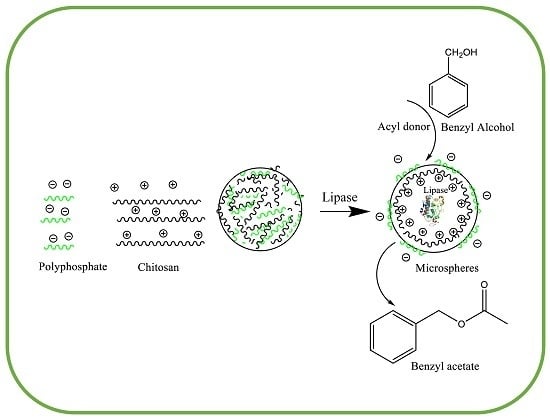
Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads
Abstract
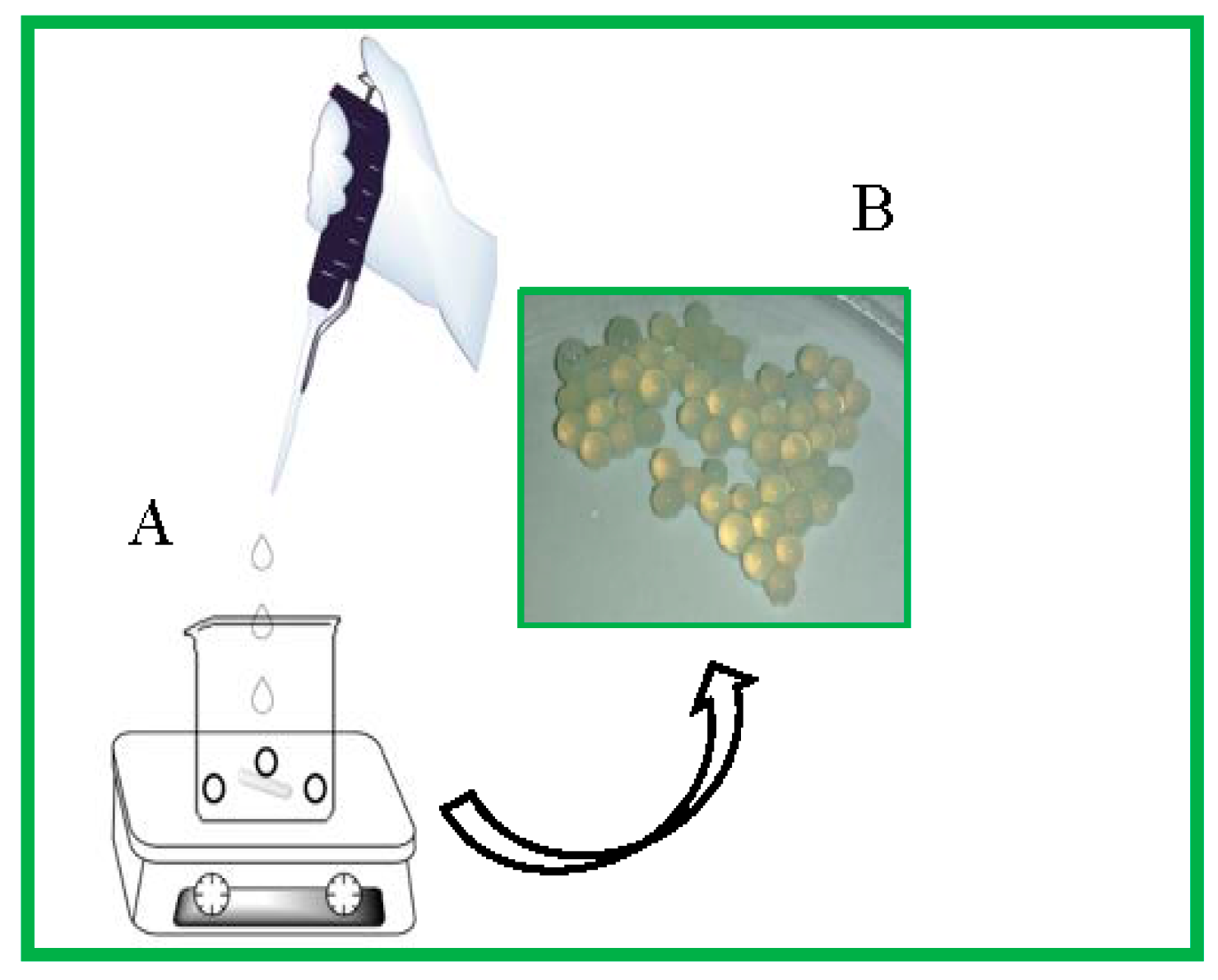
:1. Introduction
2. Results and Discussion
2.1. Characterization of Lipase Immobilized on Polyphosphate and Chitosan Support (LPCS) Microspheres
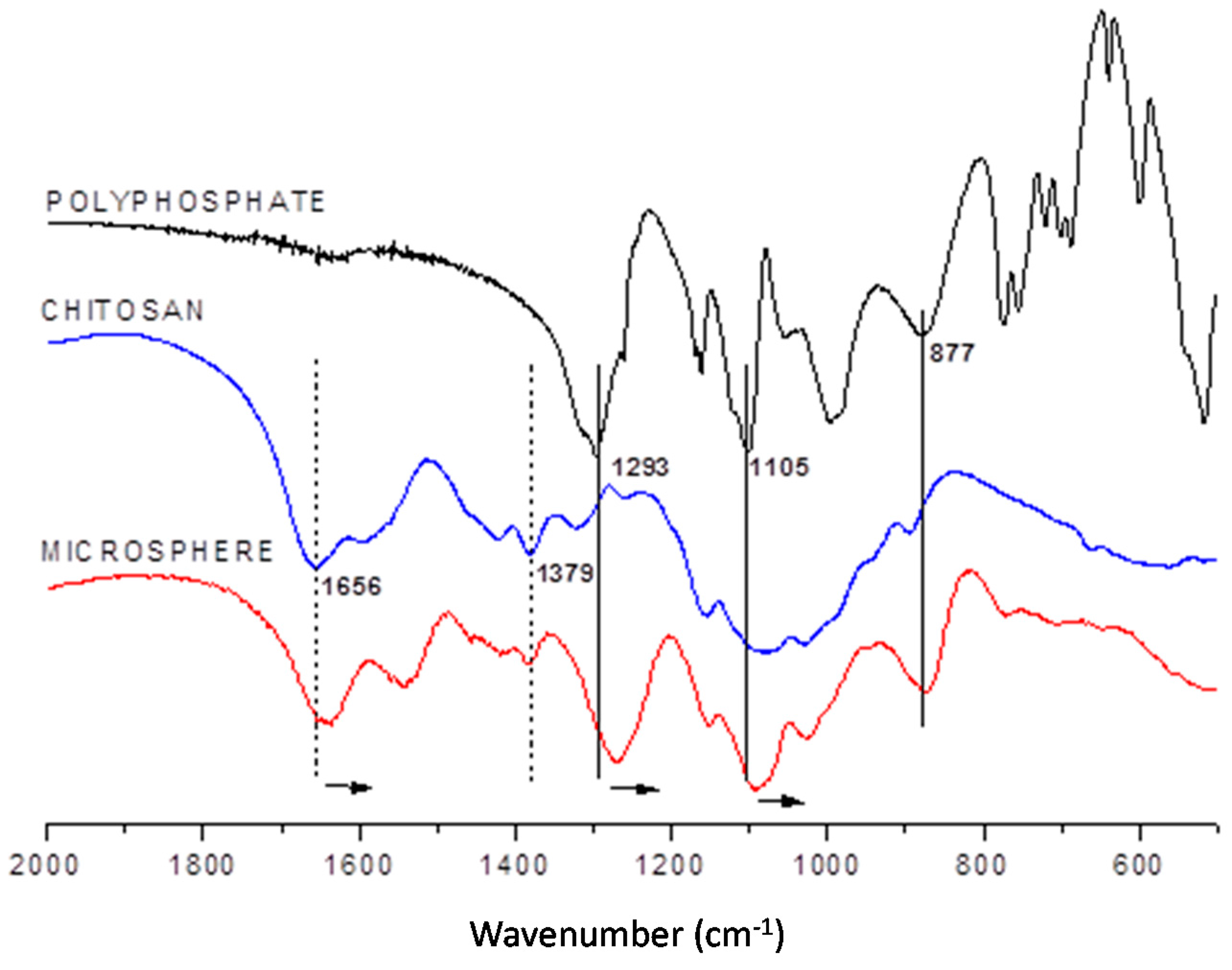
2.1.1. Infrared Spectroscopy
2.1.2. Thermogravimetric Analysis
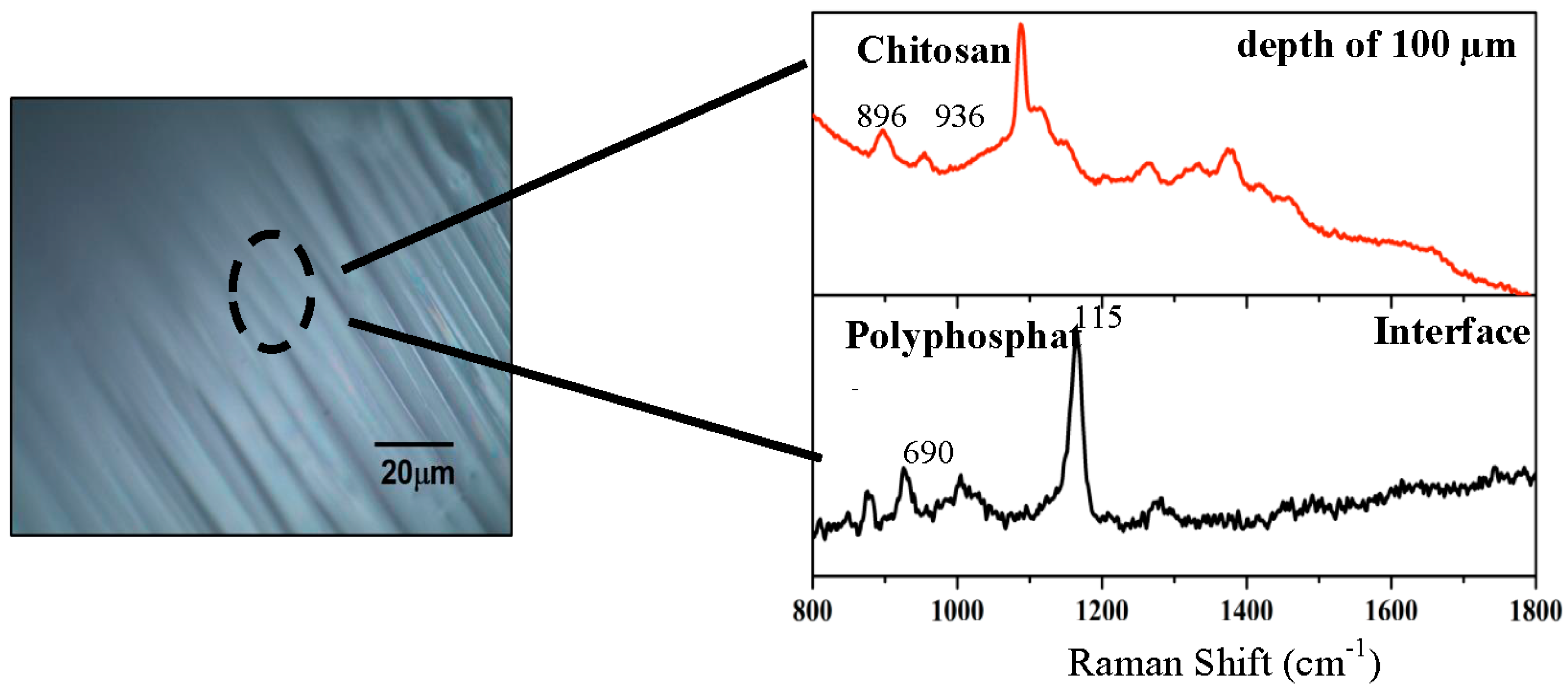
2.1.3. Confocal Raman Microscopy
2.2. Characterization of LPCS
2.2.1. Preservation of the Catalytic Activity of the Lipase by LPCS Immobilization
2.2.2. Effect of Reaction Temperature on the Activity of LPCS
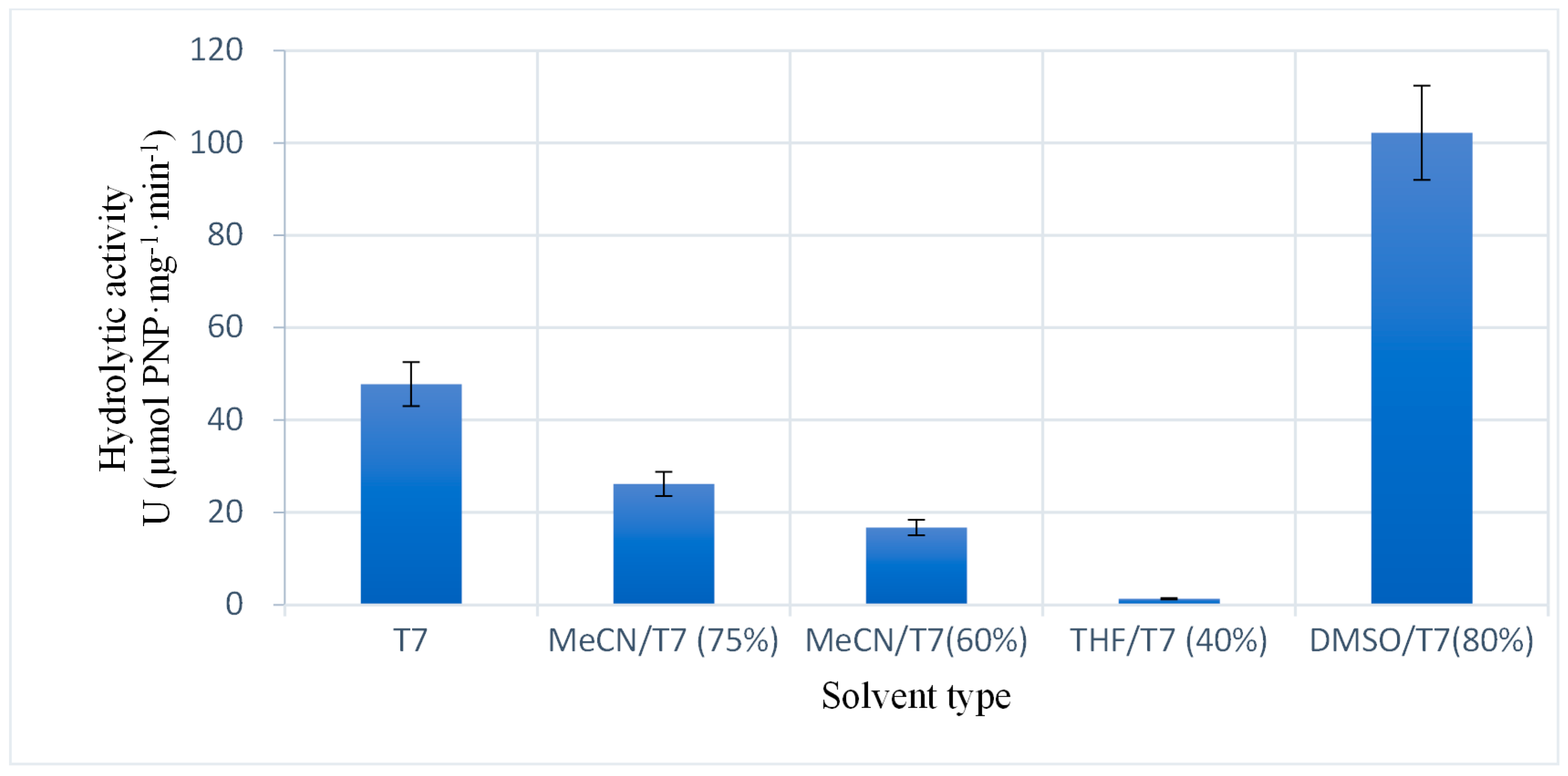
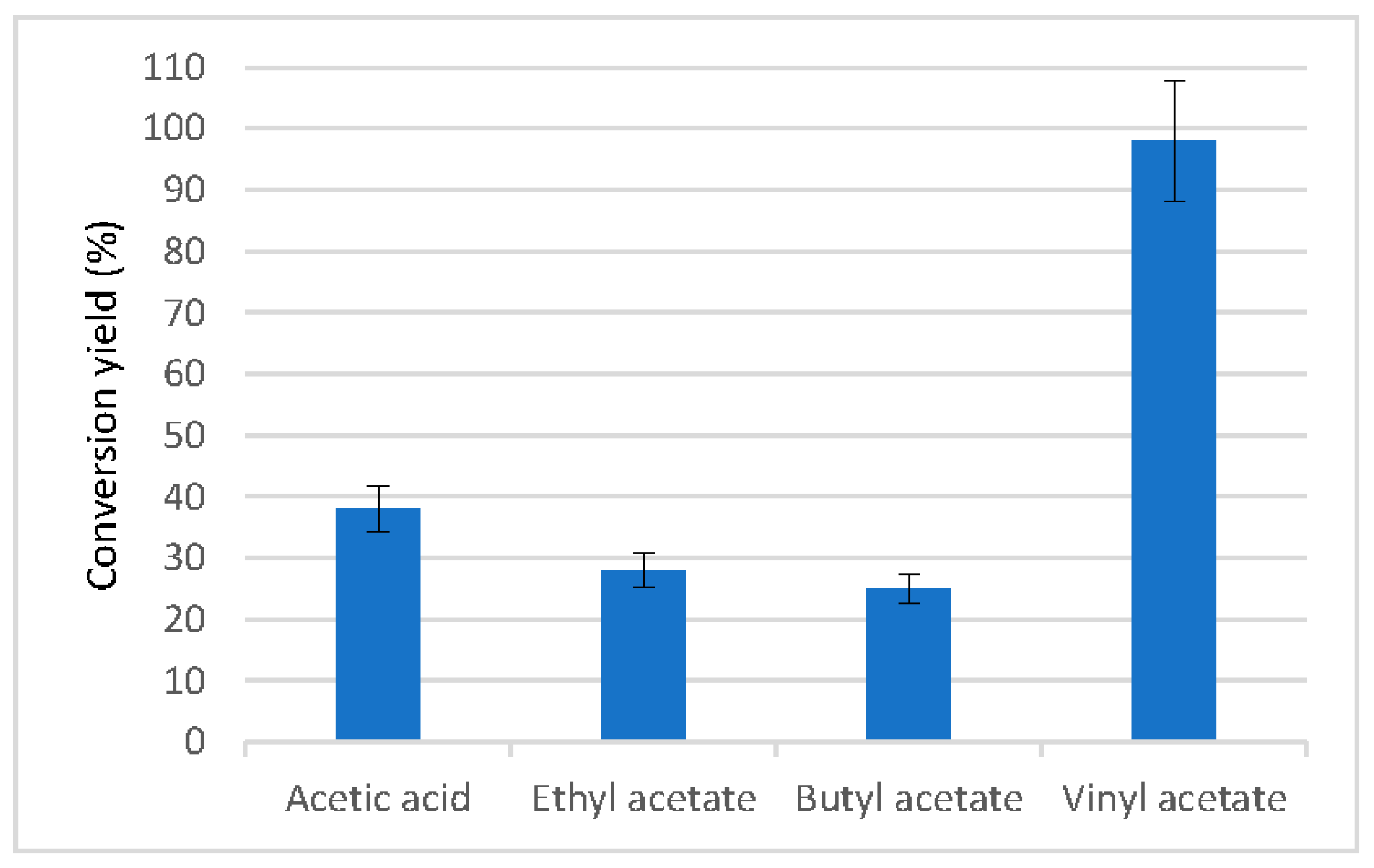
2.2.3. Catalytic Ability of the Enzyme Immobilized Using Different Solvents
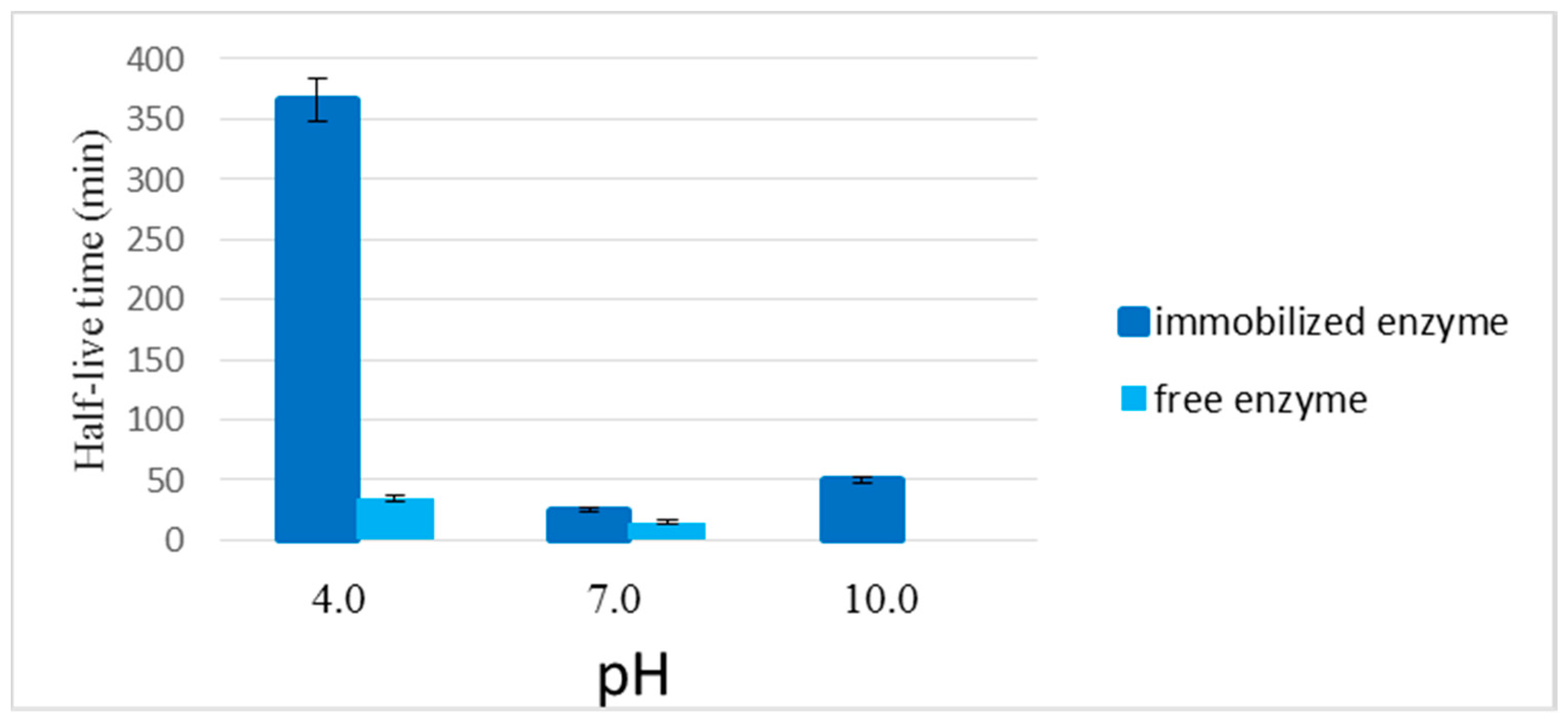
2.2.4. Stability of the Enzyme Immobilized in the LPCS Support at Different Values of pH
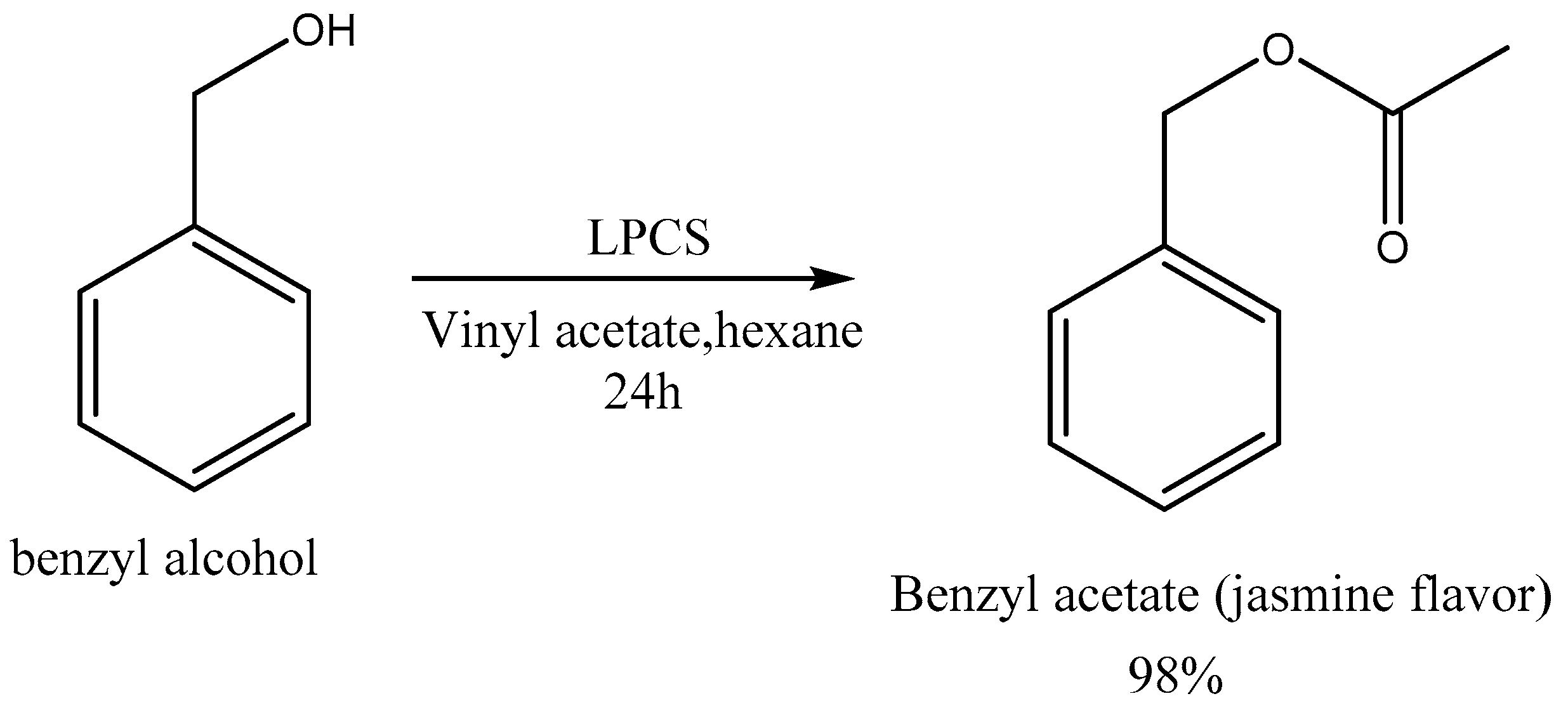
2.2.5. Enzymatic Synthesis Catalyzed by LPCS of the Compound Responsible for the Aroma of Jasmine
2.2.6. LPCS Recycling
3. Materials and Methods
3.1. Materials
3.2. Equipments Used for the Characterization of the Biocatalyst
3.3. Synthesis of the Microspheres from Chitosan and Polyphosphate
3.4. Immobilization of CALB
3.5. Determination of the Concentration of the Immobilized Enzyme
3.6. Hydrolytic Activity
3.7. Activity in Organic Solvents
3.8. Effect of pH
3.9. Enzymatic Synthesis of Esters—Effect of the Acyl Donor Groups and Enzymatic Synthesis of the Compound Responsible for the Aroma of Jasmine
3.10. Reuse of the Catalyst
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; Ribeiro, M.F.P.; Dos Santos, J.C.S.; Coelho, M.A.Z.; Simas, A.B.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia: Application to the kinetic resolution of myo-inositol derivatives. Process Biochem. 2015, 50, 1557–1564. [Google Scholar] [CrossRef]
