Effect of Oxylipins, Terpenoid Precursors and Wounding on Soft Corals’ Secondary Metabolism as Analyzed via UPLC/MS and Chemometrics
Abstract
:1. Introduction
2. Results
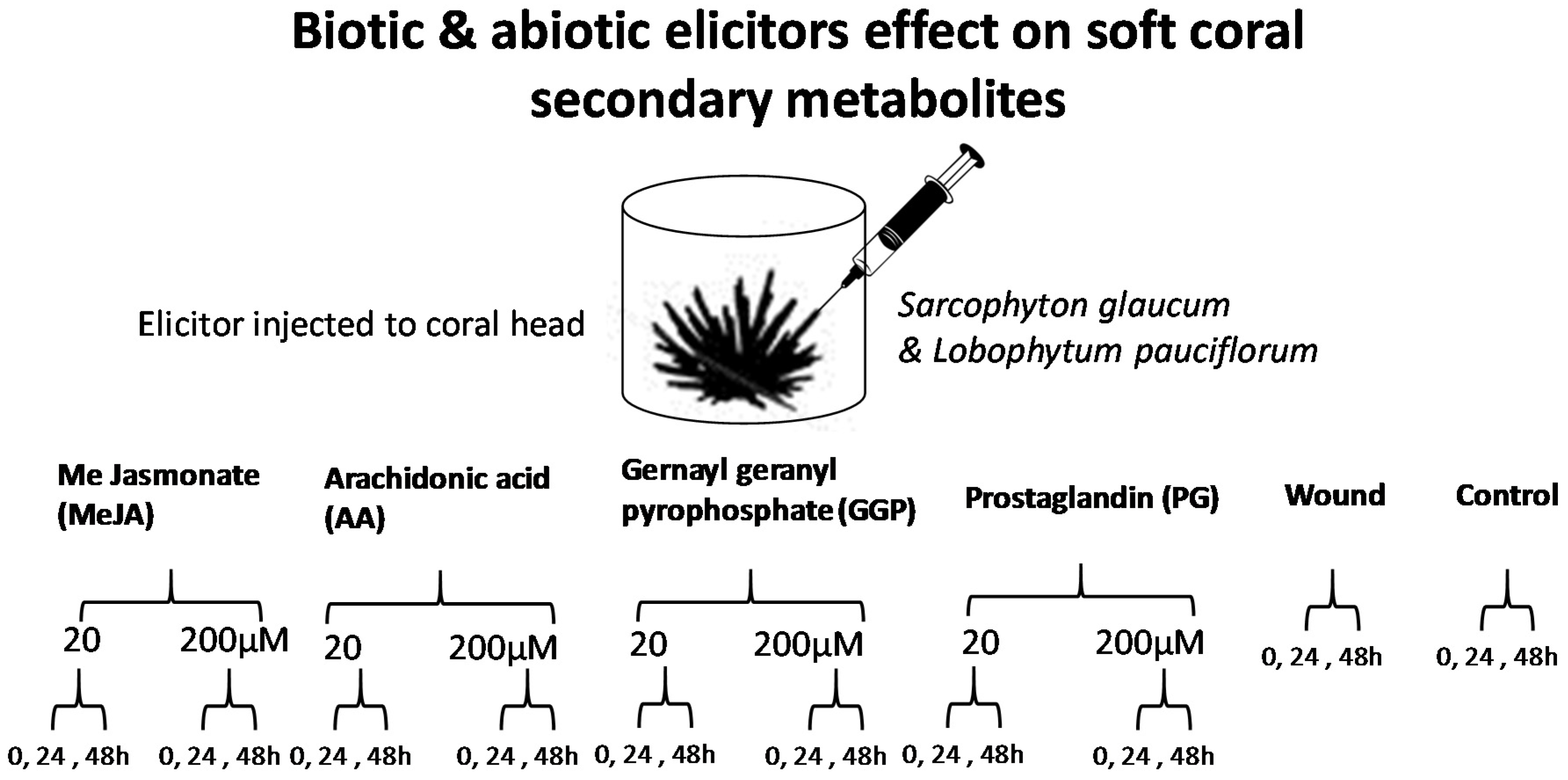
2.1. Experimental Design and Analytical Parameters
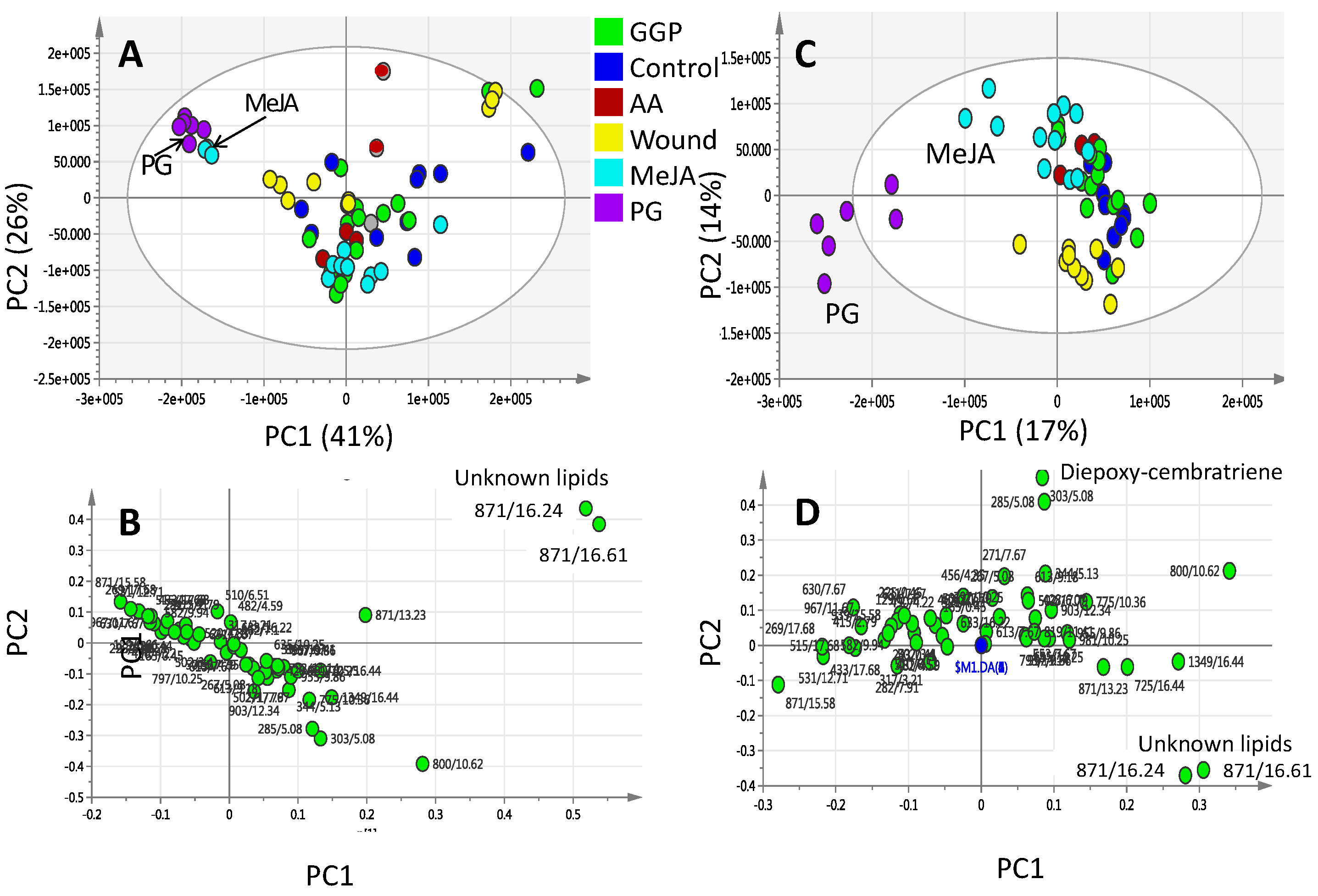
2.2. Effect of Elicitors on S. glaucum Metabolism as Analyzed via PCA & OPLS Analysis
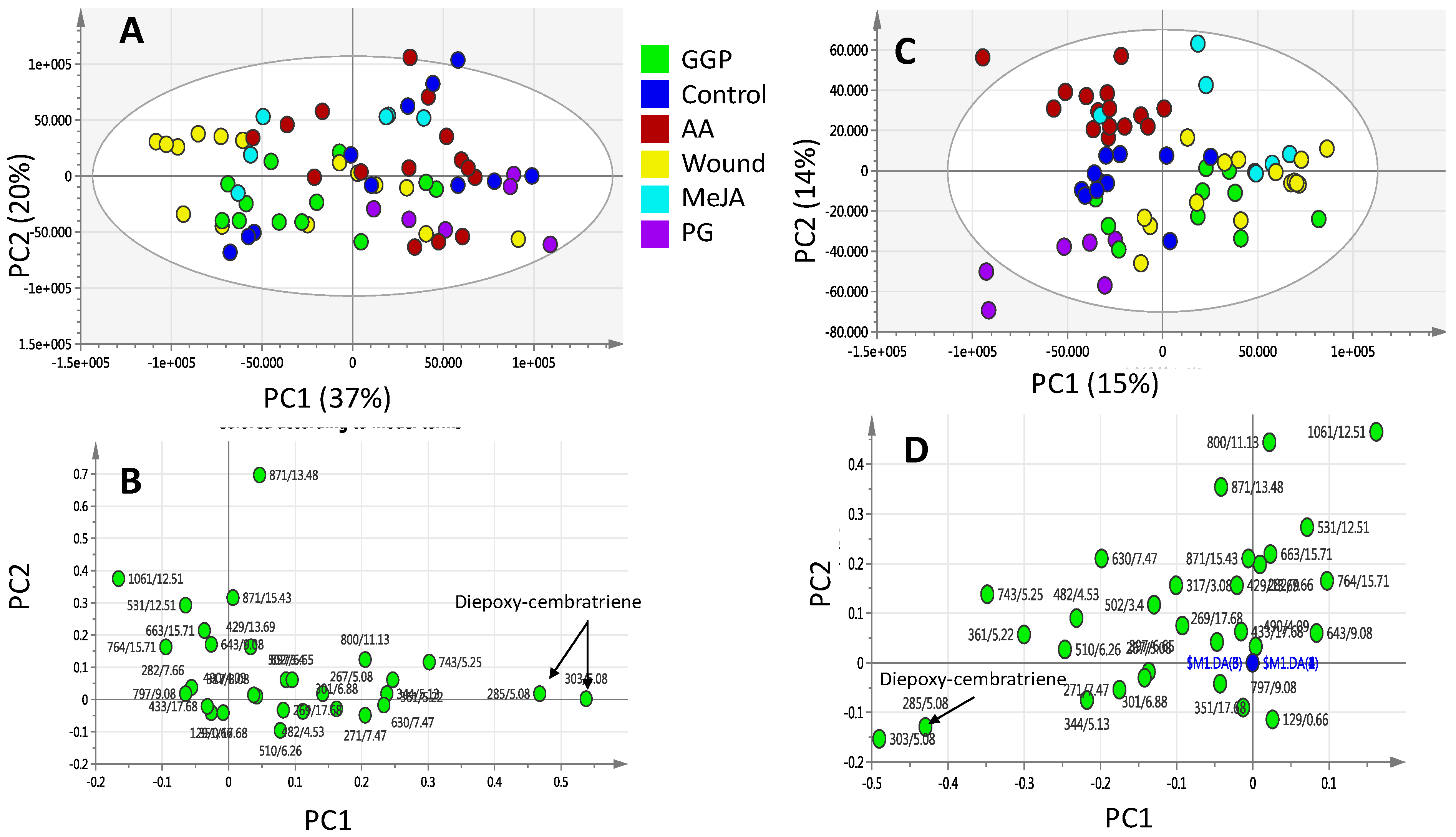
2.3. Effect of Biotic and Abiotic Elicitors on L. pauciliforum Soft Coral Metabolism via PCA & OPLS
2.4. Relative Quantification of S. glaucum & L. pauciflorum in Response to Elicitation
3. Discussion
4. Materials and Methods
4.1. Soft Coral Materials
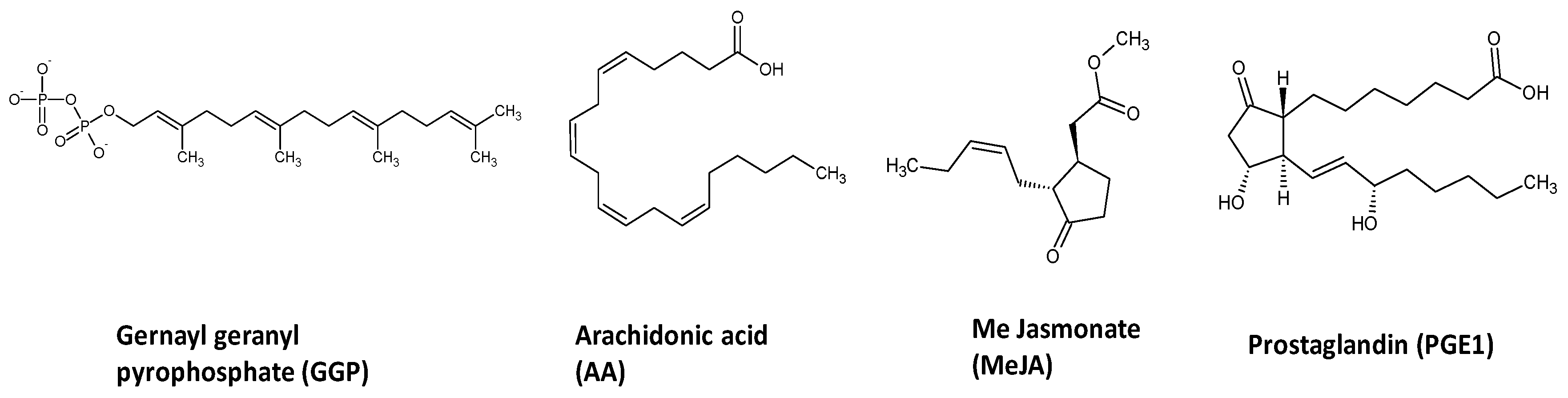
4.2. Chemicals and Reagents
4.3. Elicitation
4.4. Soft Coral Metabolites Extraction Procedure and Sample Preparation for UPLC-MS Analysis
4.5. UPLC-MS Analysis
4.6. UPLC-MS Data Processing for Multivariate Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| UPLC-MS | Ultrahigh performance coupled to mass spectrometry |
| PG-E1 | prostaglandin |
| MeJA | methyl jasmonate |
| GGP | geranylgeranlypyrophosphate |
| AA | arachidonic acid |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal projection to latent structures-discriminant analysis |
References
- Wang, W.; Zhao, Z.J.; Xu, Y.; Qian, X.; Zhong, J.J. Efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogeneity in cell cultures of Panax notoginseng by using chemically synthesized 2-hydroxyethyl jasmonate. Appl. Microbiol. Biotechnol. 2006, 70, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and Action of Jasmonates in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, H.; Muller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Achnine, L.; Huhman, D.V.; Farag, M.A.; Sumner, L.W.; Blount, J.W.; Dixon, R.A. