Evaluation on the Photosensitivity of 2,2′-Azobis(2,4-Dimethyl)Valeronitrile with UV
Abstract
:1. Introduction
2. Results and Discussion
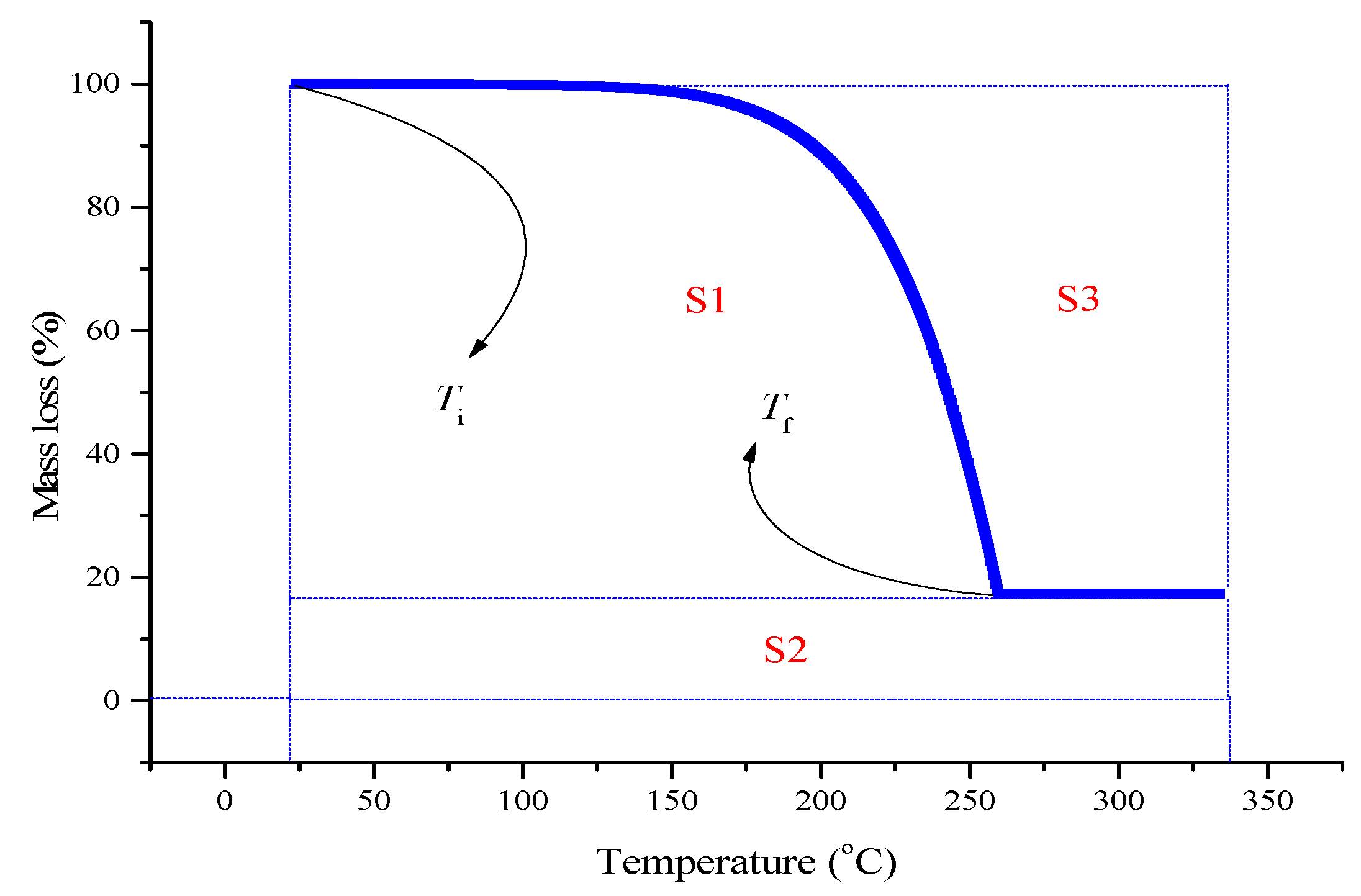
2.1. TG Results
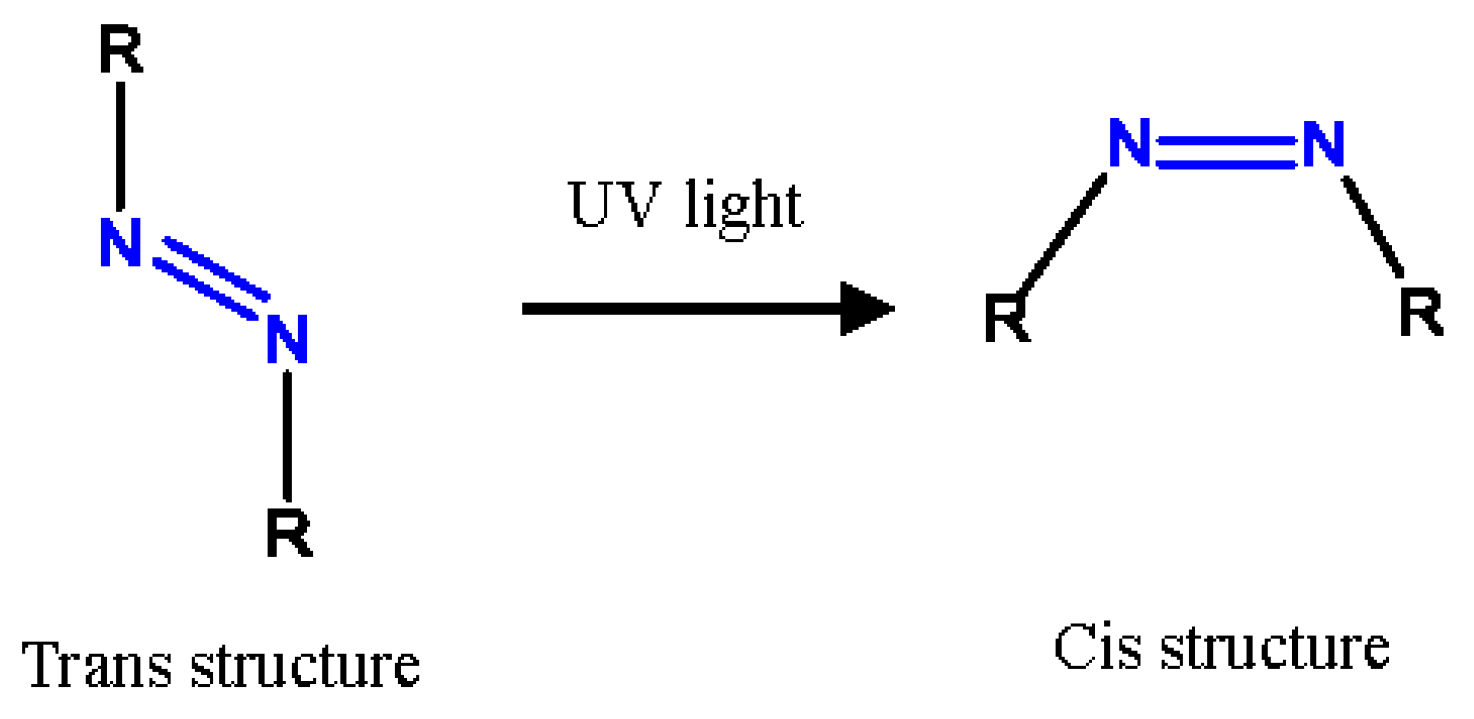
2.2. Determination of Kinetic Models for ADVN With UV Irradiation
3. Materials and Methods
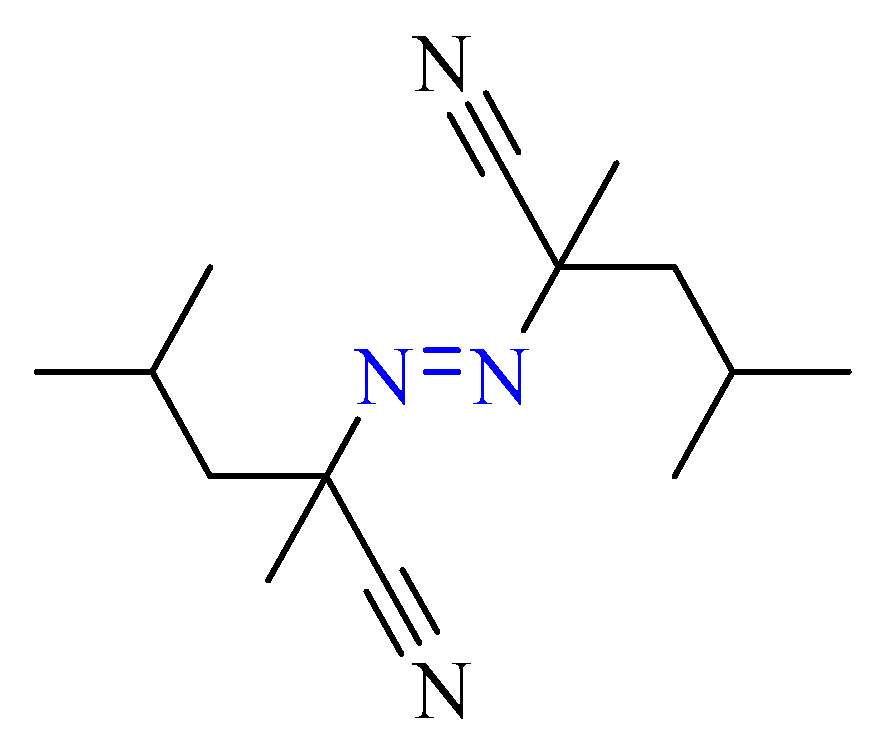
3.1. Sample
3.2. Differential Scanning Calorimetry (DSC)
3.3. Thermogravimetry (TG)
3.4. Evaluation of Integral Procedural Decomposition Temperature (IPDT)
3.5. Non-Linear Regression Method to Decide the Reaction Kinetics of ADVN/UV Irradiation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.W.; Duh, Y.S.; Shu, C.M. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC. J. Therm. Anal. Calorim. 2006, 85, 225–234. [Google Scholar] [CrossRef]