- Villalba, M.; Verdasco-Martín, C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Otero, C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzym. Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.M.; Neto, D.M.A.; Galvão, W.S.; Rios, N.S.; Carvalho, A.C.L.d.M.; Correa, M.A.; Bohn, F.; Fernandez-Lafuente, R.; Fechine, P.B.A.; de Mattos, M.C.; et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem. Eng. J. 2017, 125, 104–115. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, G.-J.; Chen, C.-I.; Liu, Y.-C.; Shieh, C.-J. Kinetics and optimization of lipase-catalyzed synthesis of rose fragrance 2-phenylethyl acetate through transesterification. Process Biochem. 2014, 49, 437–444. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Li, Y.; Liu, X.; Lei, L.; Xuan, S. Novel magnetic microspheres of P (GMA-b-HEMA): Preparation, lipase immobilization and enzymatic activity in two phases. Appl. Microbiol. Biotechnol. 2012, 95, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wei, J.; Du, X.; Zhou, X. Preparation and Evaluation of Self-Assembled Porous Microspheres-Fibers for Removal of Bisphenol A from Aqueous Solution. Ind. Eng. Chem. Res. 2016, 55, 1566–1574. [Google Scholar] [CrossRef]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszynski, D.; Kaczorek, E.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Spongin-Based Scaffolds from Hippospongia communis Demosponge as an Effective Support. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Guterman, I.; Masci, T.; Chen, X.; Negre, F.; Pichersky, E.; Dudareva, N.; Weiss, D.; Vainstein, A. Generation of phenylpropanoid pathway-derived volatiles in transgenic plants: Rose alcohol acetyltransferase produces phenylethyl acetate and benzyl acetate in petunia flowers. Plant Mol. Biol. 2006, 60, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.S.