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. Cell Mol. Biol. 2005, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Huhman, D.V.; Dixon, R.A.; Sumner, L.W. Metabolomics reveals novel pathways and differential mechanistic and elicitor-specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiol. 2008, 146, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Zlotek, U.; Swieca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Satheeshkumar, K. Cell line selection combined with jasmonic acid elicitation enhance camptothecin production in cell suspension cultures of Ophiorrhiza mungos L. Appl. Microbiol. Biotechnol. 2017, 101, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Oh, D.K. Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 2017, 66, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, D.J. Prostaglandin A2 in the caribbean gorgonian Plexaura homomalla: Evidence against allelopathic and antifouling roles. Biochem. Syst. Ecol. 1986, 14, 417–421. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and their receptors in insect biology. Front. Endocrinol. 2011, 2, 105. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Porzel, A.; Al-Hammady, M.A.; Hegazy, M.E.; Meyer, A.; Mohamed, T.A.; Westphal, H.; Wessjohann, L.A. Soft Corals Biodiversity in the Egyptian Red Sea: A Comparative MS and NMR Metabolomics Approach of Wild and Aquarium Grown Species. J. Proteome Res. 2016, 15, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, P.W.; Strychar, K.B. Responses to high seawater temperatures in zooxanthellate octocorals. PLoS ONE 2013, 8, e54989. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.M.; Xu, R.F.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Biological and chemical diversity of coral-derived microorganisms. Curr. Med. Chem. 2015, 22, 3707–3762. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Puga, J.; Serodio, J.; Gomes, N.C.