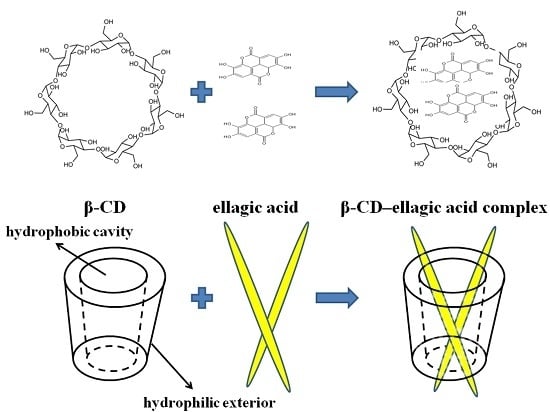
Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation
Abstract
:1. Introduction
2. Results
2.1. Identification of β-CD-Ellagic Acid Microspheres by SEM
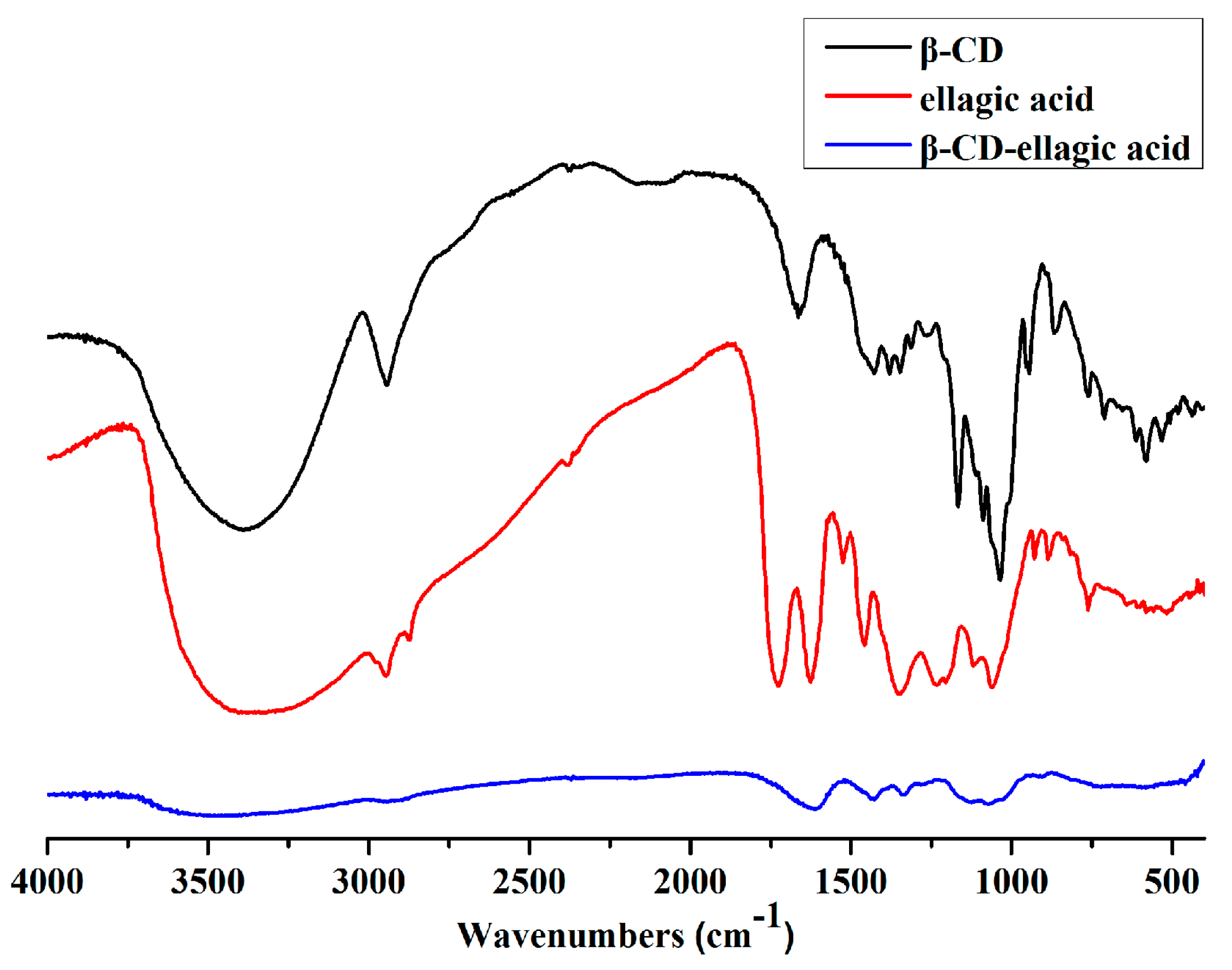
2.2. Identification of β-CD-Ellagic Acid Microspheres by FTIR
2.3. Determination of Ellagic Acid Release Rates from Ellagic Acid Microspheres
2.4. Effect of Ellagic Acid Microspheres on Tumor Cell Proliferation
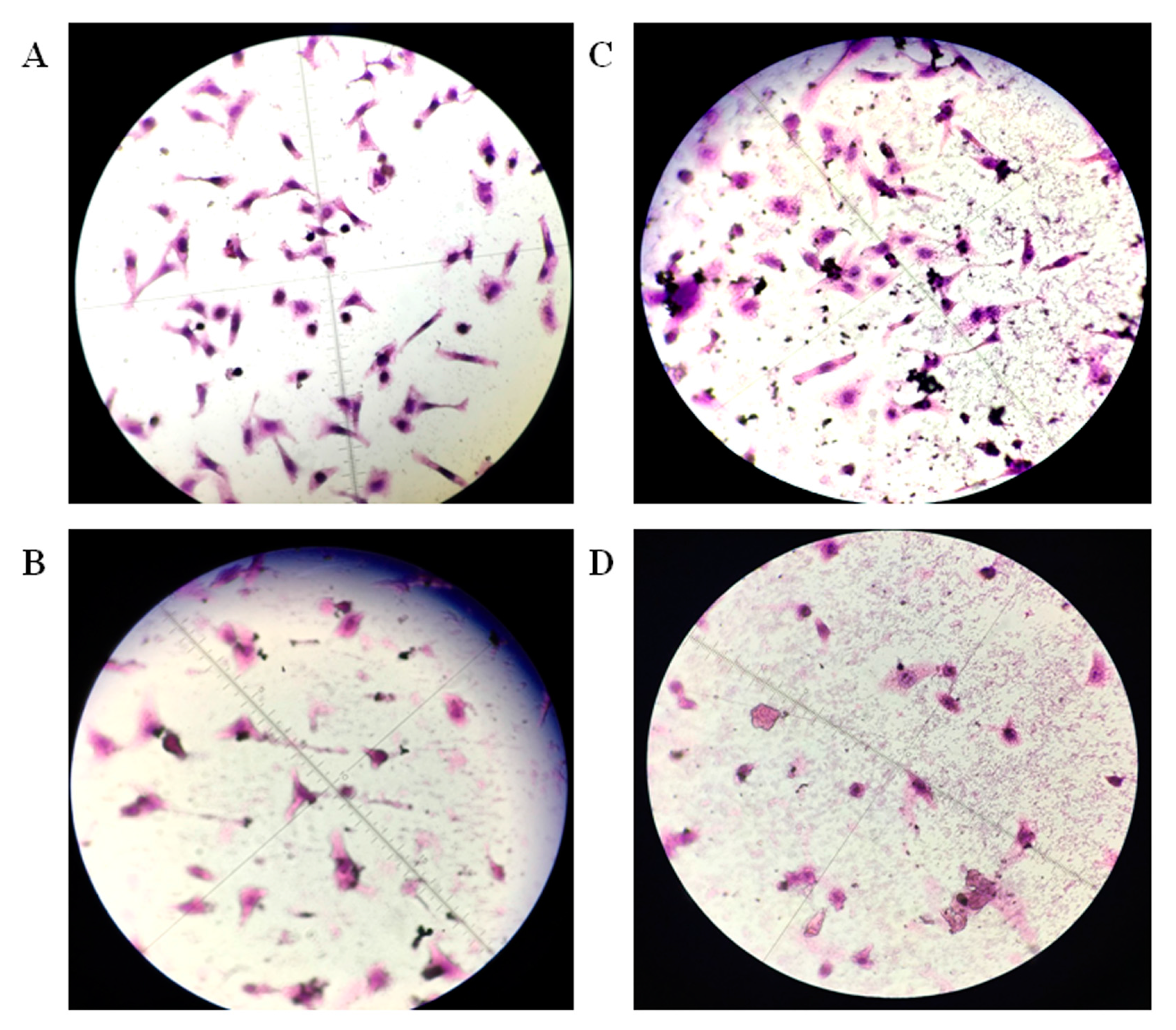
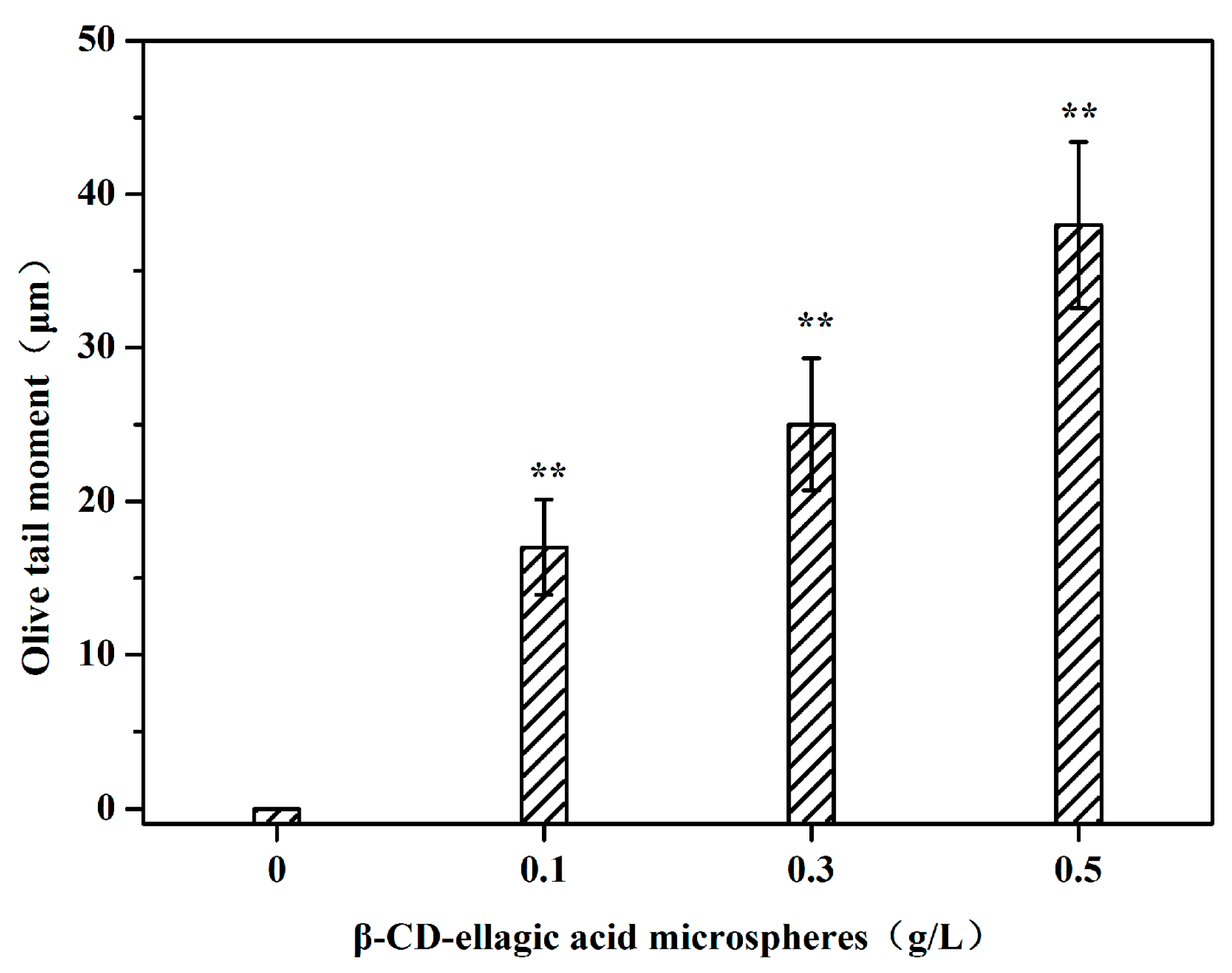
2.5. DNA Damage of Tumor Cells Caused by Ellagic Acid Microspheres
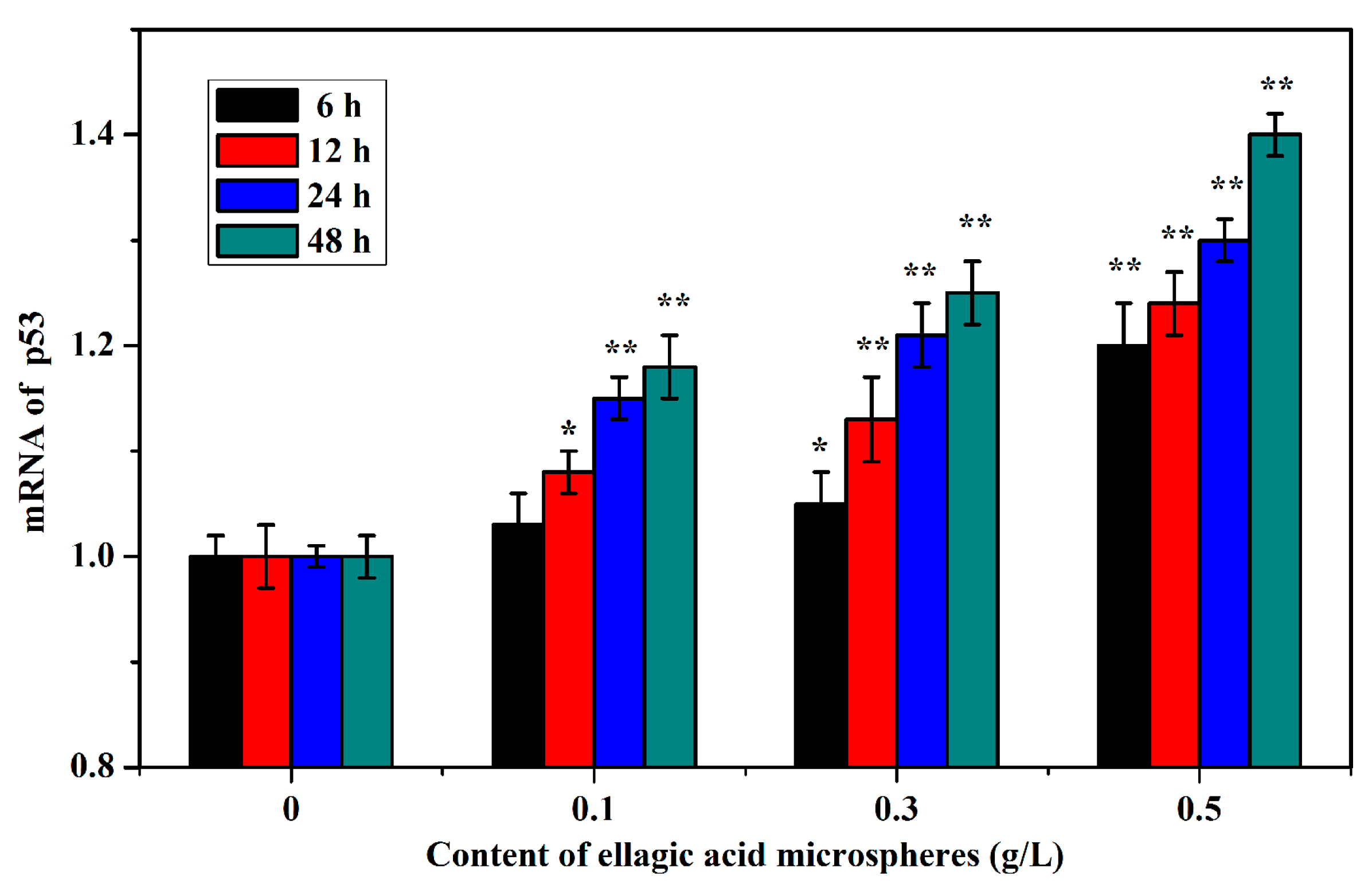
2.6. The mRNA expression of p53 Caused by Ellagic Acid Microspheres
3. Discussion
4. Material and Methods
4.1. Cell Line and Reagents
4.2. Preparation of β-CD–Ellagic Acid Microspheres
4.3. SEM Analysis
4.4. FTIR Spectroscopy
4.5. Determination of Entrapment Efficiency and Release Rate of Ellagic Acid in Ellagic Acid Microspheres
4.6. Effect of Ellagic Acid Microspheres on HepG2 Cell Proliferation
4.7. Single-Cell Electrophoresis
4.8. Reverse Transcription-Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dell’Agli, M.; Galli, G.V.; Corbett, Y.; Taramelli, D.; Lucantoni, L.; Habluetzel, A.; Maschi, O.; Caruso, D.; Giavarini, F.; Romeo, S.; et al. Antiplasmodial activity of Punica granatum L. fruit rind. J. Ethnopharmacol. 