Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acid Composition of Samples
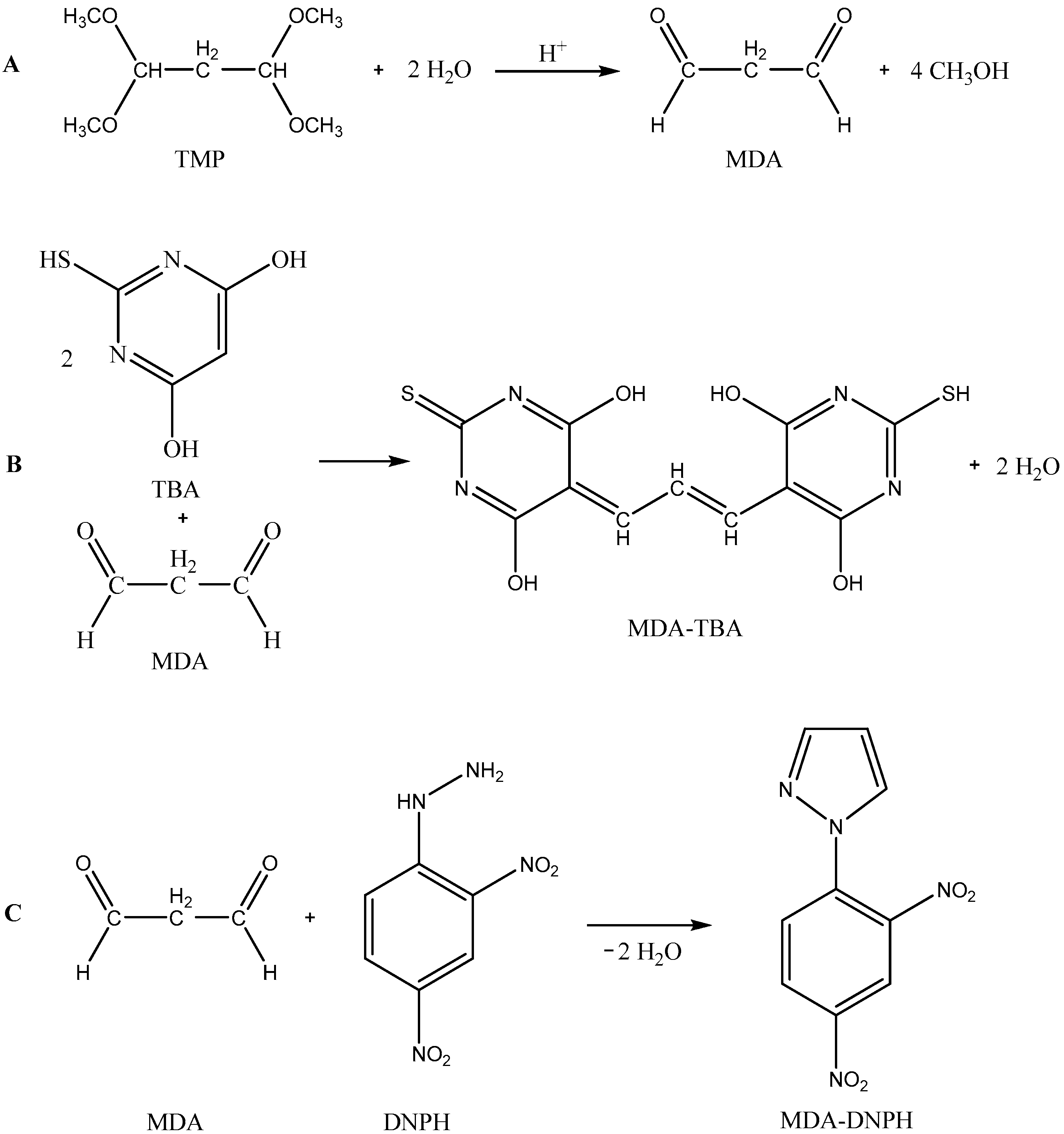
2.2. Results of Malondialdehyde Determinations
3. Materials and Methods
3.1. Fatty Acid Composition
3.2. Chemicals
3.3. Meat and Meat Product Samples
3.4. Determination of Malondialdehyde by the 2-Thiobarbituric Spectrophotometric Method
3.4.1. Preparation of Stock Solution and Calibration Curve
3.4.2. Sample Preparation and Measurement
3.5. Determination of Malondialdehyde by Reverse-Phase High-Performance Liquid Chromatography
3.5.1. Standard Preparation
3.5.2. Derivatization Reagent Preparation
3.5.3. Sample Preparation
3.5.4. Determination of MDA-DNPH by RP-HPLC
3.5.5. Chromatographic Analysis
3.6. Statistical Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruban, S.W. Lipid peroxidation in muscle foods—An overview. Glob. Vet. 2009, 3, 509–513. [Google Scholar]
- Püssa, T.; Raudsepp, P.; Toomik, P.; Pällin, R.; Mäeorg, U.; Kuusik, S.; Soidla, R.; Rei, M.A. Study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J. Food Compost. Anal. 2009, 22, 307–314. [Google Scholar] [CrossRef]
- Bigolin, J.; Weber, C.; Alfaro, A. Lipid oxidation in mechanically deboned chicken meat: Effect of the addition of different agents. Food Nutr. Sci. 2013, 4, 219–223. [Google Scholar] [CrossRef]
- Trindade, M.A.; Felício, P.E.; Castillo, C.J.C. Mechanically separated meat of broiler breeder and white layer spent hens. Sci. Agric. 2004, 61, 234–239. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 1–15. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Remacle, J. Cytotoxicity of linoleic acid peroxide, malondialdehyde and 4-hydroxynonenal towards human fibroblasts. Toxicology 1991, 66, 225–234. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Okolie, N.P.; Akioyamen, M.O.; Okpoba, N.; Okonkwo, C. Malondialdehyde levels of frozen fish, chicken and turkey on sale in Benin City markets. Afr. J. Biotechnol. 2009, 8, 6638–6640. [Google Scholar]
- Czauderna, M.; Kowalczyk, J.; Marounek, M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J. Chromatogr. B 2011, 879, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Park, P.W. Toxic compounds derived from lipids. In Analyzing Foods for Nutrition Labeling and Hazardous Contaminants; Jeon, I.J., Ikins, W.G., Dekker, M., Eds.; CRC Press LLC: New York, NY, USA, 1995; pp. 363–434. ISBN 9780824793494. [Google Scholar]
