Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa)
Abstract
:1. Introduction
2. Results
2.1. Basic Chemical Composition
2.2. Bioactive Compounds Content
2.3. Antioxidant Capacity
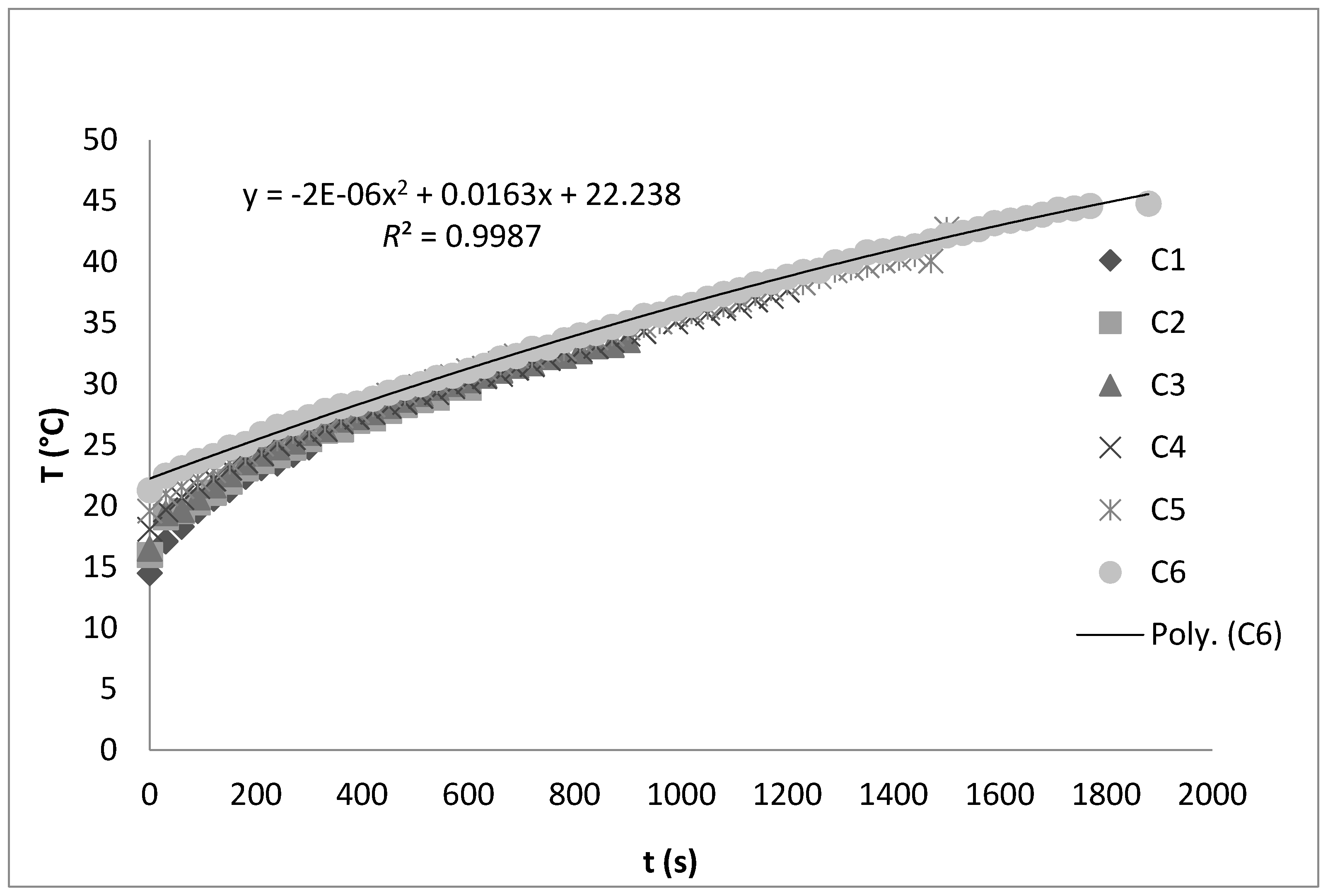
3. Materials and Methods
3.1. Plant Material
3.2. Chokeberry Powder Preparation
3.3. Sample Preparation for the Classic Extraction
3.4. Sample Preparation for Ultrasonic Extraction
3.5. The Determination of Basic Chemical Composition and Bioactive Compounds Content
3.6. Vitamin C Determination (HPLC Method)
3.7. HPLC Determination of Individual Anthocyanins and Polyphenols
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Ciocoiu, M.; Badescu, L.; Miron, A.; Badescu, M. The Involvement of a Polyphenol-Rich Extract of Black Chokeberry in Oxidative Stress on Experimental Arterial Hypertension. J. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Landeka Jurčević, I.; Panjkota Krbavčić, I.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotech. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Enriquez, E.; Salmerón, I.; Gutierrez-Mendez, N.; Ortega-Rivas, E.; Lacroix, M. Ultraviolet Irradiation Effect on Apple Juice Bioactive Compounds during Shelf Storage. Foods 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Tech. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Tripalo, B.; Ježek, D.; Brnčić, M.; Karlović, S.; Dujmić, F. Influence of High Intensity Ultrasound Treatments on Physical Properties of Sheep Milk. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 44–48. [Google Scholar]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia rebaudiana Bertoni. J. Agric Food Chem. 2015, 12, 6835–6846. [Google Scholar] [CrossRef] [PubMed]
- Šic Žlabur, J.; Voća, S.; Dobričević, N.; Pliestić, S.; Galić, A.; Boričević, A.; Borić, N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophys. 2016, 30, 95–104. [Google Scholar] [CrossRef]