2009, 125, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Xu, C.; Ming, R.; Tang, H.; Guyot, R.; Kramer, E.M.; Hu, Y.; Yi, X.; Qi, Y.; Xu, X.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Cao, S.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhang, F.; Zhao, D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). PLoS ONE 2017, 12, e0178809. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Rahman, H.; Abd El-Salam, N.M.; Tawab, A.; Hussain, A.; Khan, T.A.; Khan, U.A.; Qasim, M.; Adnan, M.; Azizullah, A.; et al. Punica granatum peel extracts: HPLC fractionation and LCMS analysis to quest compounds having activity against multidrug resistant bacteria. BMC Complement. Altern. Med. 2017, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Park, D.W.; Kwon, J.E.; Jeong, Y.J.; Kim, T.; Kim, I.; Kang, S.C.; Chi, K.W. Investigation of the biological and anti-cancer properties of ellagic acid-encapsulated nano-sized metalla-cages. Int. J. Nanomed. 2015, 10, 227–240. [Google Scholar]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [PubMed]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.Y.; Mo, S.L.; Loo, T.Y.; Wang, D.M.; Luo, H.B.; Yang, D.P.; Chen, Y.L.; Shen, J.G.; Chen, J.P. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res. Treat. 2012, 134, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Li, Y.; Yang, F.; Zeng, A.; Yang, S.; Luo, Y.; Zhang, Y.; Xie, Y.; Ye, T.; Xia, Y.; et al. The extract from Punica granatum (pomegranate) peel induces apoptosis and impairs metastasis in prostate cancer cells. Biomed. Pharmacother. 