- Okolie, N.P.; Okugbo, T.O. A comparative study of malondialdehyde contents of some meat and fish samples processed by different methods. J. Pharm. Sci. Innov. 2013, 2, 26–29. [Google Scholar] [CrossRef]
- Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem. 2009, 112, 1038–1045. [Google Scholar] [CrossRef]
- Bergamo, P.; Fedele, E.; Balestrieri, M.; Abrescia, P.; Ferrara, L. Measurement of malondialdehyde levels in food by high-performance liquid chromatography with fluorometric detection. J. Agric. Food Chem. 1998, 46, 2171–2176. [Google Scholar] [CrossRef]
- Mateos, R.; Lucemberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B 2005, 827, 76–82. [Google Scholar] [CrossRef]
- Marcinčák, S.; Sokol, J.; Turek, P.; Róžaňská, H.; Dičakova, Z.; Máté, D.; Popelka, P.; Korim, P. Comparative evaluation of analytical techniques to quantify malondialdehyde in broiler meat. Bull. Vet. Inst. Pulawy 2003, 47, 491–496. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Fenaille, F.; Mottier, P.; Turesky, R.J.; Ali, S.; Guy, P.A. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J. Chromatogr. A 2001, 921, 237–245. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Cansian, R.L.; Oliveira, D.; Gaio, I.; Saggirato, A.G. Lipid and protein oxidation in the internal part of Italian type salami containing basil essential oil (Ocimum basilicum L.). Ciênc. Technol. Aliment 2011, 31, 436–442. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Marcinčák, S.; Sokol, J.; Turek, P.; Popelka, P.; Nagy, J. Stanovenie malóndialdehydu v bravčovom mase s použitím extrakcie tuhej fáze a HPLC. Chem. Listy 2006, 100, 528–532. [Google Scholar]
- Mielnik, M.B.; Aaby, K.; Rolfsen, K.; Ellekjær, M.R.; Nilsson, A. Quality of comminuted sausages formulated from mechanically deboned poultry meat. Meat Sci. 2002, 61, 73–84. [Google Scholar] [CrossRef]
- Ross, C.F.; Smith, D.M. Use of volatiles as indicators of lipid oxidation in muscle foods. Comp. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef]
- Marcinčák, S.; Sokol, J.; Bystrický, P.; Popelka, P.; Turek, P.; Bhide, M.; Máté, D. Determination of lipid oxidation level in broiler meat by liquid chromatography. J. AOAC Int. 2004, 87, 1148–1152. [Google Scholar] [PubMed]
- Warriss, P.D. Meat Science: An Introductory Text; CABI International: New York, NY, USA, 2000; p. 234. ISBN 0851994245. [Google Scholar]