- You, M.L. Thermal hazard evaluation of cumene hydroperoxide-metal ion mixture using DSC, TAM III, and GC/MS. Molecules 2016, 21, 562. [Google Scholar] [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2’-azobis (2-amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions. J. Pharm. Sci. 2013, 100, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Chen, W.C.; You, M.L.; Tsai, Y.T.; Shu, C.M. Evaluation of thermal decomposition phenomenon for 1,1-bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane by DSC and VSP2. J. Therm. Anal. Calorim. 2015, 122, 1125–1133. [Google Scholar] [CrossRef]
- Li, K.Y.; Tsai, S.Y.; Lin, C.P.; Tsai, Y.Y.; Shu, C.M. Smart technology for evaluating fire extinguishing effect of tert-butyl hydroperoxide. Ind. Eng. Chem. Res. 2013, 52, 10969–10976. [Google Scholar] [CrossRef]
- Tong, J.W.; Chen, W.C.; Tsai, Y.T.; Cao, Y.; Chen, J.R.; Shu, C.M. Incompatible reaction for (3-4-epoxycyclohexane) methyl-3’-4’-epoxycyclohexyl-carboxylate (EEC) by calorimetric technology and theoretical kinetic mode. J. Therm. Anal. Calorim. 2014, 116, 1445–1452. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Tsai, Y.T.; Deng, J.; Shu, C.M. Spontaneous combustion in six types of coal by using the simultaneous thermal analysis-Fourier transform infrared spectroscopy technique. J. Therm. Anal. Calorim. 2016, 126, 1591–1602. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Lin, S.Y.; Tong, J.W.; Chen, W.C.; Chen, W.T.; Shu, C.M. Incompatible hazard investigation of a cycloaliphatic epoxy resin using green analytical method. J. Therm. Anal. Calorim. 2015, 122, 1135–1141. [Google Scholar] [CrossRef]
- Wanasekara, N.; Chalivendra, V.; Calverta, P. Sub-micron scale mechanical properties of polypropylene fibers exposed to ultraviolet and thermal degradation. Polym. Degrad. Stab. 2011, 96, 432–437. [Google Scholar] [CrossRef]
- Wang, L.X.; Wang, X.G. Progress of the trans-cis isomerization mechanism of azobenzene. Chemistry 2008, 71, 243–248. [Google Scholar] [CrossRef]
- Wang, L.X.; Tuo, X.L.; Xu, J.; Li, S.N.; Wang, X.G. Effect of trans-cis isomerization on the sensitivity of energetic azo-compounds. Acta Phys. Chim. Sin. 2008, 24, 1756–1760. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2011. [Google Scholar] [CrossRef]
- Albrektienė, R.; Rimeika, M.; Zalieckienė, E. Determination of organic matter by UV absorption in the ground Water. J. Environ. Eng. Landsc. 2012, 20, 163–167. [Google Scholar] [CrossRef]
- Lu, K.M.; Lee, W.J.; Chen, W.H.; Lin, T.C. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl. Energy 2013, 105, 57–65. [Google Scholar] [CrossRef]
- Malow, M.; Wehrstedt, K.D. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J. Hazard. Mater. 2005, 120, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; You, M.L.; Qian, X.M.; Shu, C.M. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind. Eng. Chem. Res. 2013, 52, 8206–8215. [Google Scholar] [CrossRef]