2017, 93, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Teel, R.W.; Martin, R.M. Disposition of the plant phenol ellagic acid in the mouse following oral administration by gavage. Xenobiotica 1988, 18, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.C.; Huang, M.T.; Chang, R.L.; Sayer, J.M.; Jerina, D.M.; Conney, A.H. Disposition of the naturally occurring antimutagenic plant phenol, ellagic, and its synthetic derivatices, 3-O-decylellagic acid and 3,3′-di-O-methylellagic acid in mice. Carcinogenesis 1986, 7, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Piešťanský, J.; Maráková, K.; Mikuš, P. Two-dimensional capillary electrophoresis with On-Line sample preparation and cyclodextrin separation environment for direct determination of serotonin in human urine. Molecules 2017, 22, 1668. [Google Scholar] [CrossRef] [PubMed]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum onites L.) essential oil in β-cyclodextrin (β-CD): Synthesis and characterization of the inclusion complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Hyun, H.; Lee, D.W.; Yang, D.H. Visible light-cured glycol chitosan hydrogel containing a beta-cyclodextrin-curcumin inclusion complex improves wound healing in vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Ding, N.; Gao, X.L. Determination of ellagic acid in the extract of pomegranate peel by HPLC. J. Xinjiang Med. Univ. 2012, 35, 770–772. [Google Scholar]
