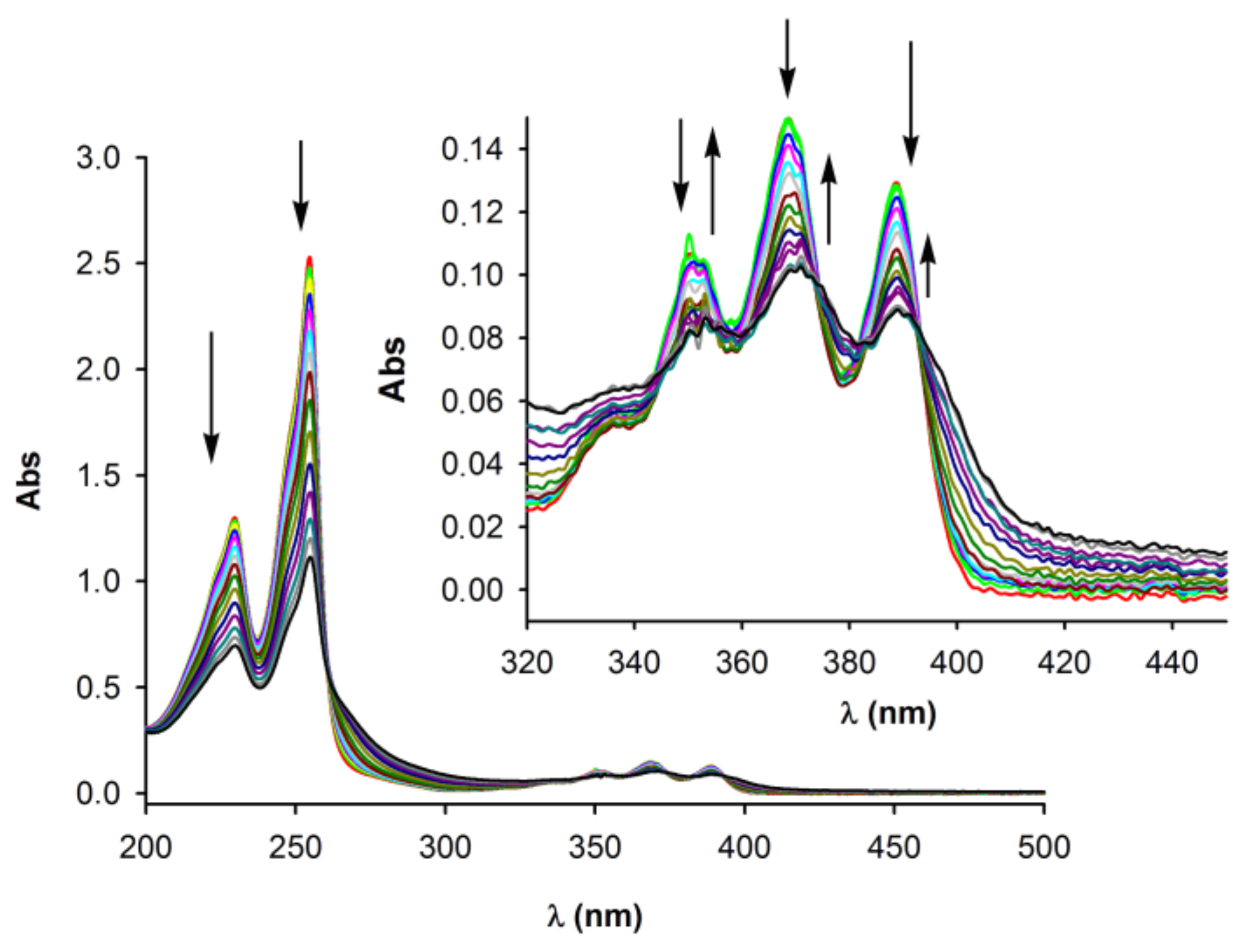
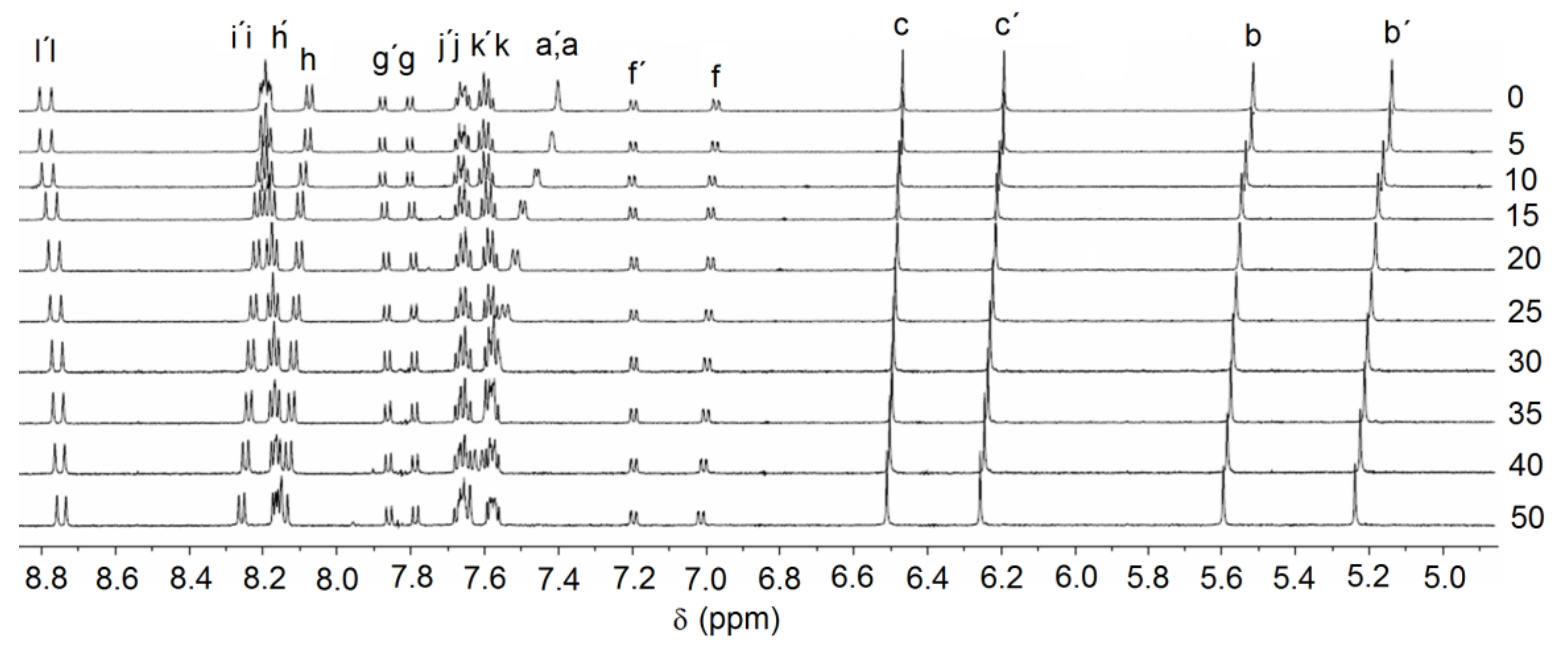
2.2. Anion Binding Studies
3. Experimental Section
3.1. General Comments
3.2. General Procedure for Synthesis of Compounds 2 and 4
3.3. Synthesis of Compound 3+·Br−
3.4. Synthesis of Receptor 52+·2Br−
3.5. Synthesis of Receptor 52+·2PF6−
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, N.H.; Beer, P.D. Advances in anion supramolecular chemistry: From recognition to chemical applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, P.A.; Busschaert, N.; Haynes, C.J.E.; Karagiannidis, L.E.; Kirby, I.L. Anion receptor chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Santos-Figueroa, L.E.; Moragues, M.E.; Climent, E.; Agostini, A.; Martinez-Mañez, R.; Sancenon, F. Chromogenic and fluorogenic chemosensors and reagents for anions: A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion receptor chemistry. Chem. Commun. 2016, 1, 351–422. [Google Scholar] [CrossRef]
- Bondy, C.R.; Loeb, S.J. Amide Based Receptors for Anions. Coord. Chem. Rev. 2003, 240, 77–99. [Google Scholar] [CrossRef]
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Anion recognition by hydrogen bonding: Urea-based receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Camiolo, S.; Gale, P.A. Pyrrolic and polypyrrolic anion binding agents. Coord. Chem. Rev. 2003, 240, 17–55. [Google Scholar] [CrossRef]
- Molina, P.; Tarraga, A.; Oton, F. Imidazole derivatives: A comprehensive survey of their recognition properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Halogen bonds formed between substituted imidazolium and N bases of varying N-hybridization. Molecules 2017, 22, 1634. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Zapata, F.; Caballero, A. Anion recognition strategies based on combined noncovalent interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.J.; Davis, J.J.; Beer, P.D. Interlocked host rotaxane and catenane structures for sensing charged guest species via optical and electrochemical methodologies. Org. Biomol. Chem. 2009, 7, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Lankshear, M.D.; Beer, P.D. Interweaving anion templation. Acc. Chem. Res. 2007, 40, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Kilah, N.L.; Wise, M.D.; Serpell, C.J.; Thompson, A.L.; White, N.G.; Christensen, K.E.; Beer, P.D. Enhancement of anion recognition exhibited by a halogen-bonding rotaxane host system. J. Am. Chem. Soc. 2010, 132, 11893–11895. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.R.; Partridge, B.E.; Beer, P.D. A halogen-bonding bis-triazolium rotaxane for halide-selective anion recognition. Chemistry 2015, 21, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.J.; Robinson, S.W.; Marques, I.; Félix, V.; Beer, P.D. Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts. Nat. Chem. 2014, 6, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.J.; Marques, I.; Robinson, S.W.; Félix, V.; Beer, P.D. Iodide recognition and sensing in water by a halogen-bonding ruthenium(II)-based rotaxane. Chemistry 2016, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Swan, L.; Zapata, F.; Beer, P.D. Iodide-induced shuttling of a halogen- and hydrogen-bonding two-station rotaxane. Angew. Chem. Int. Ed. 2014, 53, 11854–11858. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Beer, P.D. Halogen- and hydrogen-bonding catenanes for halide-anion recognition. Chemistry 2014, 20, 8379–8385. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, J.M.; Caballero, A.; Cookson, J.; Beer, P.D. A halogen- and hydrogen-bonding [2]catenane for anion recognition and sensing. RSC Adv. 2015, 5, 9298–9306. [Google Scholar] [CrossRef]
- Robinson, S.W.; Mustoe, C.L.; White, N.G.; Brown, A.; Thompson, A.L.; Kennepohl, P.; Beer, P.D. Evidence for halogen bond covalency in acyclic and interlocked halogen-bonding receptor anion recognition. J. Am. Chem. Soc. 2015, 137, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chudzinski, M.G.; McClary, C.A.; Taylor, M.S. Anion receptors composed of hydrogen- and halogen-bond donor groups: Modulating selectivity with combinations of distinct noncovalent interactions. J. Am. Chem. Soc. 2011, 133, 10559–10567. [Google Scholar] [CrossRef] [PubMed]