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.M. Ecology: Deep and complex ways to survive bleaching. Nature 2015, 518, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K. Modern plant metabolomics: Advanced natural product gene discoveries, improved technologies, and future prospects. Nat. Prod. Rep. 2015, 32, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A. Comparative mass spectrometry & nuclear magnetic resonance metabolomic approaches for nutraceuticals quality control analysis: A brief review. Recent Pat. Biotechnol. 2014, 8, 17–24. [Google Scholar] [PubMed]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass spectrometry strategies in metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Al-Mahdy, D.A.; Meyer, A.; Westphal, H.; Wessjohann, L.A. Metabolomics reveals biotic and abiotic elicitor effects on the soft coral Sarcophyton ehrenbergi terpenoid content. Sci. Rep. 2017, 7, 648. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateff, A.; Alarif, W.M.; Ayyad, S.E.; Al-Lihaibi, S.S.; Basaif, S.A. New cytotoxic isoprenoid derivatives from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Res. 2015, 29, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, Biscembranoids with New Structural Type from a Cultured Soft Coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones U—Z(1), biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Biodivers. 2011, 8, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, P.; Zhou, Y.J.; Wang, X.J.; Zhao, Y.J.; Liu, Y.J.; Tong, Y.R.; Hu, T.Y.; Huang, L.Q.; Gao, W. Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep. 2015, 34, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Boehnlein, J.M.; Santiago-Vázquez, L.Z.; Kerr, R.G. Diterpene biosynthesis by the dinoflagellate symbiont of the Caribbean gorgonian Pseudopterogorgia bipinnata. Mar. Ecol. Prog. Ser. 2005, 303, 105–111. [Google Scholar] [CrossRef]
