The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Filtrated Chokeberry Juice
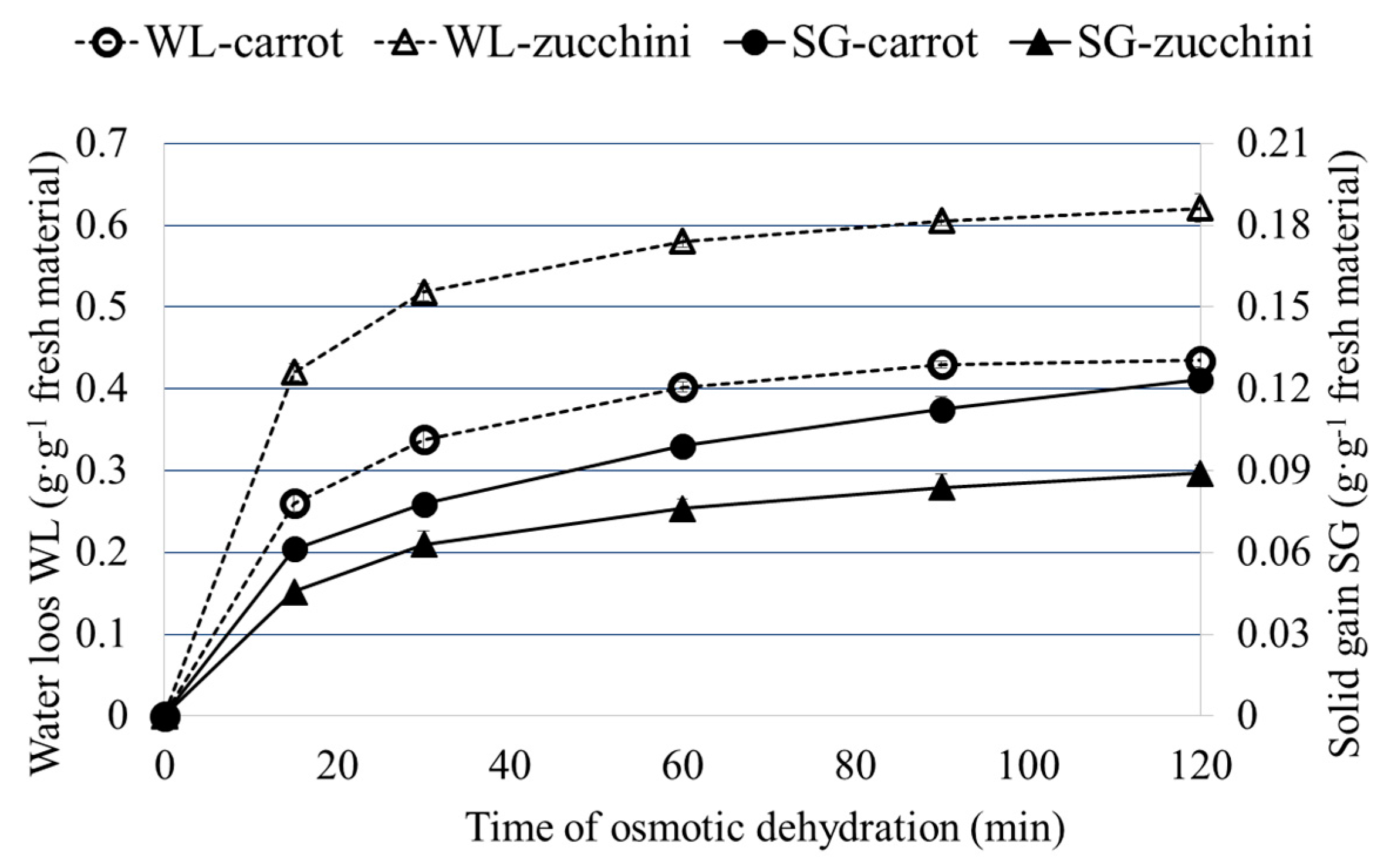
2.2. Osmotic Dehydration (OD) of Carrot and Zucchini
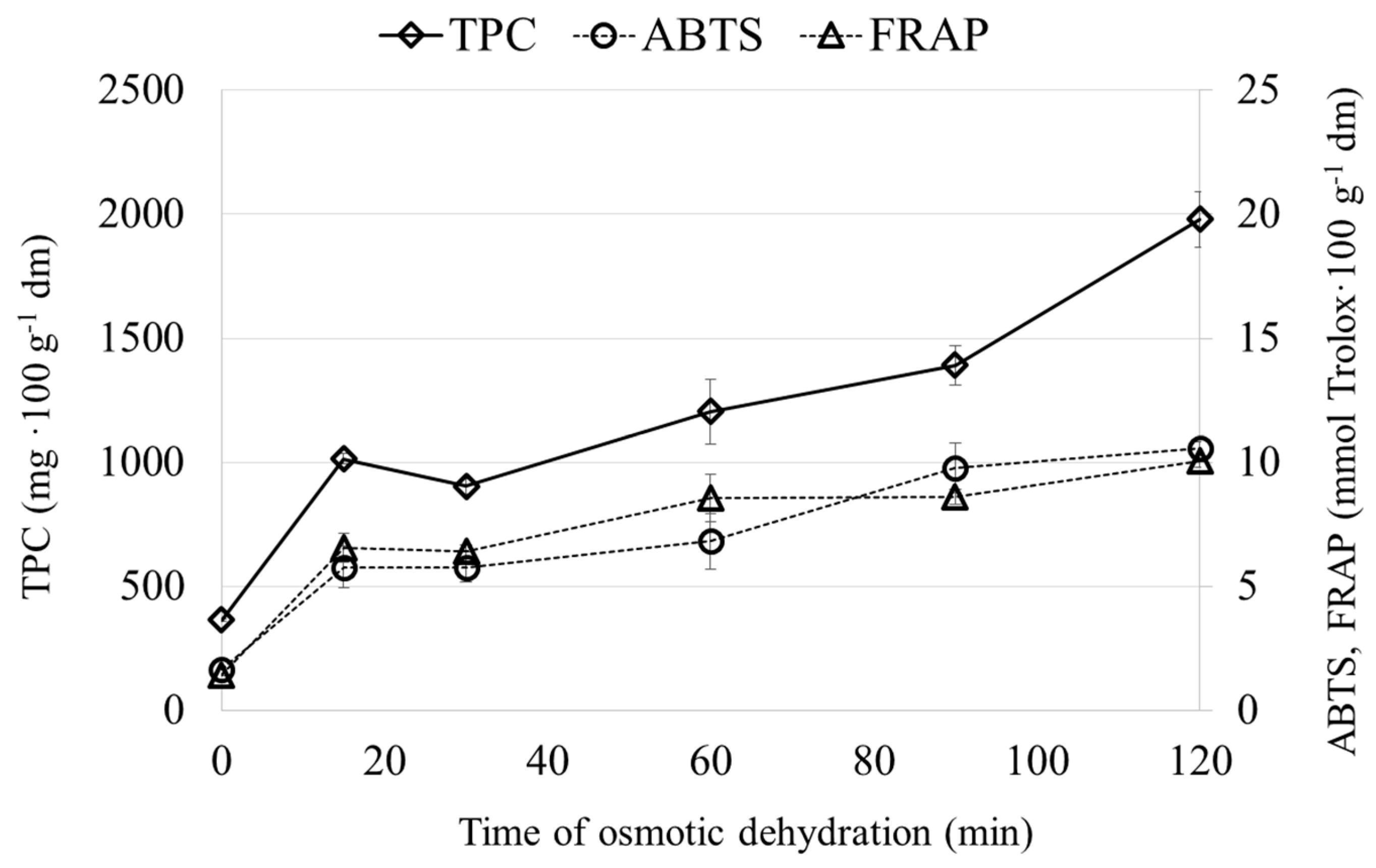
2.3. Properties of Osmotic Solution (OS) after Osmotic Dehydration
3. Materials and Methods
3.1. Materials
3.2. Filtration of Chokeberry Juice
3.3. Osmotic Dehydration
3.4. Physical and Chemical Analyses
3.4.1. Moisture Content
3.4.2. Concentration of Chokeberry Juice
3.4.3. Water Activity (aw) of Chokeberry Juice
3.4.4. Density of Chokeberry Juice
3.4.5. Viscosity of Chokeberry Juice
3.5. Identification and Quantification of Polyphenols by the LC-PDA-MS Method
3.6. Antioxidant Capacity (TEAC ABTS and FRAP Methods)
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lenart, A.; Lewicki, P. Osmotic Dehydration of Fruits and Vegetables. In Handbook of Industrial Drying, 3rd ed.; CRC Press: London, UK, 2006; pp. 661–680. [Google Scholar]
- Sareban, M.; Abbasi Souraki, B. Anisotropic diffusion during osmotic dehydration of celery stalks in salt solution. Food Bioprod. Process. 2016, 98, 161–172. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT-Food Sci. Technol. 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Li, M.; Ye, B.; Guan, Z.; Ge, Y.; Li, J.; Ling, C. Impact of ultrasound-assisted osmotic dehydration as a pre-treatment on the quality of heat pump dried tilapia fillets. Energy Procedia 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Szadzińska, J. Convective-intermittent drying of cherries preceded by ultrasonic assisted osmotic dehydration. Chem. Eng. Process. Process Intensif. 2014, 82, 65–70. [Google Scholar] [CrossRef]
- Emam-Djomeh, Z.; Dehghannya, J.; Gharabagh, R.S. Assessment of Osmotic Process in Combination with Coating on Effective Diffusivities during Drying of Apple Slices. Dry. Technol. 2006, 24, 1159–1164. [Google Scholar] [CrossRef]
- Souraki, B.A.; Ghavami, M.; Tondro, H. Correction of moisture and sucrose effective diffusivities for shrinkage during osmotic dehydration of apple in sucrose solution. Food Bioprod. Process. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Osmotic dehydration with sorbitol combined with hot air convective drying of apple cubes. J. Food Sci. Technol. 2017, 54, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Argaiz, A.; López-Malo, A.; Palou, E.; Welti, J. Osmotic Dehydration Op Papaya with Corn Syrdp Solids. Dry. Technol. 1994, 12, 1709–1725. [Google Scholar] [CrossRef]