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- De Souza, T.C.; Fonseca, T.d.S.; da Costa, J.A.; Rocha, M.V.P.; de Mattos, M.C.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: Application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016, 58–69. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; Santos, J.C.S.; Mammarella, E.J. Operational and Thermal Stability Analysis of Thermomyces lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl. Biochem. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; Albuquerque, T.L.; Bartolome-cabrero, R.; Barbosa, O.; Fernandez-lafuente, R. Reversible Immobilization of Lipases on Heterofunctional Octyl-Amino Agarose Beads. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Yuce-Dursun, B.; Cigil, A.B.; Dongez, D.; Kahraman, M.V.; Ogan, A.; Demir, S. Preparation and characterization of sol-gel hybrid coating films for covalent immobilization of lipase enzyme. J. Mol. Catal. B Enzym. 2016, 127, 18–25. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst many applications: The use of Candida antarctica B-lipase in organic syhthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V.; Muzzarelli, R.A.A.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and phamaceutical perspective. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, Á.A.A.; Passes, E.D.; De Brito Alves, S.; Silva, G.S.; Higa, O.Z.; Vítolo, M. Alginate-poly(vinyl alcohol) core-shell microspheres for lipase immobilization. J. Appl. Polym. Sci. 2006, 102, 1553–1560. [Google Scholar] [CrossRef]