- Bogdanov, A.; Hertzer, C.; Kehraus, S.; Nietzer, S.; Rohde, S.; Schupp, P.J.; Wagele, H.; Konig, G.M. Defensive Diterpene from the Aeolidoidean Phyllodesmium longicirrum. J. Nat. Prod. 2016, 79, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Sharron, L. The Culture of Soft Corals (Order: Alcyonacea) for the Marine Aquarium Trade; Center for Tropical and Subtropical Aquaculture: Waimanalo, HI, USA, 1999. [Google Scholar]
- Alonso, A.; Marsal, S.; Julia, A. Analytical methods in untargeted metabolomics: State of the art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.N.; Slattery, M. Terpenoids of Sinularia.: Chemistry and Biomedical Applications. Pharm. Biol. 2005, 43, 253–269. [Google Scholar] [CrossRef]
- Christian, O.E.; Compton, J.; Christian, K.R.; Mooberry, S.L.; Valeriote, F.A.; Crews, P. Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomospis asparagi. J. Nat. Prod. 2005, 68, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.S.; Rosado, P.M.; Leite, D.C.; Rosado, A.S.; Bourne, D.G. Beneficial Microorganisms for Corals (BMC): Proposed Mechanisms for Coral Health and Resilience. Front. Microbiol. 2017, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Nagel, R.; Krekling, T.; Christiansen, E.; Gershenzon, J.; Krokene, P. Induction of isoprenyl diphosphate synthases, plant hormones and defense signalling genes correlates with traumatic resin duct formation in Norway spruce (Picea abies). Plant Mol. Biol. 2011, 77, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhou, W.; Zhang, J.; Huang, S.; Wang, H.; Kai, G. Methyl jasmonate induction of tanshinone biosynthesis in Salvia miltiorrhiza hairy roots is mediated by JASMONATE ZIM-DOMAIN repressor proteins. Sci. Rep. 2016, 6, 20919. [Google Scholar] [CrossRef] [PubMed]
- Blechert, S.; Brodschelm, W.; Holder, S.; Kammerer, L.; Kutchan, T.M.; Mueller, M.J.; Xia, Z.Q.; Zenk, M.H. The octadecanoic pathway: Signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. USA 1995, 92, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Rakwal, R.; Kodama, O. Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett. 1997, 412, 61–64. [Google Scholar] [CrossRef]