- Tsai, L.C.; Tsai, Y.T.; Lin, C.P.; Liu, S.L.; Wu, T.C.; Shu, C.M. Isothermal versus non-isothermal calorimetric technique to evaluate thermokinetic parameters and thermal hazard of tert-butyl peroxy-2-ethyl hexanoate. J. Therm. Anal. Calorim. 2012, 109, 1291–1296. [Google Scholar] [CrossRef]
- Feng, B.; Yu, M.; Ji, J.; Liu, L.; Ben, M.; Sun, J. Thermal properties and thermal degradation kinetics of polysulfonamide based single polymer composites. Polym. Mater. Sci. Eng. 2016, 32, 42–47. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.; Criado, J.; Pérez-Maqueda, L.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Hou, H.Y.; Liao, T.S.; Duh, Y.S.; Shu, C.M. Thermal hazard studies for dicumyl peroxide by DSC and TAM. J. Therm. Anal. Calorim. 2006, 1, 167–171. [Google Scholar] [CrossRef]
- Liu, S.H.; Shu, C.M. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J. Therm. Anal. Calorim. 2015, 121, 1–8. [Google Scholar] [CrossRef]
- Omrani, A.; Simon, L.C.; Rostam, A.A.; Ghaemy, M. Cure kinetics, dynamic mechanical and morphological properties of epoxy resin–Im6NiBr2 system. Eur. Polym. J. 2008, 44, 769–779. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating Thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 22, 77–79. [Google Scholar] [CrossRef]
- Shu, C.M.; Chang, Y.H.; Chiu, C.W. Evaluation on the thermal stability and hazards behaviors of advn using green thermal analysis approach. J. Civ. Eng. Archit. 2016, 10, 280–290. [Google Scholar] [CrossRef]
- Málek, J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim. Acta 1995, 267, 61–73. [Google Scholar] [CrossRef]
- Tseng, J.M.; You, M.Y.; Chen, S.L.; Hwang, W.T.; Gupta, J.P.; Shu, C.M. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III tests. J. Therm. Anal. Calorim. 2009, 96, 789–793. [Google Scholar] [CrossRef]