- Wen, Y.; Grøndahl, L.; Gallego, M.R.; Jorgensen, L.; Møller, E.H.; Nielsen, H.M. Delivery of Dermatan Sulfate from Polyelectrolyte Complex-Containing Alginate Composite Microspheres for Tissue Regeneration. Biomacromolecules 2012, 13, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Xie, G.; Zhang, W.; Wang, W.; Shan, J.; Liu, H.; Zhou, X. Preparation of a novel chitosan-microcapsules/starch blend film and the study of its drug-release mechanism. Int. J. Biol. Macromol. 2016, 87, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhu, H.-Y.; Chen, H.-H.; Yao, J.; Fu, Y.-Q.; Zhang, Z.-Y.; Xu, Y.-M. Effect of calcination temperature on physical parameters and photocatalytic activity of mesoporous titania spheres using chitosan/poly(vinyl alcohol) hydrogel beads as a template. Appl. Surf. Sci. 2014, 319, 189–196. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Jing, L.; Peng, X.; Li, Y. Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide-chitosan composite for lipase immobilization. Biochem. Eng. J. 2015, 98, 75–83. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Canella, K.M.N.C.; Garcia, R.B. Caracterização de quitosana por cromatografia de permeação em gel-influência do método de preparação e do solvente. Quim. Nova 2001, 24, 13–17. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Silva, M.J.A.; Klein, M.; Feddern, V.; Feltes, M.M.C.; Oliveira, J.V.; Oliveira, D. Enzymatic Current status and trends in enzymatic nanoimmobilization. J. Mol. Catal. B Enzym. 2014, 99, 56–67. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Xia, C.; Zhao, C.; Ren, Z.; Zhang, W. Immobilization of lipase on porous monodisperse chitosan microspheres. Biotechnol. Appl. Biochem. 2015, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, X.Y.; Gao, Q.L.; Fang, F.; Wang, L.W.; Huang, X.J. Immobilization of lipase onto functional cyclomatrix polyphosphazene microspheres. J. Mol. Catal. B Enzym. 2016, 132, 67–74. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Garcia, K.C.; Bussamara, R.; Both, F.S.; Vainstein, M.H.; Soares, R.M.D. Poly (lactic acid)/chitosan fiber mats: Investigation of effects of the support on lipase immobilization. Int. J. Biol. Macromol. 2015, 72, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.L.; Gõmez Brizuela, L.; Úbeda Iranzo, J.; Arevalo-Villena, M.; Briones Pérez, A.I. Pectinase Immobilization on a Chitosan-Coated Chitin Support. J. Food Process Eng. 2016, 39, 97–104. [Google Scholar] [CrossRef]
- Kopp, W.; Barud, H.S.; Paz, M.F.; Bueno, L.A.; Giordano, R.L.C.; Ribeiro, S.J.L. Calcium polyphosphate coacervates: Effects of thermal treatment. J. Sol-Gel Sci. Technol. 2012, 63, 219–223. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Yu, F. Facile preparation of Fe3O4@MOF core-shell microspheres for lipase immobilization. J. Taiwan Inst. Chem. Eng. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Krakowiak, A.; Trzciƒska, M.; Sieliwanowicz, B.; Sawicka-Zukowska, R.; Jedrychowska, B.; Ajzenberg, V. Properties of immobilized and free lipase from Rhizopus cohnii. Pol. J. Food Nutr. Sci. 2003, 12, 39–44. [Google Scholar]
- Gupta, A.; Terrell, J.L.; Fernandes, R.; Dowling, M.B.; Payne, G.F.; Raghavan, S.R.; Bentley, W.E. Encapsulated fusion protein confers “sense and respond” activity to chitosan-alginate capsules to manipulate bacterial quorum sensing. Biotechnol. Bioeng. 2013, 110, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.S.; Bonazza, H.L.; de Matos, L.J.B.L.; Carneiro, E.A.; Barbosa, O.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; de Sant’ Ana, H.B.; Santiago-Aguiar, R.S. Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol. Rep. 2017, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans.pdf. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Srinophakun, P. Biodiesel Production from Crude Sunflower Oil and Crude Jatropha Oil Using Immobilized Lipase Biodiesel Production from Crude Sunflower Oil and Crude Jatropha. J. Chem. Eng. Jpn. 2010, 43, 104–108. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, T.; Wu, X.; Cai, Y.; Yao, D.; Xie, C.; Liu, D. Covalent immobilization of enzymes within micro-aqueous organic media. J. Mol. Catal. B Enzym. 2011, 72, 145–149. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Kyzioł, A. Colloids and Surfaces A: Physicochemical and Engineering Aspects Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: I. DPPG. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 349–358. [Google Scholar] [CrossRef]
- Krajewska, B.; Kyzioł, A.; Wydro, P. Colloids and Surfaces A: Physicochemical and Engineering Aspects Chitosan as a subphase disturbant of membrane lipid monolayers. The effect of temperature at varying pH: II. DPPC and cholesterol. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 359–364. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Kumari, A.; Mahapatra, P.; Banerjee, R. Modeling, Simulation, and Kinetic Studies of Solvent-Free Biosynthesis of Benzyl Acetate. J. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Groboillot, A.F.; Champagne, C.P.; Darling, G.D.; Poncelet, D.; Neufeld, R.J. Membrane Formation by InterfaciaI Cross-Linking of Chitosan for Microencapsulation of Lactococcus iactis. Biotechnol. Bioeng. 1993, 42, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, E.; Yan, M.; Giorno, L. Development of enzyme-loaded PVA microspheres by membrane emulsification. J. Membr. Sci. 2017, 524, 79–86. [Google Scholar] [CrossRef]