- Šic Zlabur, J.; Voća, S.; Dobričević, N.; Rimac Brnčić, S.; Dujmić, F.; Brnčić, M. Optimization of ultrasound assisted extraction of functional ingredients from Stevia rebaudiana Bertoni leaves. Int. Agrophys. 2015, 29, 231–237. [Google Scholar] [CrossRef]
- Jeppsson, N. The effects of fertilizer rate on vegetative growth, yield and fruit quality, with special respect to pigments, in black chokeberry (Aronia melanocarpa) cv. ‘Viking’. Sci. Hort. 2000, 83, 127–137. [Google Scholar] [CrossRef]
- Šnebergrová, J.; Čížková, H.; Neradová, E.; Kapci, B.; Rajchl, A.; Voldřich, M. Variability of Characteristic Components of Aronia. Czech J. Food Sci. 2014, 32, 25–30. [Google Scholar]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Robus, Ribes and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar]
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lovrić, T. Procesi u Prehrambenoj Industriji s Osnovama Prehrambenog Inžinjerstva [Processes in the Food Industry with Fundamentals of Food Engineering]; Hinus: Zagreb, Croatia, 2003. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; Le Bourvellec, C.; Renard, M.G.C.C. Lab and pilot scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J.; Tiwari, A.K. Optimization of novel method for the extraction of steviosides from Stevia rebaudiana leaves. Food Chem. 2012, 132, 1113–1120. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 22, 809–813. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensmitt. Rundsch. 2007, 103, 369–378. [Google Scholar]
- Olas, B.; Wachowicz, P.; Nowak, P.; Kedzierska, M.; Tomczak, A.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. J. Physiol. Pharmacol. 2008, 59, 823–835. [Google Scholar] [PubMed]
- Taheri, R. Polyphenol Composition of Underutilized Aronia Berries and Changes in Aronia Berry Polyphenol Content through Ripening. Master’s Thesis, University of Connecticut, Storrs, CT, USA, 2013. Available online: http://digitalcommons.uconn.edu/gs_theses/436 (accessed on 5 February 2016).