- Frankel, N.E. Lipid Oxidation, 2nd ed.; The Oily Press: Bridgewater, UK, 2005; p. 488. ISBN 9780953194988. [Google Scholar]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Olsen, E.; Vogt, G.; Ekeberg, D.; Sanbakk, M.; Pettersen, J.; Nilson, A. Analysis of the early stages of lipid oxidation in freeze-stored pork back fat and mechanically recovered poultry meat. J. Agric. Food Chem. 2005, 53, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, V.; Estévez, M.; Ventanas, J.; Ventanas, S. Impact of lipid content and composition on lipid oxidation and protein carbonylation in experimental fermented sausages. Food Chem. 2014, 147, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Čertík, M.; Sláviková, L.; Masrnová, S.; Šajbidor, J. Enhancement of nutritional value of cereals with γ-linolenic acid by fungal solid-state fermentations. Food Technol. Biotechnol. 2006, 44, 75–82. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Mechanically Deboned Chicken Meat | Frankfurter Samples | |||||
|---|---|---|---|---|---|---|
| Fatty Acid (% of Total Fatty Acid) | Low Pressure | High Pressure | p-Value | Low Pressure Deboned | High Pressure Deboned | p-Value |
| C12:0 | 4.36 ± 0.49 | 1.36 ± 0.22 | <0.001 | 0.74 ± 0.04 | 0.32 ± 0.00 | <0.001 |
| C14:0 | 1.82 ± 0.08 | 1.09 ± 0.07 | <0.001 | 1.49 ± 0.03 | 1.39 ± 0.01 | <0.001 |
| C16:0 | 22.53 ± 0.60 | 23.56 ± 0.10 | 0.004 | 21.80 ± 0.32 | 23.00 ± 0.11 | <0.001 |
| C16:1–7c | 4.95 ± 0.45 | 6.08 ± 0.12 | <0.001 | 2.69 ± 0.05 | 2.77 ± 0.03 | 0.013 |
| C18:0 | 6.92 ± 0.44 | 6.68 ± 0.16 | 0.290 | 11.03 ± 0.12 | 11.72 ± 0.05 | <0.001 |
| C18:1–9c | 36.11 ± 0.40 | 37.35 ± 0.26 | <0.001 | 40.82 ± 0.11 | 41.14 ± 0.33 | 0.069 |
| C18:1–11c | 2.72 ± 0.12 | 2.73 ± 0.02 | 0.815 | 3.09 ± 0.09 | 2.78 ± 0.25 | 0.026 |
| C18:2–9c.12c | 15.63 ± 0.62 | 16.47 ± 0.20 | 0.016 | 14.03 ± 0.08 | 12.90 ± 0.07 | <0.001 |
| C18:3–6c.9c.12c | 0.16 ± 0.00 | 0.16 ± 0.01 | 0.813 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.013 |
| C18:3–9c.12c.15c | 1.20 ± 0.03 | 1.17 ± 0.03 | 0.188 | 1.03 ± 0.01 | 0.97 ± 0.01 | <0.001 |
| C20:0 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.183 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.049 |
| C20:1–11c | 0.40 ± 0.02 | 0.42 ± 0.00 | 0.051 | 0.86 ± 0.01 | 0.88 ± 0.01 | 0.001 |
| C20:2–11c.14c | 0.23 ± 0.05 | 0.22 ± 0.01 | 0.695 | 0.54 ± 0.01 | 0.52 ± 0.01 | <0.001 |
| C20:3–8c.11c.14c | 0.32 ± 0.09 | 0.25 ± 0.03 | 0.119 | 0.15 ± 0.01 | 0.12 ± 0.00 | <0.001 |
| C20:4–5c.8c.11c.14c | 1.39 ± 0.44 | 1.27 ± 0.20 | 0.596 | 0.56 ± 0.07 | 0.42 ± 0.02 | 0.001 |
