Recyclable Magnetic Titania Nanocomposite from Ilmenite with Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
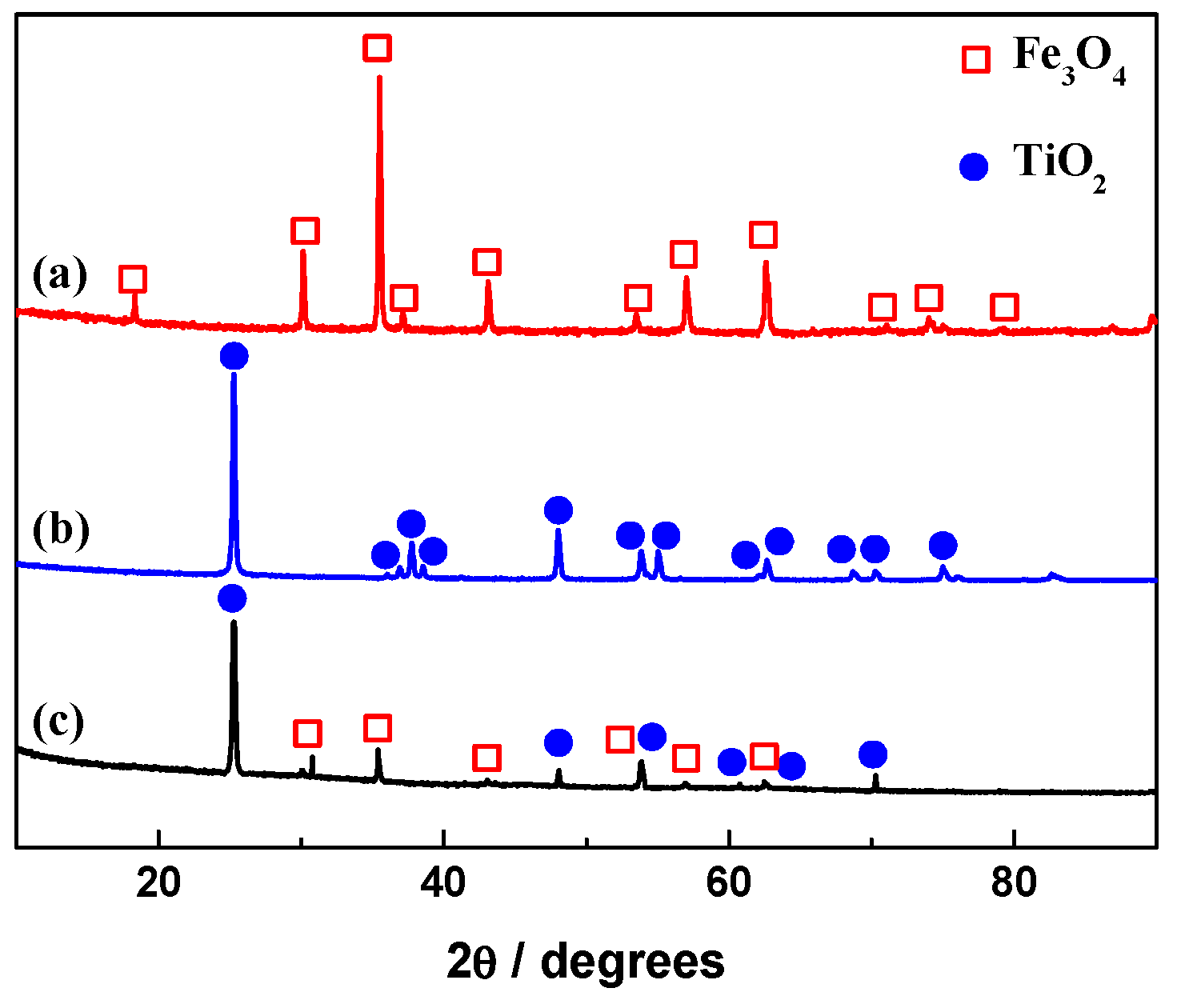
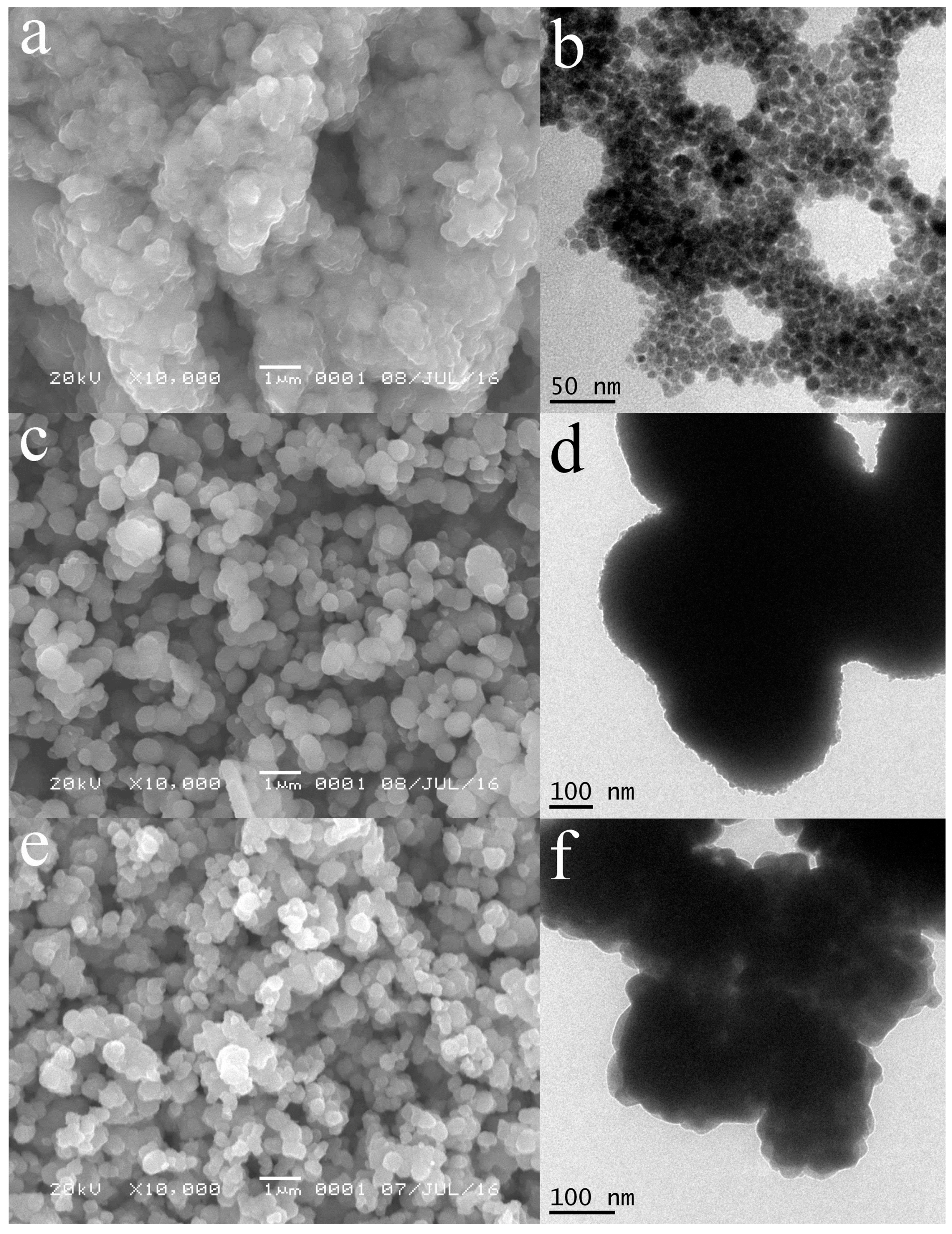
2.1. Morphologies and Structure Analysis
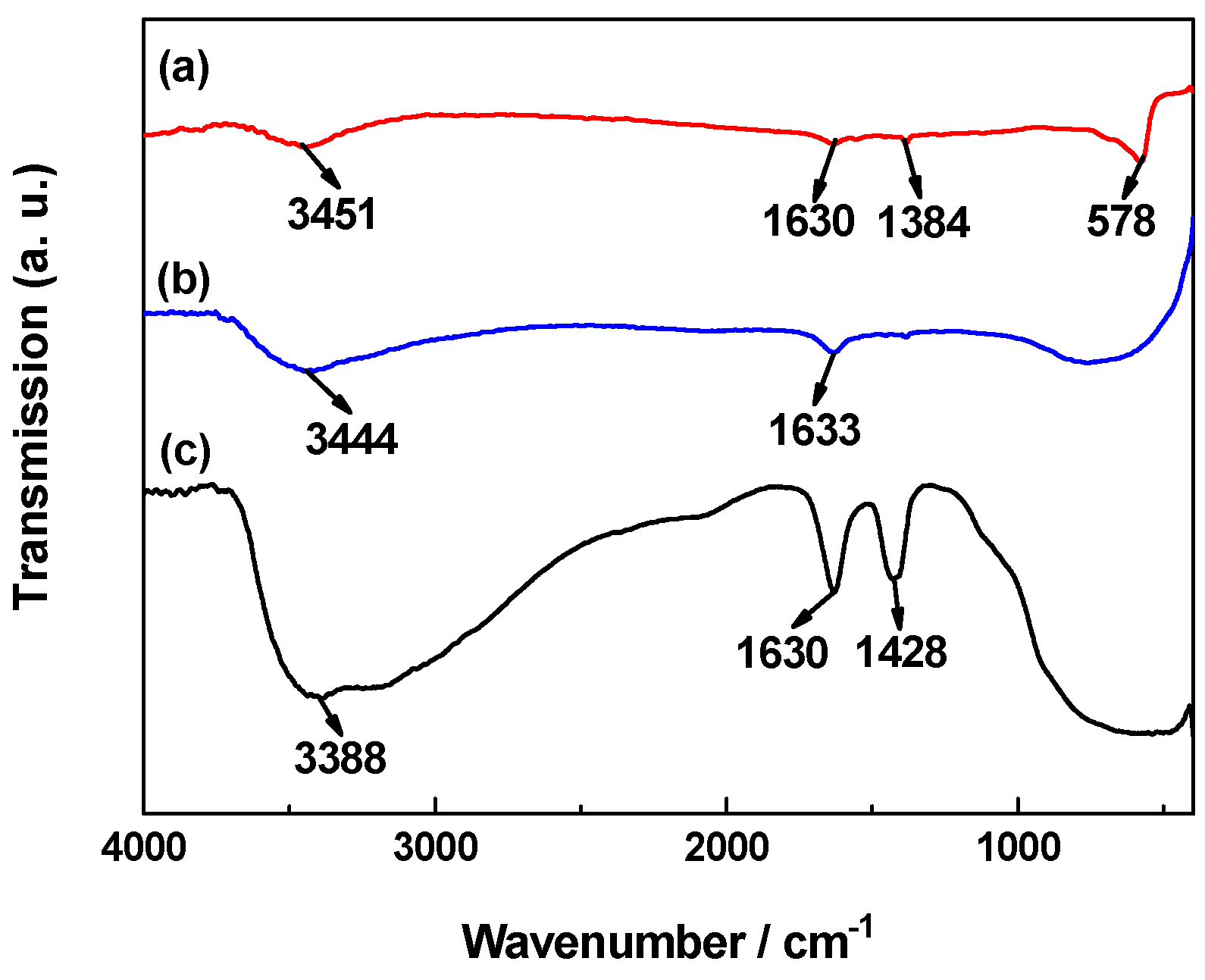
2.2. FTIR Analysis
2.3. UV-Vis Analysis
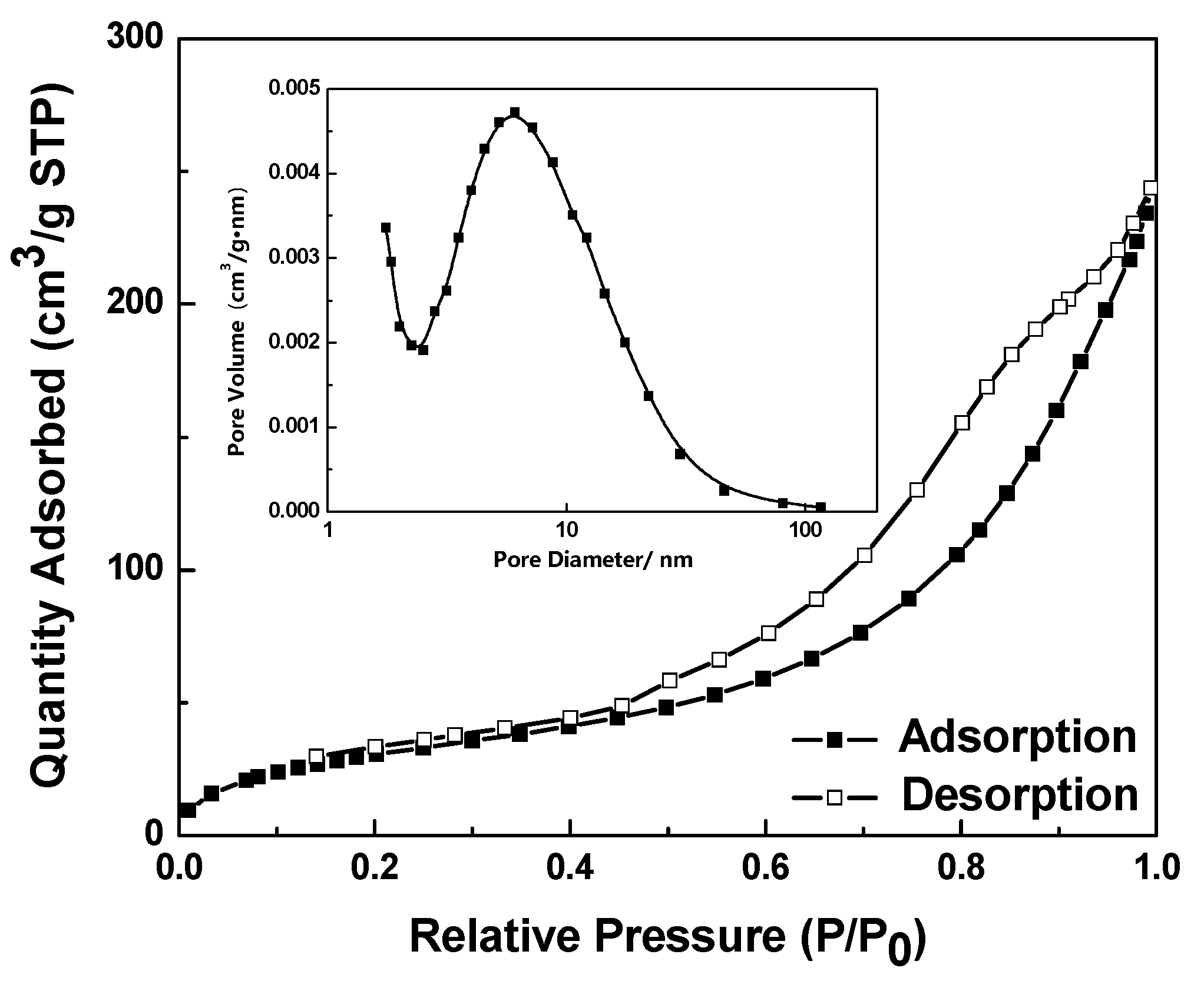
2.4. Nitrogen Adsorption–Desorption Isotherms
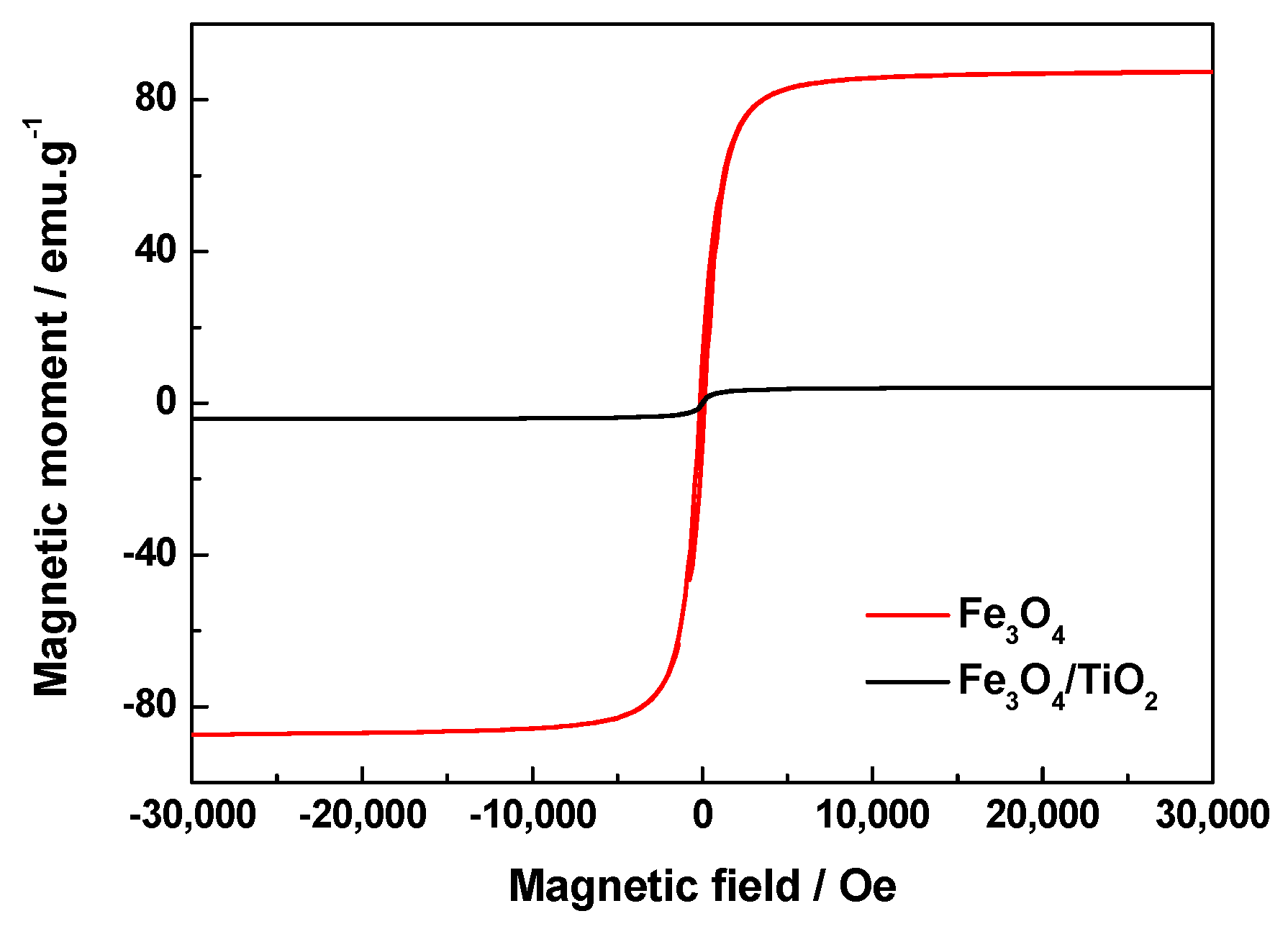
2.5. Magnetic Properties
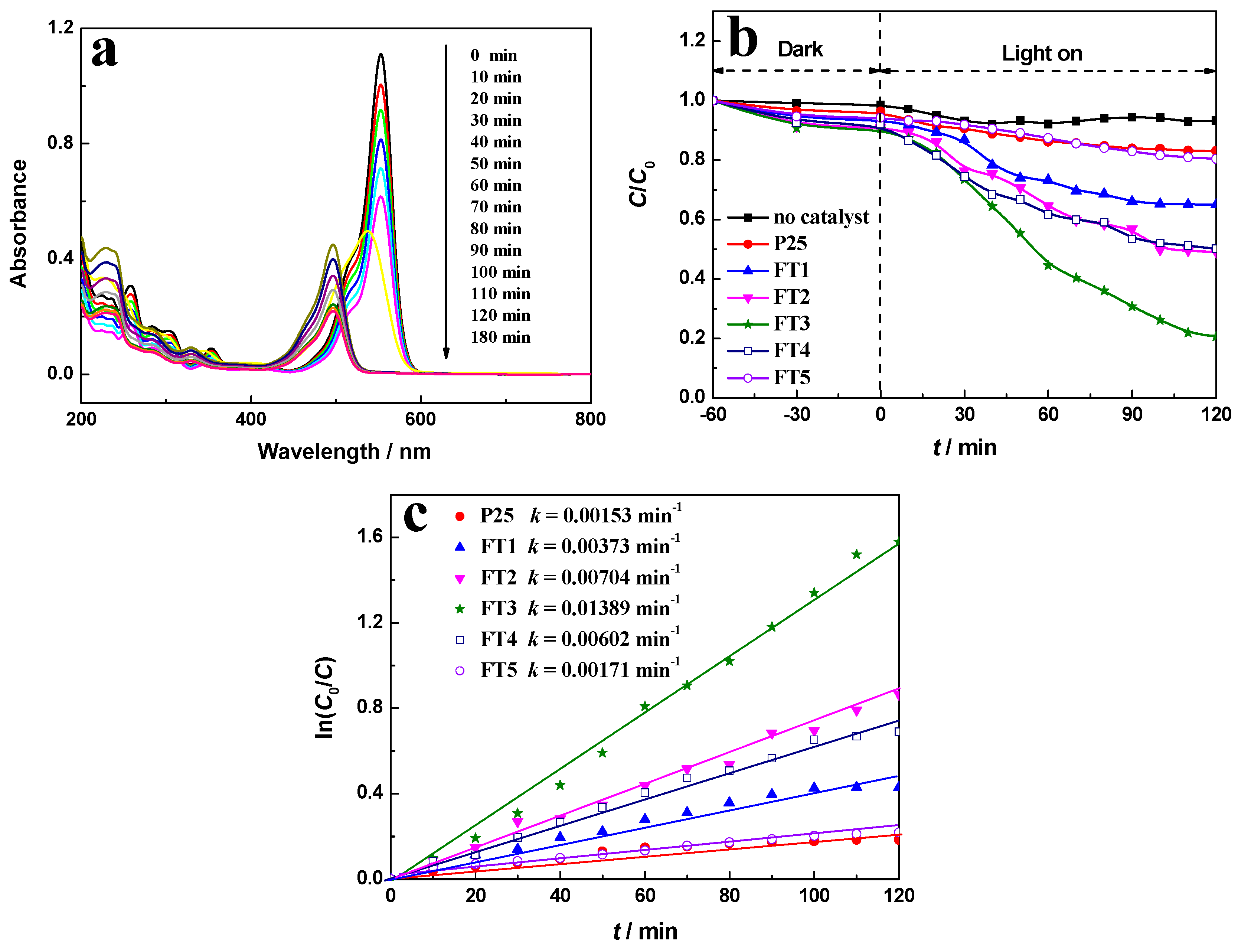
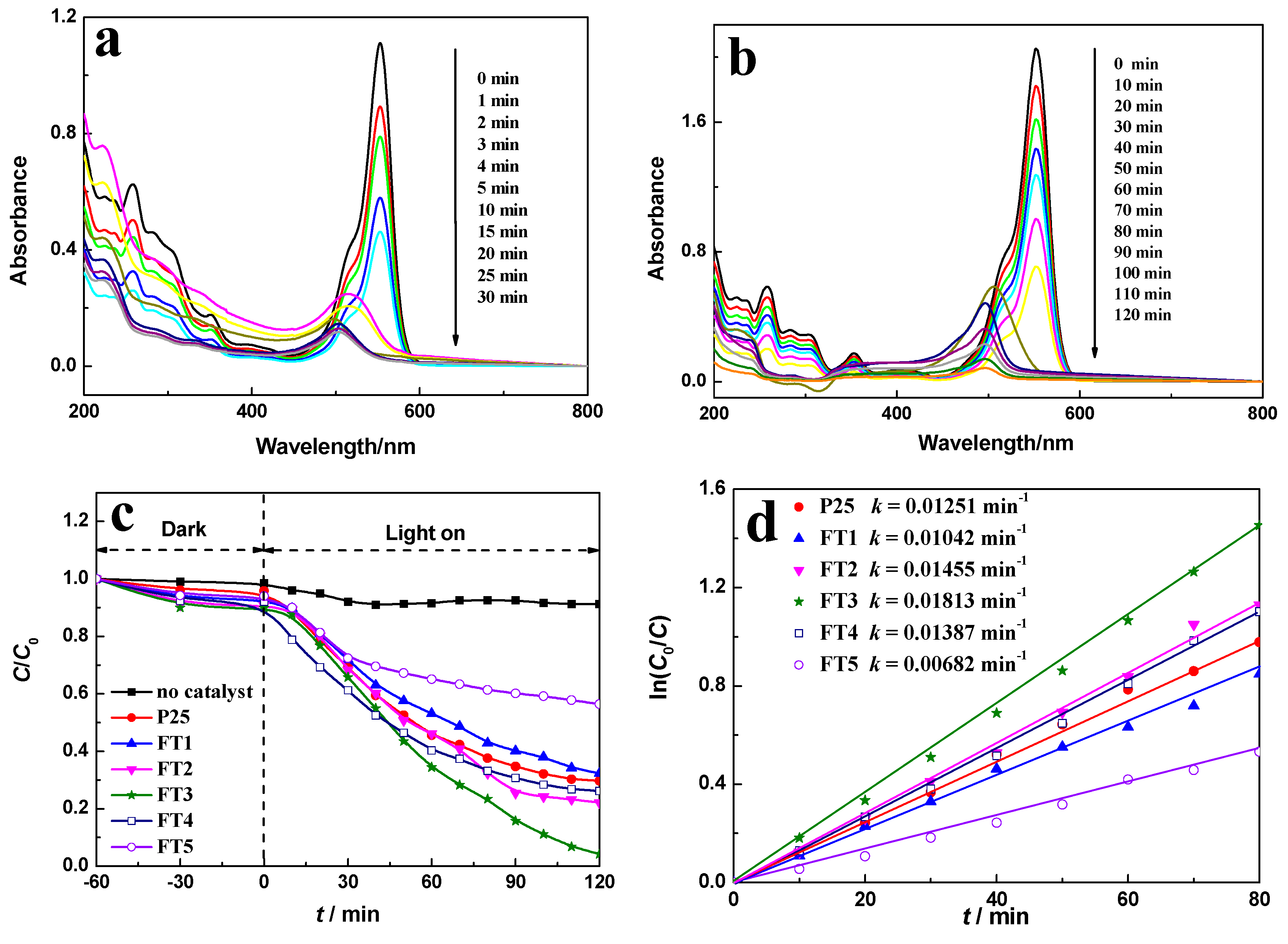
2.6. Photocatalytic Performance
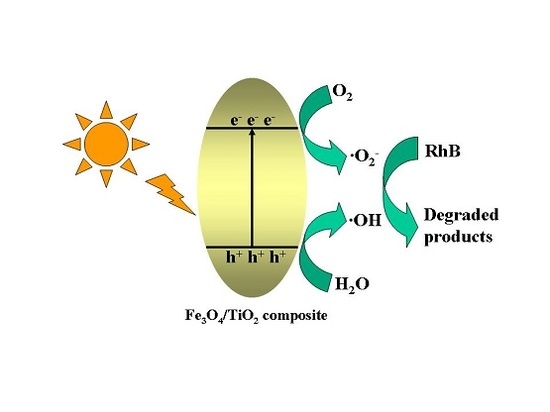
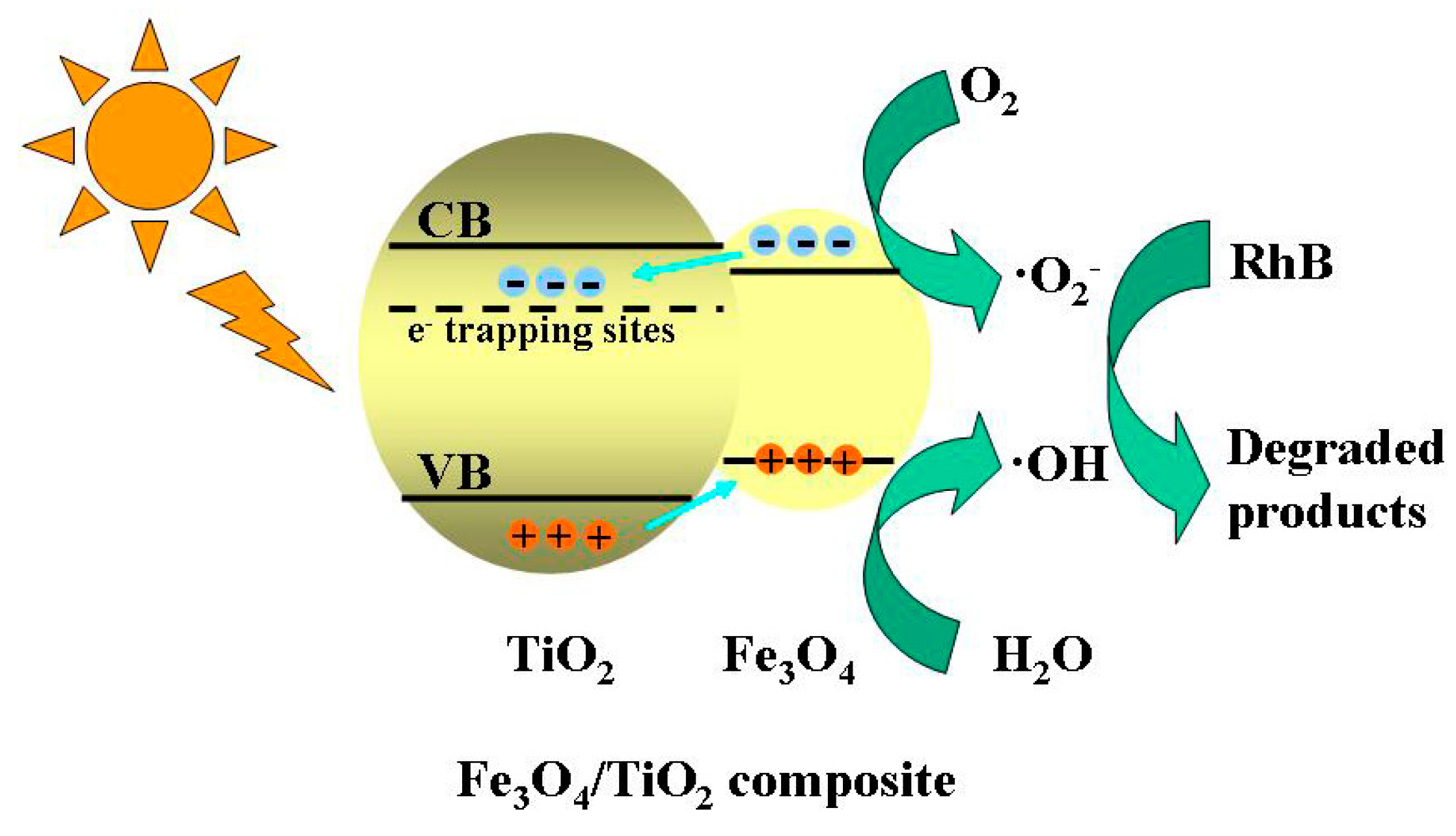
2.7. Proposed Photocatalytic Degradation Mechanism
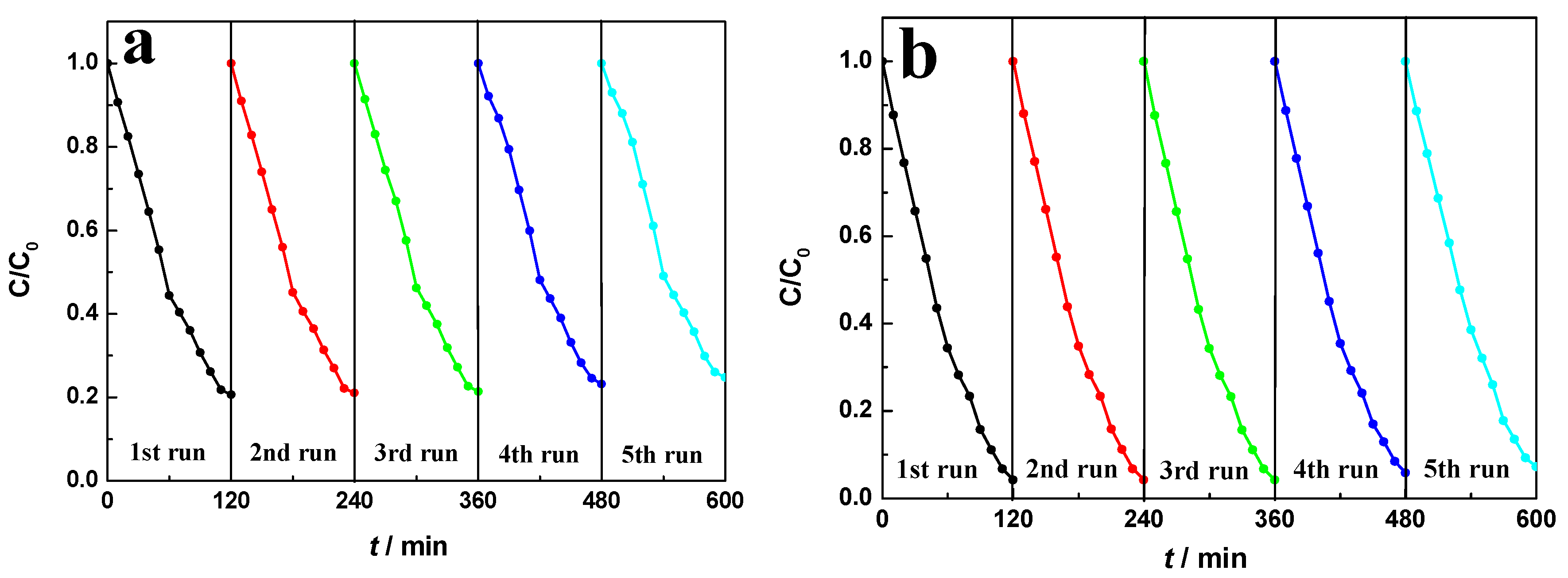
2.8. Magnetic Recyclability
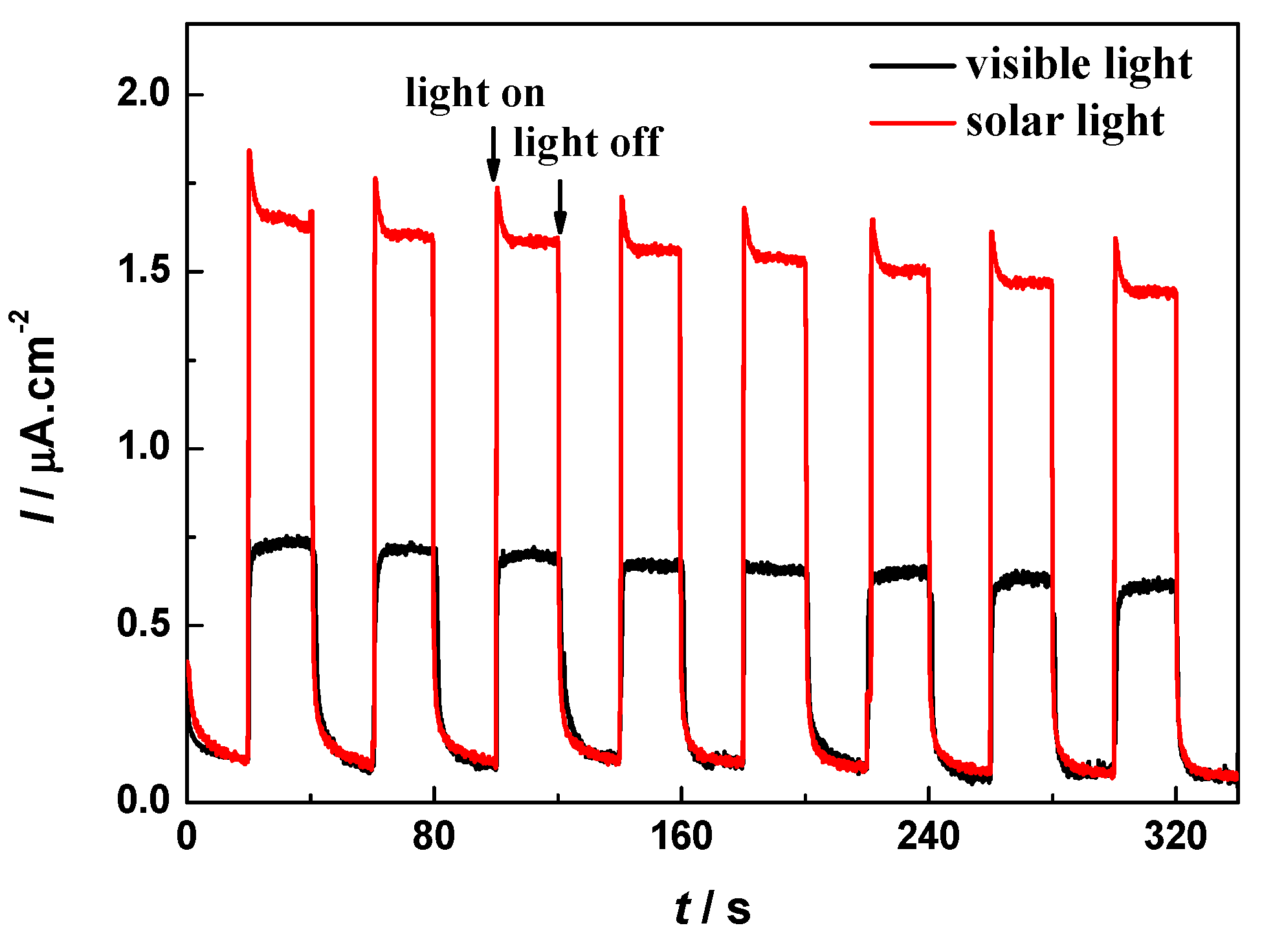
2.9. Photocurrent Responses
3. Materials and Methods
3.1. Materials
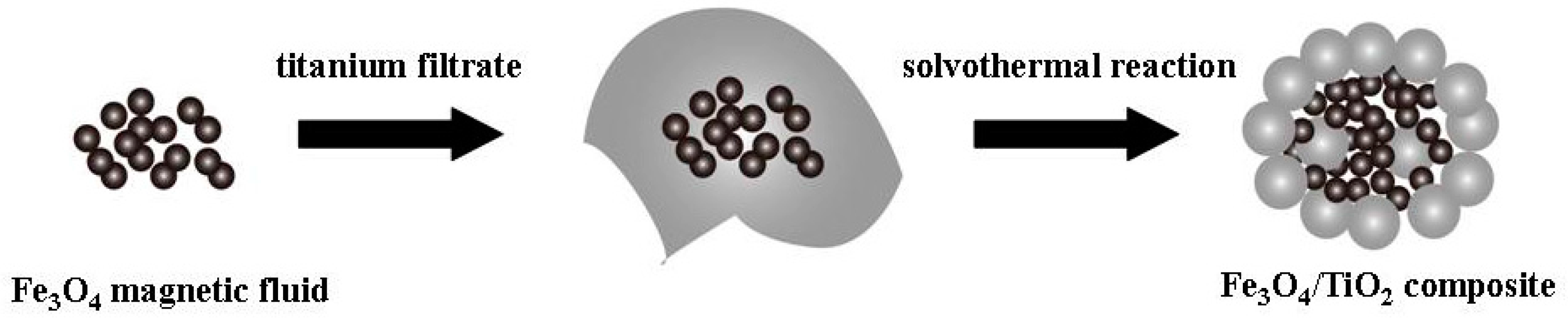
3.2. Synthesis
3.3. Characterization
3.4. Photocatalytic Activity
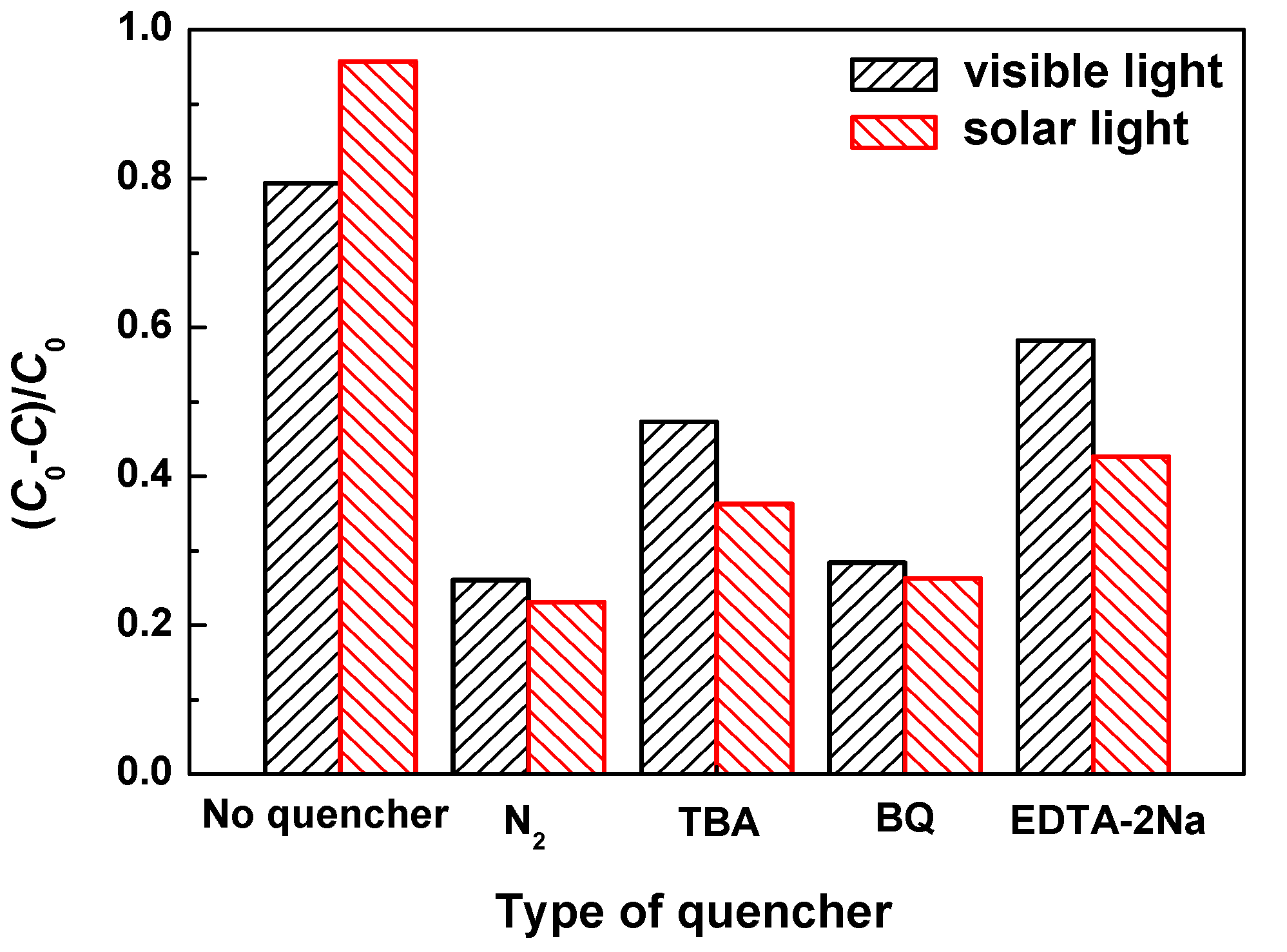
3.5. Trapping Experiments of Active Species
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Velegraki, T.; Hapeshi, E.; Fatta-kassinos, D.; Poulios, L. Solar-induced heterogeneous photocatalytic degradation of methyl-paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Versatile graphene-promoting photocatalytic performance of semiconductors: Basic principles, synthesis, solar energy