- Tiwari, B.K.; O’Donnell, C.P.; Muthukumarappan, K.; Cullen, P.J. Effect of ultrasound processing on quality of fruit juices. Stewart Postharvest Rev. 2008, 4, 1–6. [Google Scholar]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Cullen, P.J. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agric. Food Chem. 2008, 56, 10071–10077. [Google Scholar] [CrossRef] [PubMed]
- Ciccolini, L.; Taillandier, P.; Wilhem, A.M.; Delmas, H.; Strehaiano, P. Low frequency thermo-ultrasonication of Saccharomyces cerevisiae suspensions: Effect of temperature and of ultrasonic power. Chem. Eng. J. 1997, 65, 145–149. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, G.; Wyzgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plants pecies. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Method Number: 942.15; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Shukla, S.; Mehta, A.; Mehta, P.; Bajpai, V.K. Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Exp. Toxicol. Pathol. 2012, 64, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wines; John Wiley and Sons: New York, NY, USA, 1988. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant Activity and Polyphenols of Aronia in Comparison to other Berry Species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- SAS/STAT. SAS Software; Version 9.3; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
Sample Availability: Samples B1–B7 and C1–C6 are available from the authors. |
| Treatment | Density (g cm−3) p ≤ 0.0001 | TSS (%) p ≤ 0.0001 | TA (%) p ≤ 0.0001 | pH NS |
|---|---|---|---|---|
| Control Sample | ||||
| A | 1.0490 g ± 0.001 | 12.89 f ± 0.01 | 15.52 f ± 0.04 | 3.35 ± 0.01 |
| Classic Extraction | ||||
| B1 | 1.0530 f ± 0.001 | 13.80 e ± 0.01 | 17.31 de ± 0.05 | 2.89 ± 0.70 |
| B2 | 1.0527 f ± 0.001 | 13.73 e ± 0.01 | 17.10 e ± 0.07 | 3.37 ± 0.01 |
| B3 | 1.0539 e ± 0.001 | 13.99 d ± 0.01 | 17.29 de ± 0.19 | 3.38 ± 0.01 |
| B4 | 1.0539 e ± 0.001 | 14.02 cd ± 0.01 | 17.52 cd ± 0.24 | 3.38 ± 0.01 |
| B5 | 1.0547 abc ± 0.001 | 14.22 a ± 0.01 | 17.70 abc ± 0.01 | 3.37 ± 0.01 |
| B6 | 1.0543 cde ± 0.001 | 14.12 abcd ± 0.01 | 17.54 bcd ± 0.22 | 3.38 ± 0.01 |
| B7 | 1.0540 de ± 0.001 | 14.06 bcd ± 0.01 | 17.65 abc ± 0.06 | 3.38 ± 0.01 |
| Ultrasonic Extraction | ||||
| C1 | 1.0532 f ± 0.001 | 13.75 e ± 0.12 | 17.29 de ± 0.01 | 3.30 ± 0.03 |
| C2 | 1.0541 de ± 0.001 | 14.01 d ± 0.08 | 17.66 abc ± 0.04 | 3.37 ± 0.01 |
| C3 | 1.0545 abcd ± 0.001 | 14.09 abcd ± 0.08 | 17.71 abc ± 0.08 | 3.37 ± 0.01 |
| C4 | 1.0543 bcde ± 0.001 | 14.06 bcd ± 0.02 | 17.59 abcd ± 0.14 | 3.37 ± 0.01 |