- Kowalska, H.; Lenart, A.; Leszczyk, D. The effect of blanching and freezing on osmotic dehydration of pumpkin. J. Food Eng. 2008, 86, 30–38. [Google Scholar] [CrossRef]
- Delahaye, F. Should we eat less salt? Arch. Cardiovasc. Dis. 2013, 106, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Codella, R.; Terruzzi, I.; Luzi, L. Sugars, exercise and health. J. Affect. Disord. 2017, 224, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lech, K.; Michalska, A.; Wasilewska, M.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Influence of osmotic dehydration pre-treatment and combined drying method on physico-chemical and sensory properties of pomegranate arils, cultivar Mollar de Elche. Food Chem. 2017, 232, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Influence of Osmodehydration Pretreatment and Combined Drying Method on the Bioactive Potential of Sour Cherry Fruits. Food Bioprocess Technol. 2015, 8, 824–836. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Sour Cherry Fruits pre-Dehydrated in Fruit Concentrates. Food Bioprocess Technol. 2015, 8, 2076–2095. [Google Scholar] [CrossRef]
- Escriche, I.; Garcia-Pinchi, R.; Andrés, A.; Fito, P. Osmotic Dehydration of Kiwifruit (Actinidia chinensis): Fluxes and Mass Transfer Kinetics. J. Food Process Eng. 2000, 23, 191–205. [Google Scholar] [CrossRef]
- Dehghannya, J.; Hosseinlar, S.-H.; Heshmati, M.K. Multi-stage continuous and intermittent microwave drying of quince fruit coupled with osmotic dehydration and low temperature hot air drying. Innov. Food Sci. Emerg. Technol. 2017, 45, 132–151. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Kostaropoulos, A.E.; Saravacos, G.D. Air-Drying Kinetics of Osmotically Dehydrated Fruits. Dry. Technol. 1995, 13, 1503–1521. [Google Scholar] [CrossRef]