| C20:3–11c.14c.17c | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.073 | 0.14 ± 0.00 | 0.14 ± 0.00 | 1.000 |
| C22:0 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.646 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.009 |
| C20:5–5c.8c.11c.14c.17c | 0.11 ± 0.03 | 0.08 ± 0.01 | 0.040 | 0.04 ± 0.01 | 0.02 ± 0.00 | <0.001 |
| C22:5–7c.10c.13c.16c.19c | 0.33 ± 0.09 | 0.25 ± 0.05 | 0.113 | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.072 |
| C22:6–4c.7c.10c.13c.16c.19c | 0.21 ± 0.07 | 0.18 ± 0.03 | 0.397 | 0.10 ± 0.02 | 0.08 ± 0.01 | 0.216 |
| Ʃ SFA | 35.71 ± 1.63 | 32.77 ± 0.55 | 0.003 | 35.27 ± 0.52 | 36.64 ± 0.18 | <0.001 |
| Ʃ MUFA | 44.66 ± 1.00 | 47.11 ± 0.42 | <0.001 | 47.89 ± 0.27 | 47.97 ± 0.62 | 0.809 |
| Ʃ PUFA | 19.61 ± 1.43 | 20.08 ± 0.56 | 0.507 | 16.82 ± 0.22 | 15.38 ± 0.13 | <0.001 |
| Ʃ PUFAn-3 | 1.52 ± 0.13 | 1.43 ± 0.07 | 0.204 | 1.16 ± 0.03 | 1.08 ± 0.02 | 0.001 |
| Ʃ PUFAn-6 | 17.02 ± 1.07 | 17.74 ± 0.40 | 0.158 | 14.60 ± 0.15 | 13.32 ± 0.09 | <0.001 |
| Parameters | Mechanically Deboned Chicken Meat | Frankfurter Samples | ||||
|---|---|---|---|---|---|---|
| Low Pressure | High Pressure | p-Value | Low Pressure | High Pressure | p-Value | |
| Total lipids (%) | 16.08 ± 0.26 | 16.65 ± 0.28 | 0.008 | 38.07 ± 0.68 | 42.73 ± 0.57 | <0.001 |
| FFA (%) | 8.58 ± 0.59 | 12.52 ± 1.32 | <0.001 | 18.42 ± 1.46 | 16.34 ± 0.76 | <0.001 |
| Samples | Methods of MDA Determination (mg/kg) | p-Value | |
|---|---|---|---|
| Spectrophotometric Method | RP-HPLC | ||
| Raw material for meat production: | |||
| Low-pressure MDCM | 0.090 ± 0.012 | 0.071 ± 0.011 | 0.017 |
| High-pressure MDCM | 0.112 ± 0.011 | 0.090 ± 0.010 | 0.004 |
| Frankfurters: | |||
| Low-pressure MDCM | 0.161 ± 0.022 | 0.126 ± 0.015 | 0.006 |
| High-pressure MDCM | 0.156 ± 0.013 | 0.124 ± 0.011 | 0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography. Molecules 2017, 22, 1988. https://doi.org/10.3390/molecules22111988
Reitznerová A, Šuleková M, Nagy J, Marcinčák S, Semjon B, Čertík M, Klempová T. Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography. Molecules. 2017; 22(11):1988. https://doi.org/10.3390/molecules22111988
Chicago/Turabian StyleReitznerová, Anna, Monika Šuleková, Jozef Nagy, Slavomír Marcinčák, Boris Semjon, Milan Čertík, and Tatiana Klempová. 2017. "Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography" Molecules 22, no. 11: 1988. https://doi.org/10.3390/molecules22111988