| C5 | 1.0548 ab ± 0.001 | 14.16 abc ± 0.07 | 17.87 ab ± 0.04 | 3.35 ± 0.01 |
| C6 | 1.0549 a ± 0.001 | 14.20 ab ± 0.04 | 17.93 a ± 0.05 | 3.37 ± 0.02 |
| Treatment | Vitamin C (mg 100 g−1) p ≤ 0.0001 | TPC (mg L−1) p ≤ 0.0001 | TFC (mg L−1) p ≤ 0.0001 | TAC (mg L−1) p ≤ 0.0001 | Antioxidant Capacity (µmol TE L−1) p ≤ 0.0001 |
|---|---|---|---|---|---|
| Control Sample | |||||
| A | 13.16 i ± 0.88 | 512.64 k ± 0.09 | 205.89 m ± 0.40 | ND | 2164.54 e ± 0.01 |
| Classic Extraction | |||||
| B1 | 12.83 i ± 0.86 | 828.04 i ± 0.70 | 328.99 l ± 0.91 | 687.88 h ± 3.04 | 2208.62 cd ± 0.03 |
| B2 | 15.5 hi ± 1.68 | 829.23 i ± 0.51 | 360.47 i ± 0.59 | 714.02 gh ± 0.87 | 2213.79 bcd ± 0.01 |
| B3 | 17.46 gh ± 0.85 | 831.16 hi ± 0.29 | 390.18 f ± 1.16 | 846.25 fg ± 0.87 | 2215.59 bcd ± 0.01 |
| B4 | 20.57 fg ± 5.54 | 838.54 gh ± 0.19 | 350.62 k ± 0.43 | 919.12 ef ± 3.04 | 2228.86 abc ± 0.01 |
| B5 | 22.49 ef ± 4.29 | 840.81 g ± 0.21 | 353.67 j ± 0.95 | 927.42 ef ± 1.73 | 2233.81 abc ± 0.01 |
| B6 | 28.76 d ± 3.39 | 851.30 f ± 0.70 | 381.25 h ± 0.80 | 991.08 e ± 9.13 | 2237.36 abc ± 0.04 |
| B7 | 36.78 bc ± 0.88 | 761.11 j ± 0.80 | 421.22 e ± 4.59 | 1579.94 bc ± 18.26 | 2189.05 de ± 0.01 |
| Ultrasonic Extraction | |||||
| C1 | 15.57 hi ± 0.98 | 861.71 e ± 14.94 | 384.51 g ± 6.41 | 747.23 gh ± 15.65 | 2234.94 abc ± 0.01 |
| C2 | 24.07 ef ± 3.92 | 894.04 d ± 14.64 | 443.01 d ± 1.15 | 997.54 e ± 56.53 | 2235.49 abc ± 0.01 |
| C3 | 25.03 de ± 0.69 | 912.98 c ± 4.19 | 444.08 d ± 6.10 | 1393.90 d ± 0.44 | 2238.24 ab ± 0.01 |
| C4 | 33.03 c ± 0.98 | 934.82 b ± 2.43 | 446.98 c ± 1.81 | 1452.02 cd ± 133.94 | 2239.55 ab ± 0.02 |
| C5 | 40.87 ab ± 1.62 | 934.60 b ± 5.52 | 456.70 b ± 0.99 | 1707.86 ab ± 71.31 | 2241.84 a ± 0.01 |
| C6 | 42.81 a ± 2.71 | 955.03 a ± 7.09 | 482.44 a ± 0.91 | 1823.79 a ± 77.85 | 2250.31 a ± 0.01 |
| Treatment | Cyanidin 3-Galactoside | Cyanidin 3-Glucoside | Cyanidin 3-Arabinoside | Cyanidin 3-Xyloside | Chlorogenic Acid | Epicatechin | Quercetin | Myricetin |
|---|---|---|---|---|---|---|---|---|
| p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | |
| Control Sample | ||||||||
| A | ND | ND | ND | ND | 6.23 k | 0.03 h | 0.04 l | ND |
| Classic Extraction | ||||||||
| B1 | 34.34 m | 2.10 i | 4.37 l | 2.30 h | 6.47 j | 0.05 h | 0.08 k | 0.80 d |
| B2 | 36.32 l | 2.10 i | 4.37 l | 2.30 h | 6.47 j | 1.22 g | 0.28 j | 0.80 d |
| B3 | 44.84 j | 2.22 h | 5.54 j | 2.61 g | 7.71 i | 1.22 g | 0.58 h | 0.92 c |
| B4 | 47.67 i | 2.34 g | 6.12 i | 2.61 g | 7.71 i | 1.22 g | 1.18 g | 0.92 c |
| B5 | 49.09 h | 2.34 g | 6.26 h | 2.92 f | 8.34 h | 2.39 f | 1.28 f | 0.92 c |