- Zou, K.; Teng, J.; Huang, L.; Dai, X.; Wei, B. Effect of osmotic pretreatment on quality of mango chips by explosion puffing drying. LWT Food Sci. Technol. 2013, 51, 253–259. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Kharaghani, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Tsotsas, E. Drying Kinetics and Microstructural and SensoryProperties of Black Chokeberry (Aronia melanocarpa) as Affected by Drying Method. Food Bioprocess Technol. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Moraga, M.J.; Moraga, G.; Fito, P.J.; Martínez-Navarrete, N. Effect of vacuum impregnation with calcium lactate on the osmotic dehydration kinetics and quality of osmodehydrated grapefruit. J. Food Eng. 2009, 90, 372–379. [Google Scholar] [CrossRef]
- Lombard, G.E.; Oliveira, J.C.; Fito, P.; Andrés, A. Osmotic dehydration of pineapple as a pre-treatment for further drying. J. Food Eng. 2008, 85, 277–284. [Google Scholar] [CrossRef]
- Shi, J.; Jun Xue, S. Application and Development of Osmotic Dehydration Technology in Food Processing. In Advance in Food Dehydration; CRC Press: London, UK, 2009; pp. 187–208. [Google Scholar]
- García, M.; Díaz, R.; Martínez, Y.; Casariego, A. Effects of chitosan coating on mass transfer during osmotic dehydration of papaya. Food Res. Int. 2010, 43, 1656–1660. [Google Scholar] [CrossRef]
- Gomes Alves, D.; Lucena Barbosa, J.; Colato Antonio, G.; Xidieh Murr, F.E. Osmotic dehydration of acerola fruit (Malpighia punicifolia L.). J. Food Eng. 2005, 68, 99–103. [Google Scholar] [CrossRef]
- El-Aouar, Â.A.; Azoubel, P.M.; Barbosa, J.L.; Xidieh Murr, F.E. Influence of the osmotic agent on the osmotic dehydration of papaya (Carica papaya L.). J. Food Eng. 2006, 75, 267–274. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Spanswick, R.M. Symplasmic Transport in Tissues. In Transport in Plants II; Springer: Berlin, Heidelberg, 1976; pp. 35–53. [Google Scholar]
- Rózek, A.; Achaerandio, I.; Güell, C.; López, F.; Ferrando, M. Grape phenolic impregnation by osmotic treatment: Influence of osmotic agent on mass transfer and product characteristics. J. Food Eng. 2009, 94, 59–68. [Google Scholar] [CrossRef]
- Akharume, F.U.; Singh, K.; Sivanandan, L. Characteristics of apple juice and sugar infused fresh and frozen blueberries. LWT-Food Sci. Technol. 2016, 73, 448–457. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Food Properties Handbook, 2nd ed.; CRC Press: London, UK, 2009; ISBN 978-1-4200-0309-3. [Google Scholar]