conversion, and environmental applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Liu, C.; Dong, X.; Hao, Y.; Wang, X.; Ma, H.; Zhang, X. Efficient photocatalytic dye degradation over Er-doped BiOBr hollow microspheres wrapped with graphene nanosheets: Enhanced solar energy harvesting and charge separation. RSC Adv. 2017, 7, 22415–22423. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakrabarty, S.; Pal, T.; Ghosh, S. Synergistic effect of zinc selenide-reduced graphene oxide towards enhanced solar light-responsive photocurrent generation and photocatalytic 4-nitrophenol degradation. New J. Chem. 2017, 41, 4662–4671. [Google Scholar] [CrossRef]
- An, H.-R.; Park, S.Y.; Huh, J.Y.; Kim, H.; Lee, Y.-C.; Lee, Y.B.; Hong, Y.C.; Lee, H.U. Nanoporous hydrogenated TiO2 photocatalysts generated by underwater discharge plasma treatment for solar photocatalytic applications. Appl. Catal. B Environ. 2017, 211, 126–136. [Google Scholar] [CrossRef]
- Sricharan, K.; Jang, E.; Park, T.J. Deformation assisted fabrication of uniform spindle, tube and rod shaped nanoscale 3D TiO2 architectures and their photocatalytic activity. CrystEngComm 2013, 15, 8241–8245. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, X.-X.; Su, J.; Wang, P.-P.; Zhou, L.-J.; Li, G.-D. Synthesis and photocatalytic activity of porous anatase TiO2 microspheres composed of {010}-faceted nanobelts. Dalton Trans. 