- Egerton, T.A. UV-absorption-the primary process in photocatalysis and some practical consequences. Molecules 2014, 19, 18192–18214. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Tseng, J.M. Green technology for improving process manufacturing design and storage management of organic peroxide. Chem. Eng. J. 2012, 180, 284–292. [Google Scholar] [CrossRef]
- Matthes, P.R.; Schönfeld, F.; Zottnick, S.H.; Müller-Buschbaum, K. Post-synthetic shaping of porosity and crystal structure of Ln-Bipy-MOFs by thermal treatment. Molecules 2015, 20, 12125–12153. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the ADVN are available from the authors. |

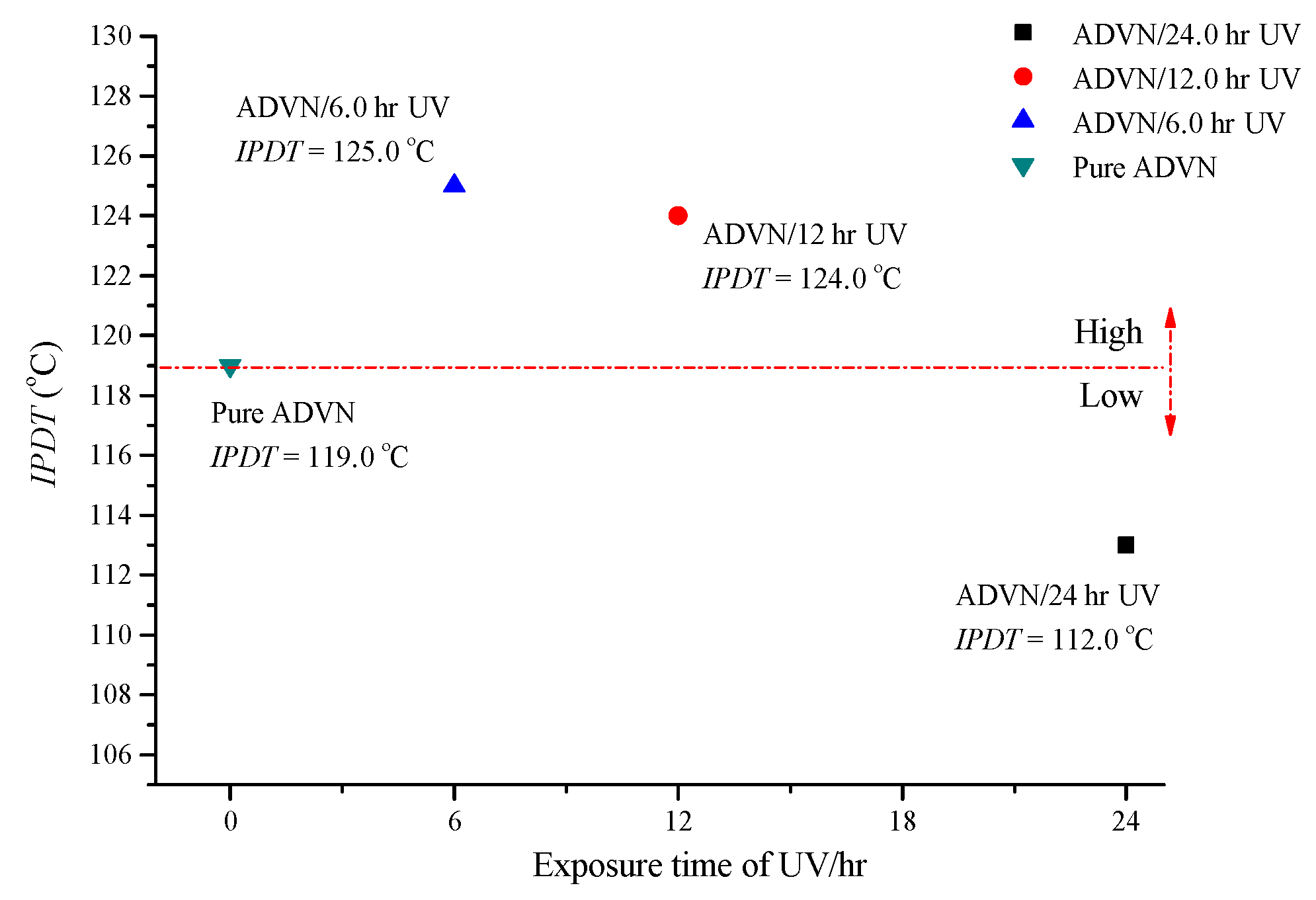
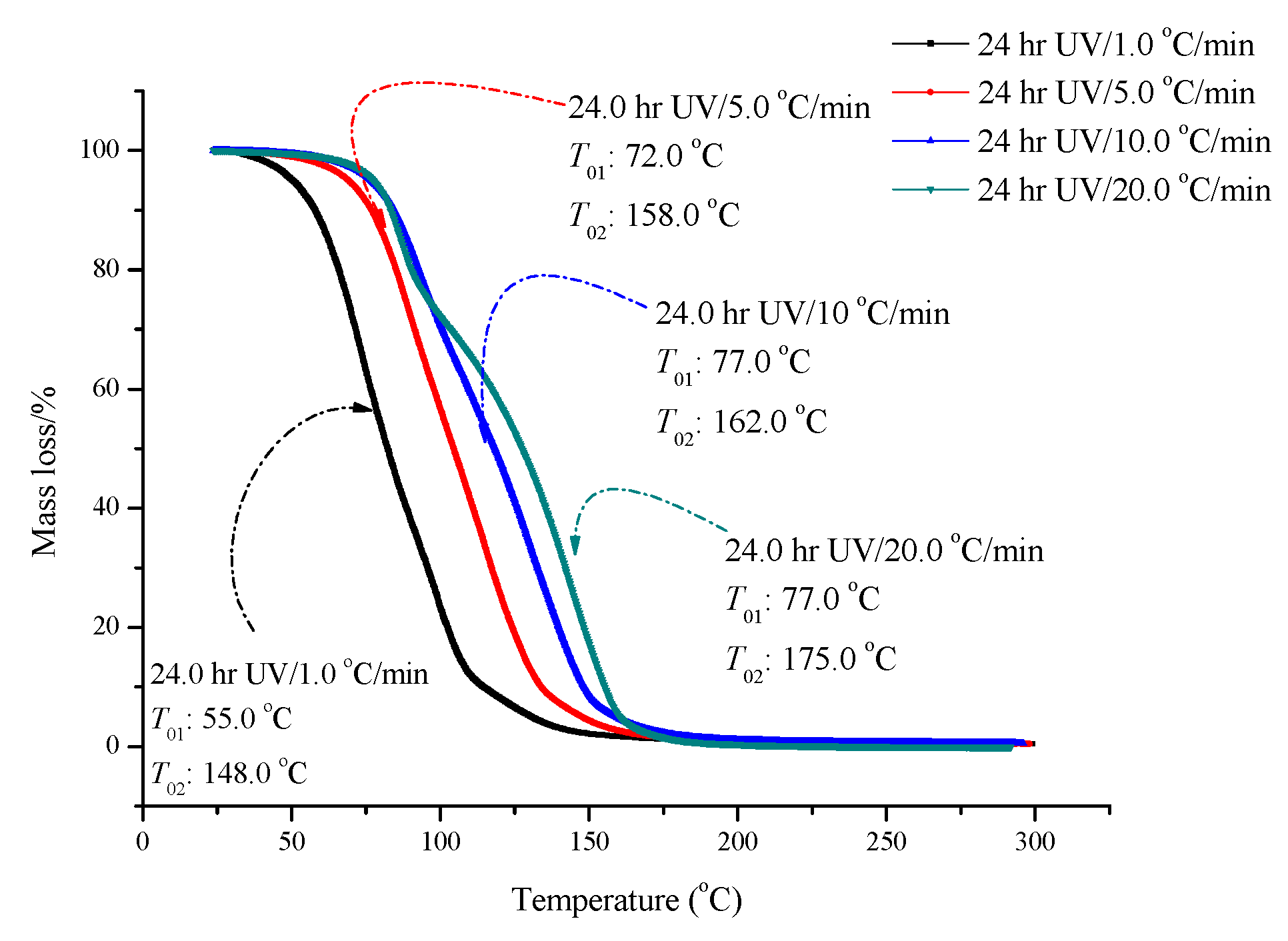
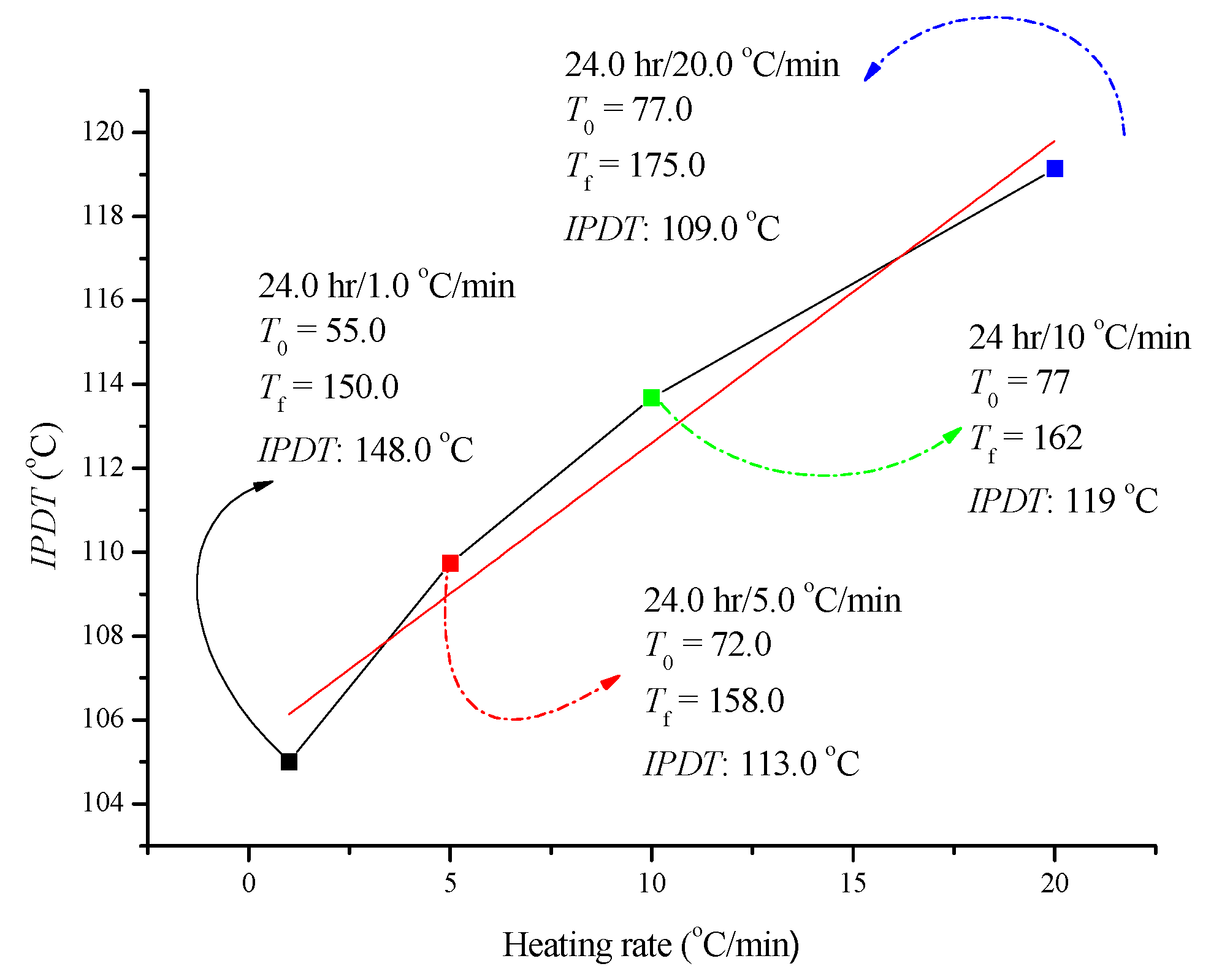
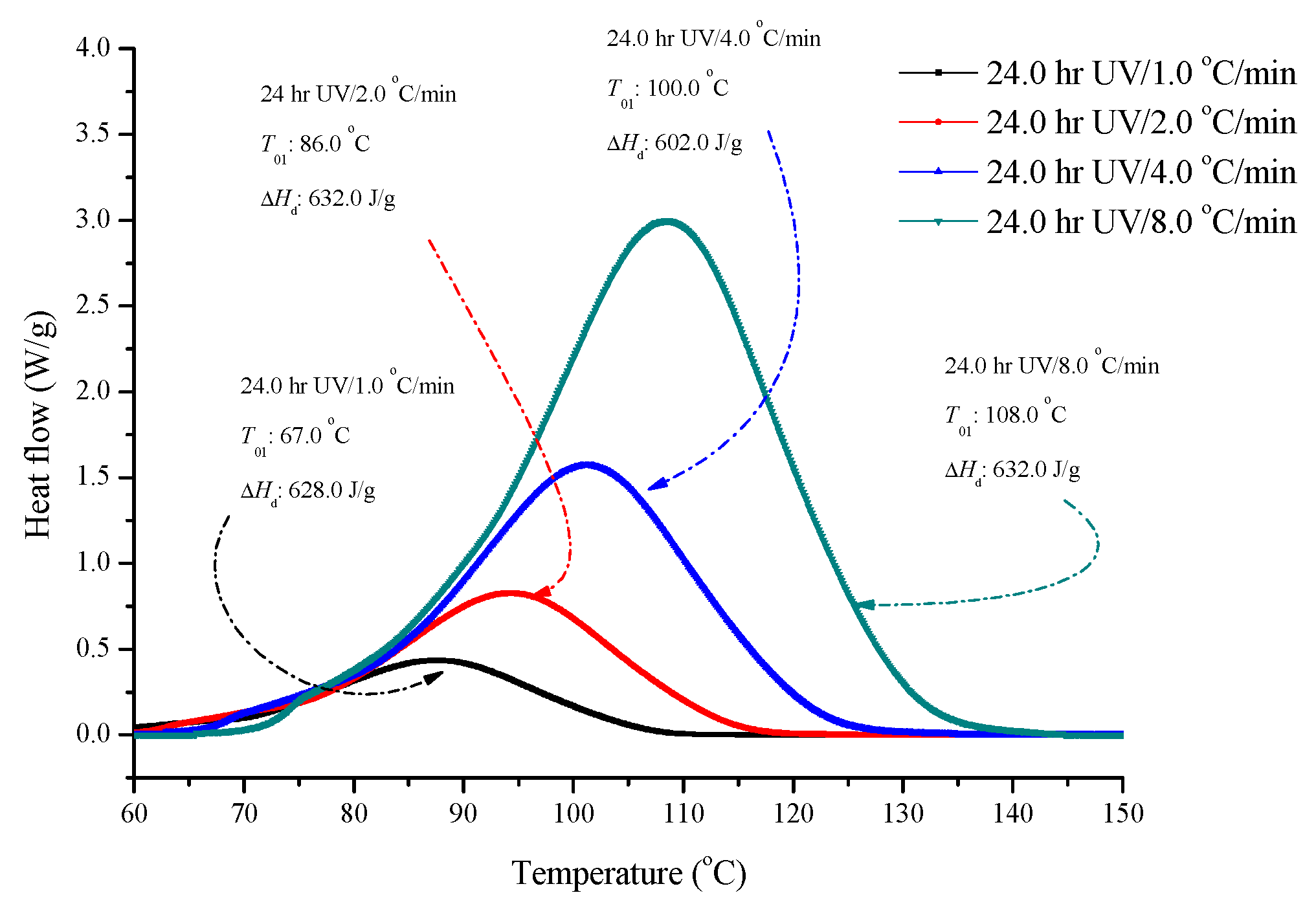

| Sample | T01 (°C) | T02 (°C) | Mass Loss (First Stage) (%) | Mass Loss (Second Stage) (%) | IPDT/°C |
|---|---|---|---|---|---|
| ADVN/without UV | 78.6 | 132.1 | 30.0 | 70.0 | 119.0 |
| ADVN/6 h of UV | 78.7 | 133.2 | 30.0 | 70.0 | 125.0 |