- Garza, S.; Ibarz, A. Effect of Temperature and Concentration on the Density of Clarified Pineapple Juice. Int. J. Food Prop. 2010, 13, 913–920. [Google Scholar] [CrossRef]
- Held, C.; Neuhaus, T.; Sadowski, G. Compatible solutes: Thermodynamic properties and biological impact of ectoines and prolines. Biophys. Chem. 2010, 152, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; Sadowski, G. Compatible solutes: Thermodynamic properties relevant for effective protection against osmotic stress. Fluid Phase Equilibria 2016, 407, 224–235. [Google Scholar] [CrossRef]
- Cepeda, E.; Villarán, M.C. Density and viscosity of Malus floribunda juice as a function of concentration and temperature. J. Food Eng. 1999, 41, 103–107. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Teleszko, M.; Sokół-Łętowska, A. Composition and quantification of major polyphenolic compounds, antioxidant activity and colour properties of quince and mixed quince jams. Int. J. Food Sci. Nutr. 2013, 64, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Pore Sizes (μm) | Polyphenols (mg·100 g−1 dm) | TEAC ABTS (mmol Trolox·100 g−1 dm) | FRAP (mmol Trolox·100 g−1 dm) | |
|---|---|---|---|---|
| no filtration | 2919.4 ± 12.2 a,b,c,* | 26.74 ± 0.43 a,b | 21.33 ± 0.50 b,c | |
| 8 | 3002.2 ± 133.9 a | 27.25 ± 0.30 b | 21.85 ± 0.06 c | |
| 5 | 3081 ± 0.3 a | 26.48 ± 0.476 a,b | 21.14 ± 0.25 a,b | |
| 3 | 3092.2 ± 24.4 a | 26.43 ± 0.41 a,b | 20.80 ± 0.19 a,d | |
| 1.2 | 3116.5 ± 5.2 a | 26.53 ± 0.27 a,b | 21.34 ± 0.15 a,b,c | |
| 0.8 | 2998 ± 85.9 a,b | 26.43 ± 0.30 a,b | 21.23 ± 0.73 a,b,c | |
| 0.45 | 2811.5 ± 13.9 b,c | 25.98 ± 0.25 a | 20.74 ± 0.16 a,d | 20.74 ± 0.16 a,d |
| 0.2 | 2719.4 ± 7 c | 25.95 ± 0.35 a | 20.44 ± 0.48 d |
| Pore Sizes (μm) | PolyMeric Procyanidins | Phenolic Acids | Flavonoids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neochlorogenic Acid | p-Coumaric Acid | Chlorogenic Acid | Q-3-Rutinoside | Q-3-Galactoside | Q-3-Glucoside | Q-Arabinoside | Cya-3-Galactoside | Cya-3-Glucoside | Cya-3-Arabinoside | Cya-3-Xyloside | Derivatives of Cyanidin | ||