2013, 42, 4365–4368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.-J.; Song, Y.; Sun, Z. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chem. Commun. 2014, 50, 2755–2757. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.H.; Amal, R.; Beydoun, D. Photodeposition of CdSe using Se-TiO2 suspensions as photocatalysts. J. Photochem. Photobiol. A 2006, 179, 57–65. [Google Scholar] [CrossRef]
- Chen, J.Y.; Qian, Y.; Wei, X.Z. Comparison of magnetic-nanometer titanium dioxide/ferriferous oxide (TiO2/Fe3O4) composite photocatalyst prepared by acid-sol and homogeneous precipitation method. J. Mater. Sci. 2010, 45, 6016–6024. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide based nanomaterials: Systhesis, characteristics, and photocatalytic application in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, C.; Xiao, G.; Wei, G.; Li, L.; Liu, C.; Su, H. Controlled synthesis and photocatalysis of sea unchin-like Fe3O4@TiO2@Ag nanocomposites. Nanoscale 2016, 8, 5313–5326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, F.; Dong, W.; Xu, L.; Wu, K.; Xu, G.; Chen, W. Recyclable silver-decorated magnetic Titania nanocomposite with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2016, 189, 192–198. [Google Scholar] [CrossRef]
- Aziz, A.B.; Cheng, C.K.; Ibrahim, S.; Matheswaran, M.; Saravanan, P. Visible light improved, photocatalytic activity of magnetically separable Titania nanocomposite. Chem. Eng. J. 2012, 183, 349–356. [Google Scholar] [CrossRef]