| ADVN/12 h of UV | 78.9 | 133.5 | 25.0 | 75.0 | 124 |
| ADVN/24 h of UV | 77.9 | 127.3 | 20.0 | 80.0 | 112.0 |
| Heating Rate (°C/min) | Ea (kJ/mol) | ln (A) (1/s) | Reaction Order (n) | ΔHd (kJ/kg) |
|---|---|---|---|---|
| 1.0 | 137.0 | 39.0 | 1.4 | 632.0 |
| 2.0 | 144.0 | 42.0 | 1.5 | 632.0 |
| 4.0 | 140.0 | 40.0 | 1.4 | 603.0 |
| 8.0 | 138.0 | 39.0 | 1.5 | 633.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tsai, Y.-T. Evaluation on the Photosensitivity of 2,2′-Azobis(2,4-Dimethyl)Valeronitrile with UV. Molecules 2017, 22, 2219. https://doi.org/10.3390/molecules22122219
Yang Y, Tsai Y-T. Evaluation on the Photosensitivity of 2,2′-Azobis(2,4-Dimethyl)Valeronitrile with UV. Molecules. 2017; 22(12):2219. https://doi.org/10.3390/molecules22122219
Chicago/Turabian StyleYang, Yi, and Yun-Ting Tsai. 2017. "Evaluation on the Photosensitivity of 2,2′-Azobis(2,4-Dimethyl)Valeronitrile with UV" Molecules 22, no. 12: 2219. https://doi.org/10.3390/molecules22122219