| no filtration | 1857.4 ± 1.1 a,b,* | 251.2 ± 2.2 a | 9.22 ± 0.39 a | 400.1 ± 5.3 a | 28.16 ± 0.84 a | 18.52 ± 1.53 a | 66.68 ± 0.86 a,b | 28.61 ± 1.94 a | 1.69 ± 0.08 a | 164.5 ± 2.7 e | 9.73 ± 0.68 a,b | 74.4 ± 0.8 c | 9.24 ± 0.35 a,b |
| 8 | 1939.6 ± 134.3 a,b | 257.9 ± 8.7 a | 9.06 ± 0.41 a | 397.2 ± 1 a,b | 28.27 ± 0.86 a | 18.92 ± 0.14 a | 67.22 ± 2.1 a,b | 29.14 ± 0.4 a | 1.66 ± 0.06 a | 161.5 ± 3.3 c,e | 10.01 ± 0.5 a | 72.2 ± 2.2 b,c | 9.42 ± 0.2 a |
| 5 | 2029.6 ± 11.5 a | 263.8 ± 15.3 a | 8.67 ± 0.44 a | 393.7 ± 1 a,b | 27.81 ± 0.5 a | 18.74 ± 0.6 a | 66.48 ± 0.59 a,b | 28.92 ± 0.06 a | 1.77 ± 0.07 a | 153.8 ± 1.1 a,c | 9.43 ± 0.02 a,b | 68.9 ± 0.01 a,b | 9.32 ± 0.15 a,b |
| 3 | 2032.7 ± 22.8 a | 259.5 ± 0.3 a | 8.68 ± 0.06 a | 399.3 ± 1.1 a | 28.72 ± 0.04 a | 19.95 ± 0.02 a | 68.05 ± 0.3 a,b | 29.23 ± 0.07 a | 1.45 ± 0.01 a | 155.3 ± 0.1 a,c | 10.09 ± 0.15 a | 69.8 ± 0.3 a,b,c | 9.38 ± 0.48 a,b |
| 1.2 | 2048.1 ± 9.4 a | 270.6 ± 13 a | 9.09 ± 0.37 a | 401.4 ± 1.1 a | 28.78 ± 0.01 a | 19.62 ± 0.11 a | 67.84 ± 0.81 a,b | 28.9 ± 0.77 a | 1.59 ± 0.14 a | 152.9 ± 1 a,b | 9.69 ± 0.11 a,b | 68.8 ± 0.9 a,b | 9.06 ± 0.52 a,b |
| 0.8 | 1931.6 ± 116.1 a,b | 261 ± 7.3 a | 9.48 ± 0.24 a | 404.7 ± 12.9 a | 29.29 ± 0.5 a | 20.09 ± 1.24 a | 69.12 ± 1.28 b | 30.23 ± 1.68 a | 1.75 ± 0.14 a | 153 ± 3.2 a,b | 9.67 ± 0.08 a,b | 68.6 ± 1.8 a,b | 9.51 ± 0.1 a |
| 0.45 | 1770.9 ± 1.6 b | 263.1 ± 6.8 a | 9.34 ± 0.31 a | 395.3 ± 4.2 a,b | 27.91 ± 1.17 a | 19.66 ± 0.11 a | 67.12 ± 0.05 a,b | 28.24 ± 0.2 a | 1.69 ± 0.09 a | 145 ± 0.5 b,d | 9.12 ± 0.01 a,b | 65.1 ± 0.1 a,d | 9.03 ± 0.07 a,b |
| 0.2 | 1726.1 ± 5.6 b | 249.9 ± 9.4 a | 8.86 ± 0.01 a | 378 ± 0.6 a | 27.56 ± 0.1 a | 18.79 ± 0.49 a | 64.63 ± 0.48 a | 28.12 ± 0.24 a | 1.55 ± 0.03 a | 137.7 ± 2.2 d | 8.69 ± 0.04 b | 61.4 ± 1.3 d | 8.18 ± 0.21 b |
| Osmotic Solution | Time of Osmotic Dehydration (min) | Concentration of Osmotic Solution (° Brix) | Polyphenols TPC (mg GA·100 g−1 dm) | ABTS (mmol Trolox·100 g−1 dm) | FRAP (mmol Trolox·100 g−1 dm) | Water Activity (-) | Density (kg·m−3) | Viscosity (mPa·s) |
|---|---|---|---|---|---|---|---|---|
| Chokeberry juice after the OD of carrot | 0 | 40 ± 0.1 e,* | 2909.4 ± 12.2 b | 26.95 ± 0.43 b | 21.54 ± 0.50 b | 0.944 ± 0.004 a | 1194.6 ± 24 a | 3.35 ± 0.1 c |