- Haw, C.; Chiu, W.; Rahman, S.A.; Khiew, P.; Radiman, S.; Shukor, R.A.; Hamid, M.Z.Z.; Ghazali, N. The design of new magnetic-photocatalyst nanocomposites (CoFe2O4-TiO2) as smart nanomaterials for recyclable-photocatalysis applications. New J. Chem. 2016, 40, 1124–1136. [Google Scholar] [CrossRef]
- Salamat, S.; Younesi, H.; Bahramifar, N. Synthesis of magnetic core-shell Fe3O4@TiO2 nanoparticles from electric arc furnace dust for photocatalytic degradation of steel mill wastewater. RSC Adv. 2017, 7, 19391–19405. [Google Scholar] [CrossRef]
- Rtimi, S.; Robyr, M.; Pulgarin, C.; Lavanchy, J.C.; Kiwi, J. A new perspective in the use of FeOx-TiO2 photocatalytic films: Indole degradation in the absence of Fe-leaching. J. Catal. 2016, 342, 184–192. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, H.; Zhu, Y.; Lin, Y.; Liu, P. High visible-light photocatalytic activity of γ-Fe2O3/TiO2 nanotube heterojunction arrays. Rare Met. Mater. Eng. 2016, 45, 1117–1121. [Google Scholar]
- Mangayayam, M.; Kiwi, J.; Giannakis, S.; Pulgarin, C.; Zivkovic, I.; Magrez, A.; Rtimi, S. FeOx magnetization enhancing E. coli inactivation by orders of magnitude on Ag-TiO2 nanotubes under sunlight. Appl. Catal. B Environ. 2017, 202, 438–445. [Google Scholar] [CrossRef]
- Tarigh, G.D.; Shemirani, F.; Maz’hari, N.S. Fabrication of a reusable magnetic multi-walled carbon nanotube-TiO2 nanocomposite by electrostatic adsorption: Enhanced photodegradation of malachite green. RSC Adv. 2015, 5, 35070–35079. [Google Scholar] [CrossRef]