- Farmer, E.E.; Almeras, E.; Krishnamurthy, V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 2003, 6, 372–378. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Song, J.T.; Cheong, J.J.; Lee, Y.H.; Lee, Y.W.; Hwang, I.; Lee, J.S.; Choi, Y.D. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.D.; Anderson, D.M.; Erdner, D.L.; Bhattacharya, D. Dinoflagellates: A remarkable evolutionary experiment. Am. J. Bot. 2004, 91, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.-Y.; Wang, S.-K.; Tseng, H.-K.; Sheu, J.-H.; Chiang, M.Y. Novel Cytotoxic Cembranoids from the Soft Coral Sinularia flexibilis. J. Nat. Prod. 1998, 61, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Péter, M.; Glatz, A.; Gudmann, P.; Gombos, I.; Török, Z.; Horváth, I.; Vígh, L.; Balogh, G. Metabolic crosstalk between membrane and storage lipids facilitates heat stress management in Schizosaccharomyces pombe. PLoS ONE 2017, 12, e0173739. [Google Scholar] [CrossRef] [PubMed]
- McFadden, C.S.; Alderslade, P.; van Ofwegen, L.P.; Johnsen, H.; Rusmevichientong, A. Phylogenetic Relationships within the Tropical Soft Coral Genera Sarcophyton and Lobophytum (Anthozoa, Octocorallia). Invertebr. Biol. 2006, 125, 288–305. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; Paul, V.J. Chemical and structural defenses in the sea fan Gorgonia ventalina: Effects against generalist and specialist predators. Coral Reefs 1992, 11, 155–159. [Google Scholar] [CrossRef]
- Sammarco, P.W.; La Barre, S.; Coll, J.C. Defensive strategies of soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef. Oecologia 1987, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sella, I.; Benayahu, Y. Rearing cuttings of the soft coral Sarcophyton glaucum (Octocorallia, Alcyonacea): Towards mass production in a closed seawater system. Aquac. Res. 2010, 41, 1748–1758. [Google Scholar] [CrossRef]