- Sabater, P.; Zapata, F.; Caballero, A.; Visitación, N.; Alkorta, I.; Elguero, J.; Molina, P. Comparative study of charge-assisted hydrogen- and halogen-bonding capabilities in solution of two-armed imidazolium receptors toward oxoanions. J. Org. Chem. 2016, 81, 7448–7458. [Google Scholar] [CrossRef] [PubMed]
- D’Sa, A.; Cohen, L.A.J. 4,5-Dimethylimidazole: A correction and alternative synthesis. Heterocycl. Chem. 1991, 28, 1819–1820. [Google Scholar] [CrossRef]
- Serpell, C.J.; Kilah, N.L.; Costa, P.J.; Felix, V.; Beer, P.D. Halogen bond anion templated assembly of an imidazolium pseudorotaxane. Angew. Chem. Int. Ed. 2010, 49, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Glynn, M.; Tocci, G.M.; Kruger, P.E.; Pfeffer, F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- De Silva, P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Kuzmic, P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 1996, 237, 260–273. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Receptor | H2PO4− | SO42− | AcO− |
|---|---|---|---|
| 52+·PF6− | β = 5.9 × 1010 M−2 (15) | K = 5.7 × 104 M−1 (14) | β = 2.16 × 109 M−2 (10) |
| 72+·2PF6− | β = 5.0 × 1011 M−2 (16) | K = 8.0 × 104 M−1 (14) | - |
| 82+·2PF6− | β = 9.8 × 1011 M−2 (13) | K = 1.4 × 106 M−1 (6) | - |
| Receptor | H2PO4− | SO42− | K(SO42−)/β(H2PO4−) |
|---|---|---|---|
| 52+·PF6− | β = 1.37 × 102 M−2 (4) | K = 2.6 × 103 M−1 (4) | 18 |
| 72+·2PF6− | β = 9.9 × 103 M−2 (3) | K = 5.5 × 103 M−1 (4) | 0.55 |
| 82+·2PF6− | β = 5.6 × 103 M−2 (4) | K = 2.2 × 103 M−1 (4) | 0.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, F.; Benítez-Benítez, S.J.; Sabater, P.; Caballero, A.; Molina, P. Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions. Molecules 2017, 22, 2273. https://doi.org/10.3390/molecules22122273
Zapata F, Benítez-Benítez SJ, Sabater P, Caballero A, Molina P. Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions. Molecules. 2017; 22(12):2273. https://doi.org/10.3390/molecules22122273
Chicago/Turabian StyleZapata, Fabiola, Sergio J. Benítez-Benítez, Paula Sabater, Antonio Caballero, and Pedro Molina. 2017. "Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions" Molecules 22, no. 12: 2273. https://doi.org/10.3390/molecules22122273