- Khashan, S.; Dagher, S.; Tit, N.; Alazzam, A.; Obaidat, I. Novel method for synthesis of Fe3O4@TiO2 core/shell nanoparticles. Surf. Coat. Technol. 2017, 322, 92–98. [Google Scholar] [CrossRef]
- Li, Z.-D.; Wang, H.-L.; Wei, X.-N.; Liu, X.-Y.; Yang, Y.-F.; Jiang, W.-F. Preparation and photocatalytic performance of magnetic Fe3O4@TiO2 core-shell microspheres supported by silica aerogels from industrial fly ash. J. Alloys Compd. 2016, 659, 240–247. [Google Scholar] [CrossRef]
- Yeh, N.; Lee, Y.C.; Chang, C.Y.; Cheng, T.C. Anti-fish bacterial pathogen effect of visible light responsive Fe3O4@TiO2 nanoparticles immobilized on glass using TiO2 sol-gel. Thin Solid Films 2013, 549, 93–97. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, C.; Yang, S.; Ding, Y.; He, H.; Wang, Z. Photocatalytic degradation of rhodamine B by Bi2WO6 with electron accepting agent under microwave irradiation: Mechanism and pathway. J. Hazard. Mater. 2009, 162, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S. TiO2 nanosheets with exposed {001} faces for photocatalytic applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, L.-B.; Zhao, Q.-B.; Frear, C.; Zheng, Y.-M. Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 14573–14583. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-S.; Wang, D.-H.; Qu, W.-G.; Lu, L.