- Kawahara, T.; Itoh, M.; Izumikawa, M.; Sakata, N.; Tsuchida, T.; Shin-ya, K. New chaetoglobosin derivatives, MBJ-0038, MBJ-0039 and MBJ-0040, isolated from the fungus Chaetomium sp. f24230. J. Antibiot. 2013, 66, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Namikoshi, M.; Kobayashi, H.; Yoshimoto, T.; Meguro, S.; Akano, K. Isolation and characterization of bioactive metabolites from marine-derived filamentous fungi collected from tropical and sub-tropical coral reefs. Chem. Pharm. Bull. 2000, 48, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; König, G.M. Terpenoids from Marine Organisms: Unique Structures and their Pharmacological Potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Wessjohann, L.A. Metabolome Classification of Commercial Hypericum perforatum (St. John’s Wort) Preparations via UPLC-qTOF-MS and Chemometrics. Planta Med. 2012, 78, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Westphal, H.; Eissa, T.F.; Wessjohann, L.A.; Meyer, A. Effect of Oxylipins, Terpenoid Precursors and Wounding on Soft Corals’ Secondary Metabolism as Analyzed via UPLC/MS and Chemometrics. Molecules 2017, 22, 2195. https://doi.org/10.3390/molecules22122195
Farag MA, Westphal H, Eissa TF, Wessjohann LA, Meyer A. Effect of Oxylipins, Terpenoid Precursors and Wounding on Soft Corals’ Secondary Metabolism as Analyzed via UPLC/MS and Chemometrics. Molecules. 2017; 22(12):2195. https://doi.org/10.3390/molecules22122195
Chicago/Turabian StyleFarag, Mohamed A., Hildegard Westphal, Tarek F. Eissa, Ludger A. Wessjohann, and Achim Meyer. 2017. "Effect of Oxylipins, Terpenoid Precursors and Wounding on Soft Corals’ Secondary Metabolism as Analyzed via UPLC/MS and Chemometrics" Molecules 22, no. 12: 2195. https://doi.org/10.3390/molecules22122195