- Peng, H.Y.; Chen, X.G.; Liu, Z.P.; He, Y.X.; Yang, W.Y.; Yang, X.; Li, Y.F. Determination of ellagic acid in the pomegranate juice by RP-HPLC. Food Sci. Technol. 2012, 37, 283–286. [Google Scholar]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med. 2017, 17, 477. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Bakhtiari, E.; Mousavi, S.H. Protective effect of Hibiscus Sabdariffa on doxorubicin-induced cytotoxicity in H9c2 cardiomyoblast cells. Iran. J. Pharm. Res. 2017, 16, 708–713. [Google Scholar] [PubMed]
- Cheng, N.; Wang, Y.; Cao, W. The protective effect of whole honey and phenolic extract on oxidative DNA damage in mice lymphocytes using comet assay. Plant Foods Hum. Nutr. 2017, 4, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Klaude, M.; Eriksson, S.; Nygren, J.; Ahnström, G. The comet assay: Mechanisms and technical considerations. Mutat. Res. 1996, 363, 89–96. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanatsu, K.; Iino, T.; Kato, S.; Jeong, Y.I.; Shibata, N.; Takada, K.; Takeuchi, K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002, 71, 827–839. [Google Scholar] [CrossRef]
- Jeong, Y.; Prasad, Y.R.; Ohno, T.; Yoshikawa, Y.; Shibata, N.; Kato, S.; Takeuchi, K.; Takada, K. Application of Eudragit P-4135F for the delivery of ellagic acid to the rat lower small intestine. J. Pharm. Pharmacol. 2001, 53, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, S.; Zhang, L.; Han, B.; Yao, X.; Chen, W.; Hu, Y. Preparation of ellagic acid molecularly imprinted polymeric microspheres based on distillation-precipitation polymerization for the efficient purification of a crude extract. J. Sep. Sci. 2016, 39, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar] [PubMed]
- Edderkaoui, M.; Odinokova, I.; Ohno, I.; Gukovsky, I.; Go, V.L.; Pandol, S.J.; Gukovskaya, A.S. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J. Gastroenterol. 2008, 14, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Chen, Y.N.; Yu, L.; Zhu, K.C.; Zeng, X.Y.; Bai, Q. Optimization of extraction technology of ellagic acid from pomegranate peels with orthogonal experiment. Agric. Sci. Technol. 2012, 13, 2404–2408. [Google Scholar]
- Sharma, G.; Italia, J.L.; Sonaje, K.; Tikoo, K.; Ravi Kumar, M.N. Biodegradable in situ gelling system for subcutaneous administration of ellagic acid and ellagic acid loaded nanoparticles: Evaluation of their antioxidant potential against cyclosporine induced nephrotoxicity in rats. J. Control. Release 2007, 118, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kharade, S.V.; Roy, N.; Ravi Kumar, M.N. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J. Drug Target. 