-Q.; Xu, A.-W. Large ultrathin anatase TiO2 nanosheets with exposed {001} facets on graphene for enhanced visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J.; Xie, F. An amorphous TiO2 sol sensitized with H2O2 with the enhancement of photocatalytic activity. J. Alloys Compd. 2010, 497, 420–427. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Lin, H.-M.; Lai, C.-L. Photo reduction of CO2 to methanol using optical-fiber photoreacor. Appl. Catal. A Gen. 2005, 296, 194–200. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Nitrogen/palladium-codoped TiO2 for efficient visible light photocatalytic dye degradation. J. Phys. Chem. C 2011, 115, 22110–22120. [Google Scholar] [CrossRef]
- Alam, J.; Riaz, U.; Ahmad, S. Effect of ferrofluid concentration on electrical and magnetic properties of the Fe3O4/PANI nanocomposites. J. Magn. Magn. Mater. 2007, 314, 93–99. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Occurrence and prevention of photodissolution at the phase junction of magnetite and titanium dioxide. J. Mol. Catal. A Chem. 2002, 180, 193–200. [Google Scholar] [CrossRef]
- Hameed, A.; Gombac, V.; Montini, T.; Graziani, M.; Fornasiero, P. Synthesis, characterization and photocatalytic activity of NiO-Bi2O3 nanocomposites. Chem. Phys. Lett. 2009, 472, 212–216. [Google Scholar] [CrossRef]
