Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes
Abstract
:1. Introduction
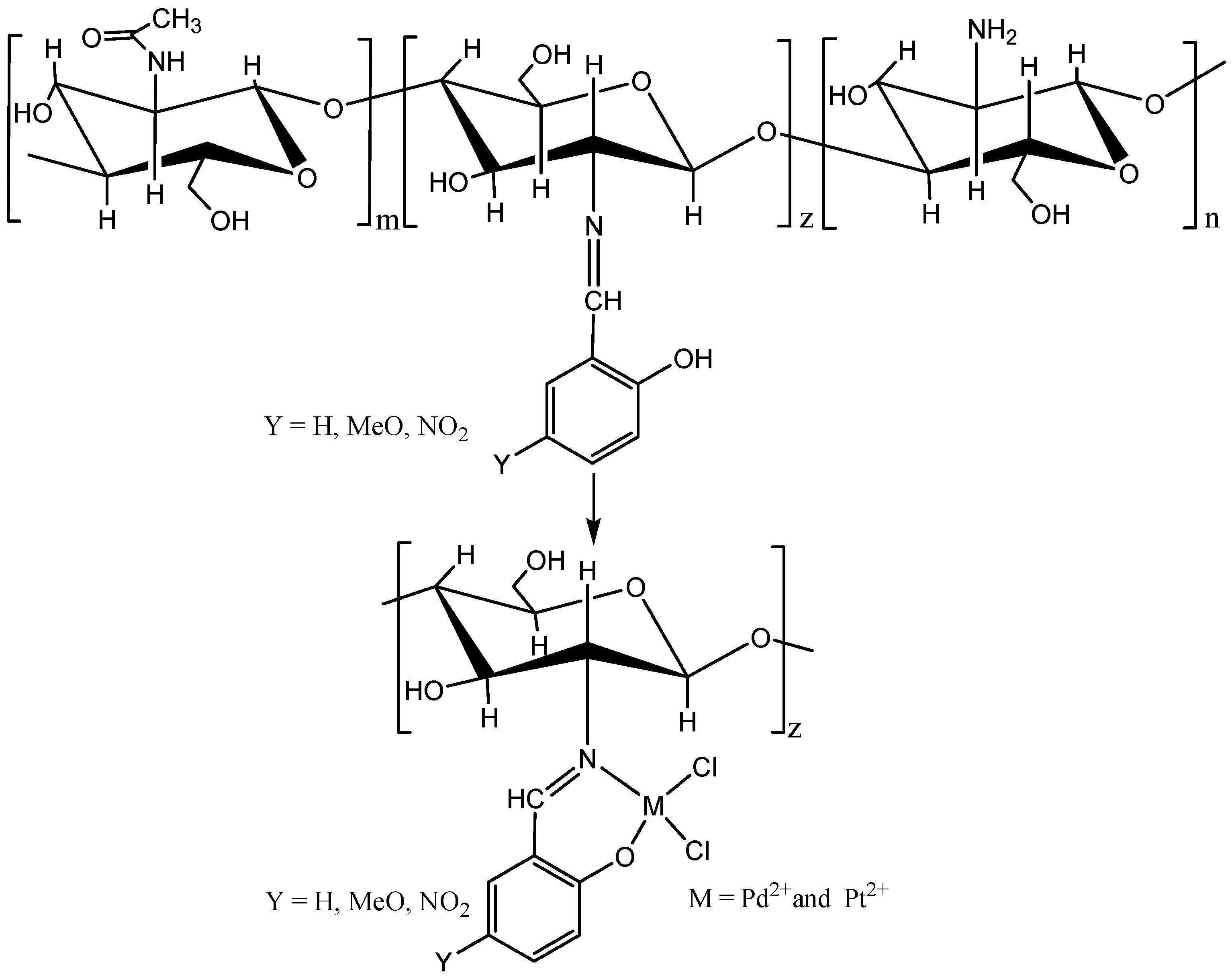
2. Results and Discussion