| 15 | 36.2 ± 0.1 d | 3626.7 ± 77.1 a | 32.00 ± 0.36 a | 26.36 ± 0.49 a | 0.954 ± 0.006 a | 1174.3 ± 26.5 a | 2.9 ± 0.15 b | |
| 30 | 35.1 ± 0.1 c | 3596.1 ± 97.1 a | 30.91 ± 0.55 a | 25.50 ± 0.77 a | 0.956 ± 0.008 a | 1168.4 ± 31.2 a | 2.77 ± 0.18 a,b | |
| 60 | 34.4 ± 0.1 b | 3679.6 ± 100.3 a | 31.29 ± 1.01 a | 25.26 ± 0.61 a | 0.958 ± 0.009 a | 1164.5 ± 20.6 a | 2.67 ± 0.15 a,b | |
| 90 | 33.4 ± 0.1 a | 3619.1 ± 60 a | 30.98 ± 0.60 a | 25.96 ± 0.19 a | 0.96 ± 0.005 a | 1159.5 ± 16 a | 2.55 ± 0.09 a,d | |
| 120 | 33.2 ± 0.1 a | 3770.4 ± 75 a | 31.72 ± 0.54 a | 27.20 ± 1.79 a | 0.96 ± 0.006 a | 1158.1 ± 28.6 a | 2.52 ± 0.1 a | |
| Chokeberry juice after the OD of zucchini | 0 | 40 ± 0.1 E,# | 2919.4 ± 12.2 C | 26.95 ± 0.43 D | 21.54 ± 0.60 A | 0.944 ± 0.004 A | 1194.6 ± 24 A | 3.35 ± 0.1 C |
| 15 | 36.5 ± 0.1 D | 3548.8 ± 54.4 B | 31.45 ± 0.60 A,B | 22.13 ± 0.56 A,B | 0.953 ± 0.008 A | 1176 ± 20.5 A | 2.94 ± 0.12 B | |
| 30 | 35.3 ± 0.1 C | 3481.9 ± 65 A,B | 32.48 ± 0.41 B | 23.31 ± 0.54 B,C | 0.956 ± 0.006 A | 1169.6 ± 30.3 A | 2.8 ± 0.11 A,B | |
| 60 | 34.9 ± 0.1 B | 3486 ± 50.3 A,B | 31.35 ± 0.55 A,B | 23.59 ± 0.51 C | 0.957 ± 0.008 A | 1167.1 ± 32.5 A | 2.73 ± 0.13 A,B | |
| 90 | 33.8 ± 0.1 A | 3387.5 ± 24.9 A | 30.05 ± 0.76 A,C | 21.97 ± 0.10 A,B | 0.959 ± 0.006 A | 1161.6 ± 25.5 A | 2.6 ± 0.09 A | |
| 120 | 33.7 ± 0.1 A | 3474.9 ± 60 A,B | 28.63 ± 0.62 C | 21.45 ± 0.65 A | 0.959 ± 0.008 A | 1160.9 ± 20.2 A | 2.58 ± 0.11 A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lech, K.; Michalska, A.; Wojdyło, A.; Nowicka, P.; Figiel, A. The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution. Molecules 2017, 22, 2246. https://doi.org/10.3390/molecules22122246
Lech K, Michalska A, Wojdyło A, Nowicka P, Figiel A. The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution. Molecules. 2017; 22(12):2246. https://doi.org/10.3390/molecules22122246
Chicago/Turabian StyleLech, Krzysztof, Anna Michalska, Aneta Wojdyło, Paulina Nowicka, and Adam Figiel. 2017. "The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution" Molecules 22, no. 12: 2246. https://doi.org/10.3390/molecules22122246