- Khojasteh, H.; Salavati-Niasari, M.; Mazhari, M.-P.; Hamadanian, M. Preparation and characterization of Fe3O4@SiO2@TiO2@Pd and Fe3O4@SiO2@TiO2@Pd-Ag nanocomposites and their utilization in enhanced degradation systems and rapid magnetic separation. RSC Adv. 2016, 6, 78043–78052. [Google Scholar] [CrossRef]
- Mortazavi-Derazkola, S.; Salavati-Niasari, M.; Mazhari, M.-P.; Khojasteh, H.; Hamadanian, M.; Bagheri, S. Magnetically separable Fe3O4@SiO2@TiO2 nanostructures supported by neodymium (III): Fabrication and enhanced photocatalytic activity for degradation of organic pollution. J. Mater. Sci. Mater. Electron. 2017, 28, 14271–14281. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, G.; Wu, J.; Li, D.; Liu, Z. Study on enhanced photocatalytic activity of magnetically recoverable Fe3O4@C@TiO2 nanocomposites with core-shell nanostructure. Opt. Mater. 2015, 46, 52–58. [Google Scholar] [CrossRef]
- Tang, W.Z.; An, H. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere 1995, 31, 4157–4170. [Google Scholar] [CrossRef]
- Arabatzis, I.M.; Stergiopoulos, T.; Andreeva, D.; Kitova, S.; Neophytides, S.G.; Falaras, P. Characterization and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Li, J.; Luo, D.; Yang, C.; He, S.; Chen, S.; Lin, J.; Zhu, L.; Li, X. Copper(II) imidazolate frameworks as highly efficient photocatalysts for reduction of CO2 into methanol under visible light irradiation. J. Solid State Chem. 2013, 203, 154–159. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Liu, Z.; Chen, J.; Wu, Y.; Guo, M.; Na, P. In-situ deposition of Ag3PO4 on TiO2 nanosheets dominated by (001) facets for enhanced photocatalytic activities and recyclability. Ceram. Int. 2017, 43, 11588–11595. [Google Scholar] [CrossRef]