| B6 | 55.62 g | 2.58 f | 7.28 g | 3.22 e | 8.96 g | 2.99 e | 1.38 e | 1.04 b |
| B7 | 93.93 c | 3.42 b | 12.67 c | 5.07 b | 13.33 d | 3.58 d | 2.18 c | 1.16 a |
| Ultrasonic Extraction | ||||||||
| C1 | 42.56 k | 2.22 h | 5.09 k | 2.30 h | 6.47 j | 0.05 h | 0.08 k | 0.80 d |
| C2 | 65.27 f | 2.70 e | 8.45 f | 3.53 d | 10.83 f | 2.99 e | 0.39 i | 0.80 d |
| C3 | 85.98 e | 3.18 d | 11.51 e | 4.45 c | 12.08 e | 2.99 e | 1.78 d | 0.92 c |
| C4 | 90.81 d | 3.30 c | 12.53 d | 5.07 b | 13.95 c | 4.16 c | 2.58 b | 0.92 c |
| C5 | 110.39 b | 3.78 a | 15.44 b | 5.99 a | 15.82 b | 5.34 b | 2.58 b | 1.04 b |
| C6 | 111.52 a | 3.78 a | 15.73 a | 5.99 a | 17.07 a | 5.93 a | 2.68 a | 1.04 b |
| Chemical Parameter | Correlation Coefficient (r) |
|---|---|
| Classic Extraction | |
| Vitamin C | −0.22 NS |
| Total phenols | 0.90 *** |
| Total flavonoids | −0.44 NS |
| Total anthocyanins | −0.48 NS |
| Ultrasonic Extraction | |
| Vitamin C | 0.83 * |
| Total phenols | 0.82 * |
| Total flavonoids | 0.79 * |
| Total anthocyanins | 0.86 * |
| Extraction Method | Solvent | Solvent Volume (mL) | Time | Ultrasonic Bath | Sample |
|---|---|---|---|---|---|
| Classic | Apple juice | 100 | 5 min | - | B1 |
| Classic | Apple juice | 100 | 10 min | - | B2 |
| Classic | Apple juice | 100 | 15 min | - | B3 |
| Classic | Apple juice | 100 | 20 min | - | B4 |
| Classic | Apple juice | 100 | 25 min | - | B5 |
| Classic | Apple juice | 100 | 30 min | - | B6 |
| Classic | Apple juice | 100 | 24 h | - | B7 |
| UAE | Apple juice | 100 | 5 min | 35 kHz 140 W | C1 |
| UAE | Apple juice | 100 | 10 min | 35 kHz 140 W | C2 |
| UAE | Apple juice | 100 | 15 min | 35 kHz 140 W | C3 |
| UAE | Apple juice | 100 | 20 min | 35 kHz 140 W | C4 |
| UAE | Apple juice | 100 | 25 min | 35 kHz 140 W | C5 |
| UAE | Apple juice | 100 | 30 min | 35 kHz 140 W | C6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šic Žlabur, J.; Dobričević, N.; Pliestić, S.; Galić, A.; Bilić, D.P.; Voća, S. Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa). Molecules 2017, 22, 2158. https://doi.org/10.3390/molecules22122158
Šic Žlabur J, Dobričević N, Pliestić S, Galić A, Bilić DP, Voća S. Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa). Molecules. 2017; 22(12):2158. https://doi.org/10.3390/molecules22122158
Chicago/Turabian StyleŠic Žlabur, Jana, Nadica Dobričević, Stjepan Pliestić, Ante Galić, Daniela Patricia Bilić, and Sandra Voća. 2017. "Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa)" Molecules 22, no. 12: 2158. https://doi.org/10.3390/molecules22122158