- Mondal, S.; Li, C.; Wang, K. Bovine Serum Albumin Adsorption on Gluteraldehyde Cross-Linked Chitosan Hydrogels. J. Chem. Eng. Data 2015, 60, 2356–2362. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Kamel, A.O.; Sammour, O.A. Injectable long acting chitosan/tripolyphosphate microspheres for the intra-articular delivery of lornoxicam: Optimization and in vivo evaluation. Carbohydr. Polym. 2016, 149, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Hanuza, J.; Wandas, M.; Dymin, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy A. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Abia, O.; Pessela, B.C.C.; Ferna, R.; Guisa, M. Co-Aggregation of Penicillin G Acylase and Polyionic Polymers: An Easy Methodology To Prepare Enzyme Biocatalysts Stable in Organic Media. Biomacromolecules 2004, 5, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Wilson, L.; Mateo, C.; Fernández-lorente, G.; Palomo, J.M.; Fernández-lafuente, R.; Guisán, J.M.; Re, D.; Tam, A.; Daminatti, M. Preparation of artificial hyper-hydrophilic micro-environments (polymeric salts) surrounding enzyme molecules New enzyme derivatives to be used in any reaction medium. J. Mol. Catal. B Enzym. 2002, 19, 295–303. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B. 2017, 106, 67–74. [Google Scholar] [CrossRef]
- Kaş, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, K.C.; Bhanage, B.M. Synthesis of geranyl acetate in non-aqueous media using immobilized Pseudomonas cepacia lipase on biodegradable polymer film: Kinetic modelling and chain length effect study. Process Biochem. 2014, 49, 1304–1313. [Google Scholar] [CrossRef]
- Majumder, A.B.; Singh, B.; Dutta, D.; Sadhukhan, S.; Gupta, M.N. Lipase catalyzed synthesis of benzyl acetate in solvent-free medium using vinyl acetate as acyl donor. Bioorg. Med. Chem. Lett. 2006, 16, 4041–4044. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Modak, J.; Madras, G. Effect of the chain length of the acid on the enzymatic synthesis of flavors in supercritical carbon dioxide. Biochem. Eng. J. 2005, 23, 199–202. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.H.; Chen, N.; Zhi, G.Y. Synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Bioresour. Technol. 2015, 198, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Banerjee, R. Solvent-free synthesis of flavour esters through immobilized lipase mediated transesterification. Enzym. Res. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.; Lourenço, N.M.T. Enzymatic kinetic resolution of sec-alcohols using an ionic liquid anhydride as acylating agent. Tetrahedron Asymmetry 2014, 25, 944–948. [Google Scholar] [CrossRef]
- Larkov, O.; Zaks, A.; Bar, E.; Lewinsohn, E.; Dudai, N.; Mayer, A.M.; Ravid, U. Enantioselective monoterpene alcohol acetylation in Origanum, Mentha and Salvia species. Phytochemistry 2008, 69, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.; Henley, J.P. Analysis of enzyme deactivations by a series-type mechanism: Influence of modification on the activity and stability of enzymes. Ann. N. Y. Acad. Sci. 1987, 501, 73–79. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the biocatalyst are available from the authors. |



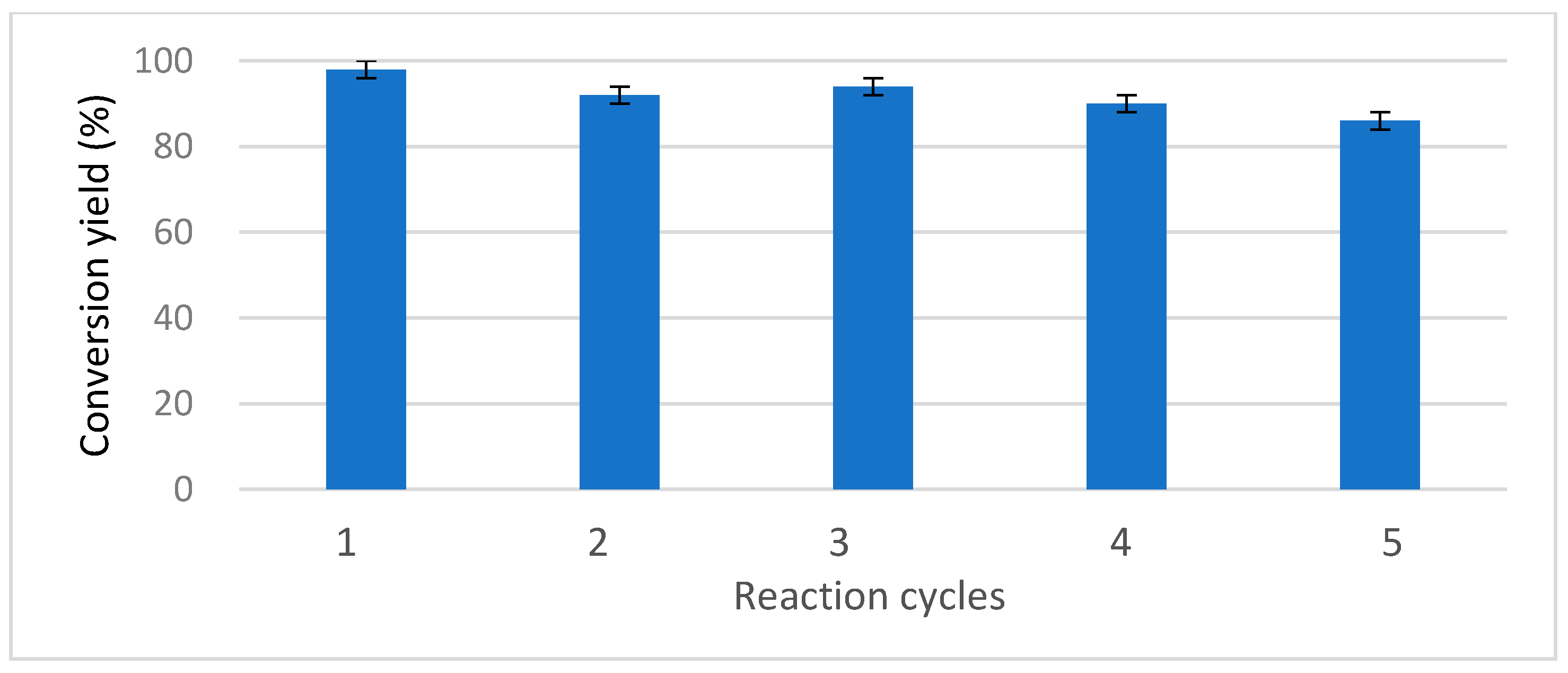

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads. Molecules 2017, 22, 2165. https://doi.org/10.3390/molecules22122165
Melo ADQ, Silva FFM, Dos Santos JCS, Fernández-Lafuente R, Lemos TLG, Dias Filho FA. Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads. Molecules. 2017; 22(12):2165. https://doi.org/10.3390/molecules22122165
Chicago/Turabian StyleMelo, Ana D. Q., Francisco F. M. Silva, José C. S. Dos Santos, Roberto Fernández-Lafuente, Telma L. G. Lemos, and Francisco A. Dias Filho. 2017. "Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads" Molecules 22, no. 12: 2165. https://doi.org/10.3390/molecules22122165