Sample Availability: Samples of the magnetic titania nanocomposites (FT1, FT2, FT3, FT4 and FT5) are available from the authors. |
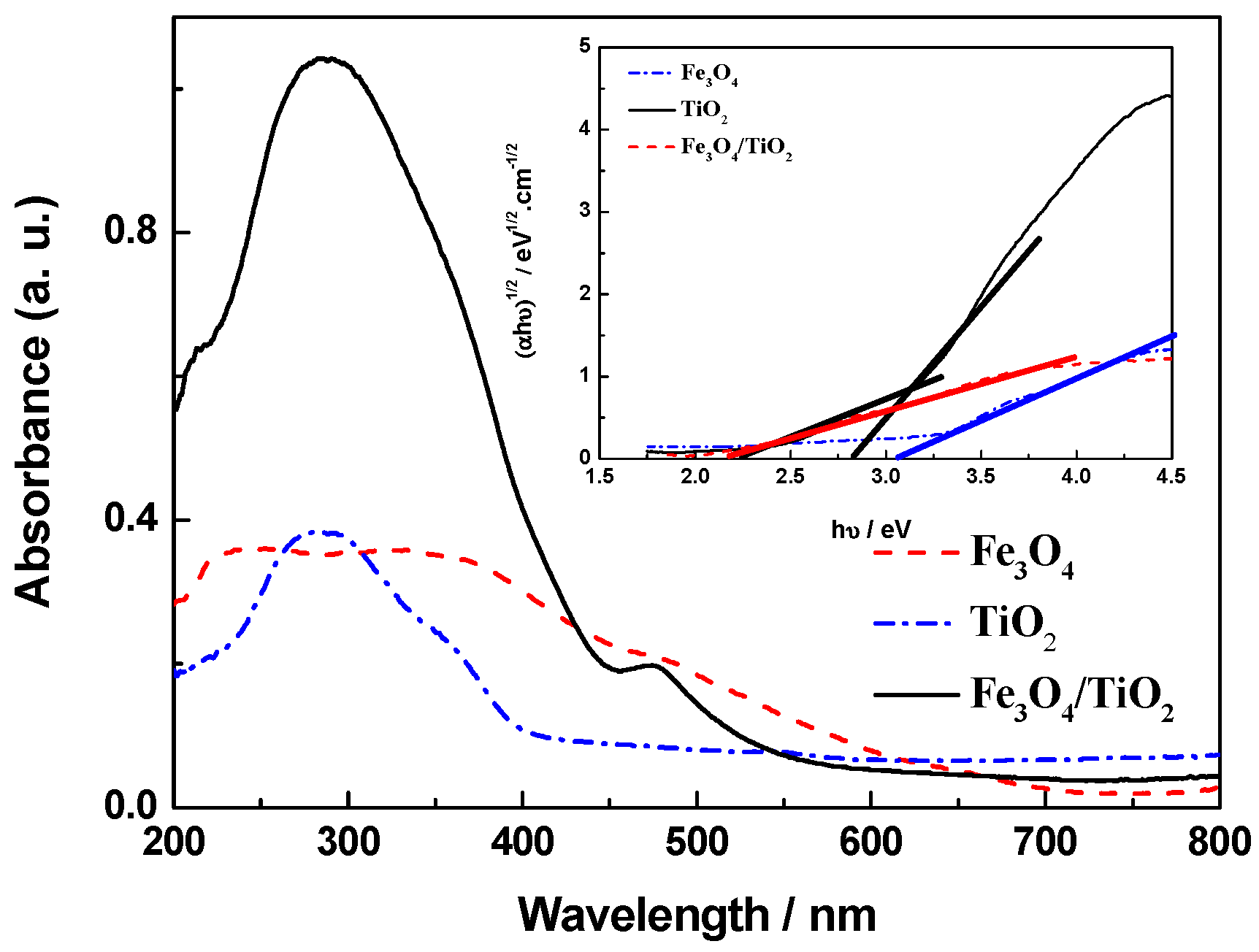
| Samples | Band Gap/eV |
|---|---|
| Fe3O4 magnetic powder | 2.17 |
| TiO2 | 3.07 |
| Fe3O4/TiO2 nanocomposite | 2.24 (for Fe3O4) 2.82 (for TiO2) |
| Samples | Fe3O4 Magnetic Fluid/mL | Fe3O4 Content/wt % | TiO2 Content/wt % |
|---|---|---|---|
| FT1 | 5 | 17.87 | 82.19 |
| FT2 | 10 | 30.02 | 69.98 |
| FT3 | 15 | 39.88 | 60.12 |
| FT4 | 20 | 47.68 | 52.32 |
| FT5 | 30 | 59.92 | 40.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, T.; Mao, J.; Tao, F.; Lan, M. Recyclable Magnetic Titania Nanocomposite from Ilmenite with Enhanced Photocatalytic Activity. Molecules 2017, 22, 2044. https://doi.org/10.3390/molecules22122044
Hong T, Mao J, Tao F, Lan M. Recyclable Magnetic Titania Nanocomposite from Ilmenite with Enhanced Photocatalytic Activity. Molecules. 2017; 22(12):2044. https://doi.org/10.3390/molecules22122044
Chicago/Turabian StyleHong, Tianjie, Jun Mao, Feifei Tao, and Mingxuan Lan. 2017. "Recyclable Magnetic Titania Nanocomposite from Ilmenite with Enhanced Photocatalytic Activity" Molecules 22, no. 12: 2044. https://doi.org/10.3390/molecules22122044