2006, 14, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, S.N.; Yu, S.M.; Tsiola, A.; Fath, K.R.; Banerjee, I.A. pH dependent spontaneous growth of ellagic acid assemblies for targeting HeLa cells. J. Nanosci. Nanotechnol. 2011, 11, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of β-CD-ellagic acid microspheres are available from the authors. |



| Dose (g/L) | Absorbance (A) | ||||
|---|---|---|---|---|---|
| 0 h | 6 h | 12 h | 24 h | 48 h | |
| 0 | 0.203 ± 0.016 | 0.232 ± 0.031 | 0.294 ± 0.026 | 0.321 ± 0.025 | 0.337 ± 0.033 |
| 0.1 | 0.212 ± 0.044 | 0.205 ± 0.021 * | 0.186 ± 0.030 ** | 0.173 ± 0.019 ** | 0.161 ± 0.035 ** |
| 0.3 | 0.198 ± 0.031 | 0.174 ± 0.042 * | 0.122 ± 0.020 ** | 0.093 ± 0.015 ** | 0.086 ± 0.014 ** |
| 0.5 | 0.204 ± 0.025 | 0.095 ± 0.018 ** | 0.079 ± 0.016 ** | 0.067 ± 0.010 ** | 0.051 ± 0.009 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, Y.; Tian, Z.; Ma, J.; Kang, M.; Ding, C.; Ming, D. Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation. Molecules 2017, 22, 2175. https://doi.org/10.3390/molecules22122175
Wang H, Zhang Y, Tian Z, Ma J, Kang M, Ding C, Ming D. Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation. Molecules. 2017; 22(12):2175. https://doi.org/10.3390/molecules22122175
Chicago/Turabian StyleWang, Hongkai, Yingxia Zhang, Zhongjing Tian, Jing Ma, Meiling Kang, Chengshi Ding, and Dongfeng Ming. 2017. "Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation" Molecules 22, no. 12: 2175. https://doi.org/10.3390/molecules22122175