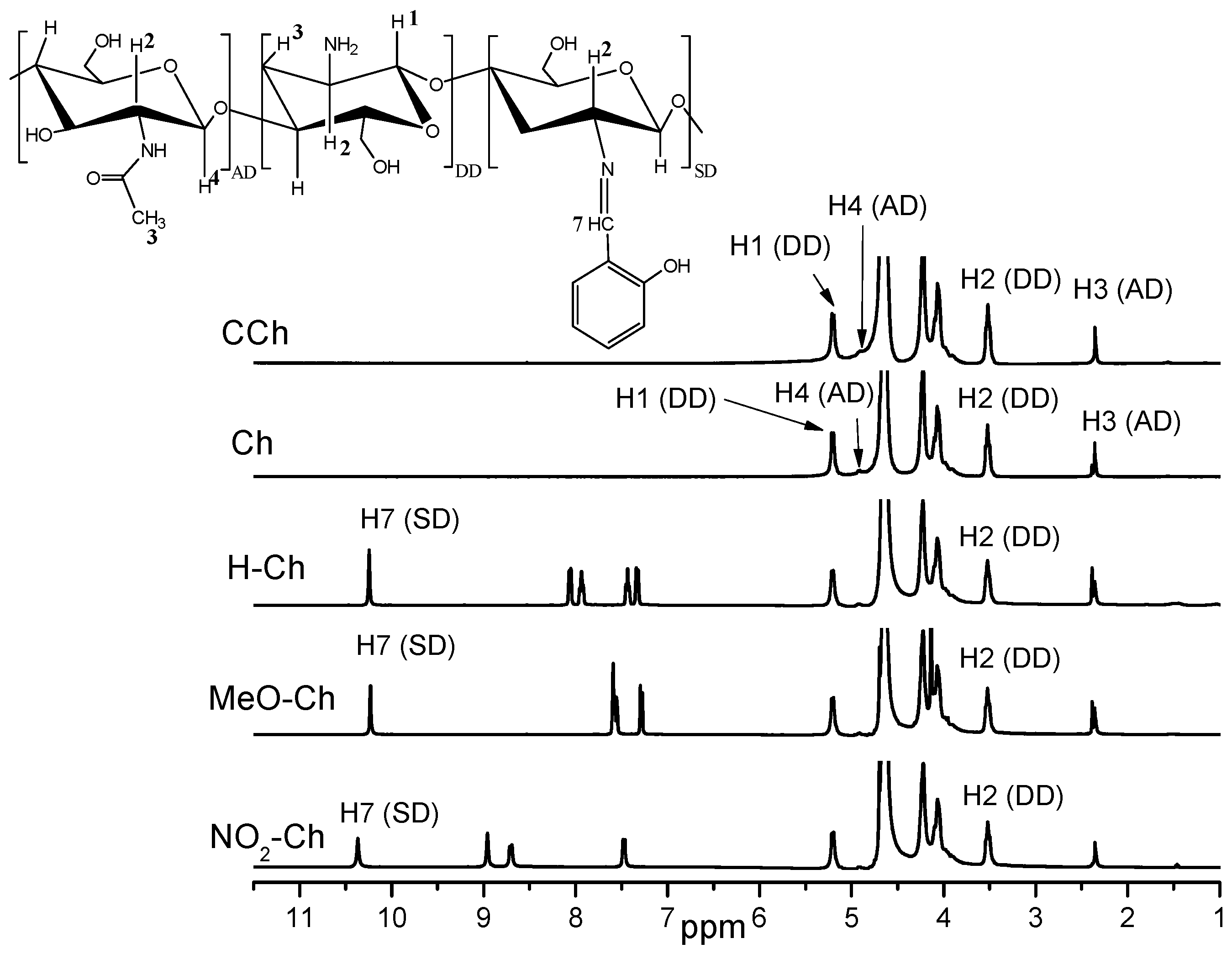
2.1. 1H-NMR Spectroscopy
2.2. Infrared Spectroscopy
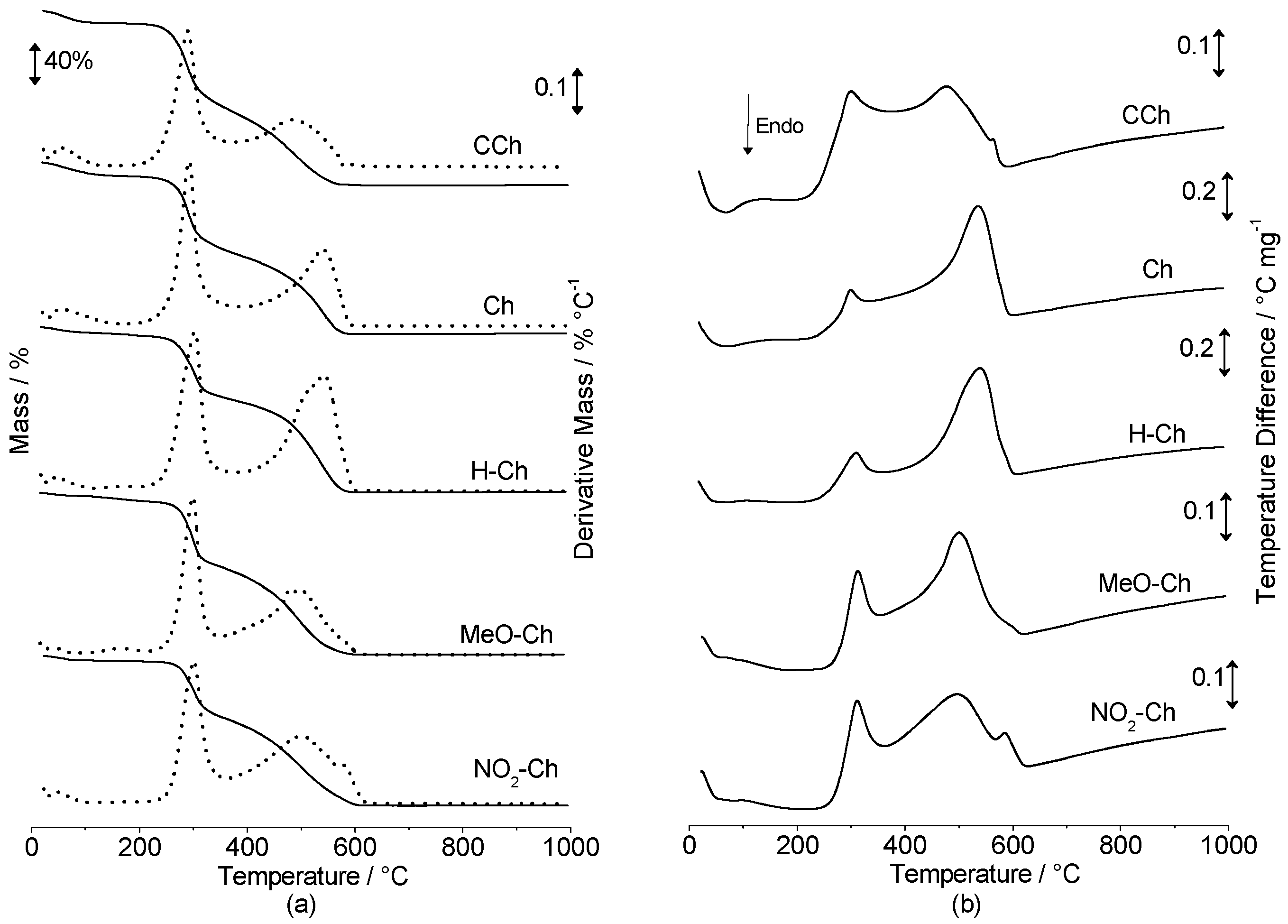
2.3. Thermal Analysis
2.4. X-ray Powder Diffraction Spectroscopy (XPRD)
2.5. Scanning Electron Microscopy (SEM) with X-ray Energy-Dispersive Analysis (EDS)
2.6. Electronic Absorption Spectra
2.7. Antimicrobial Activity
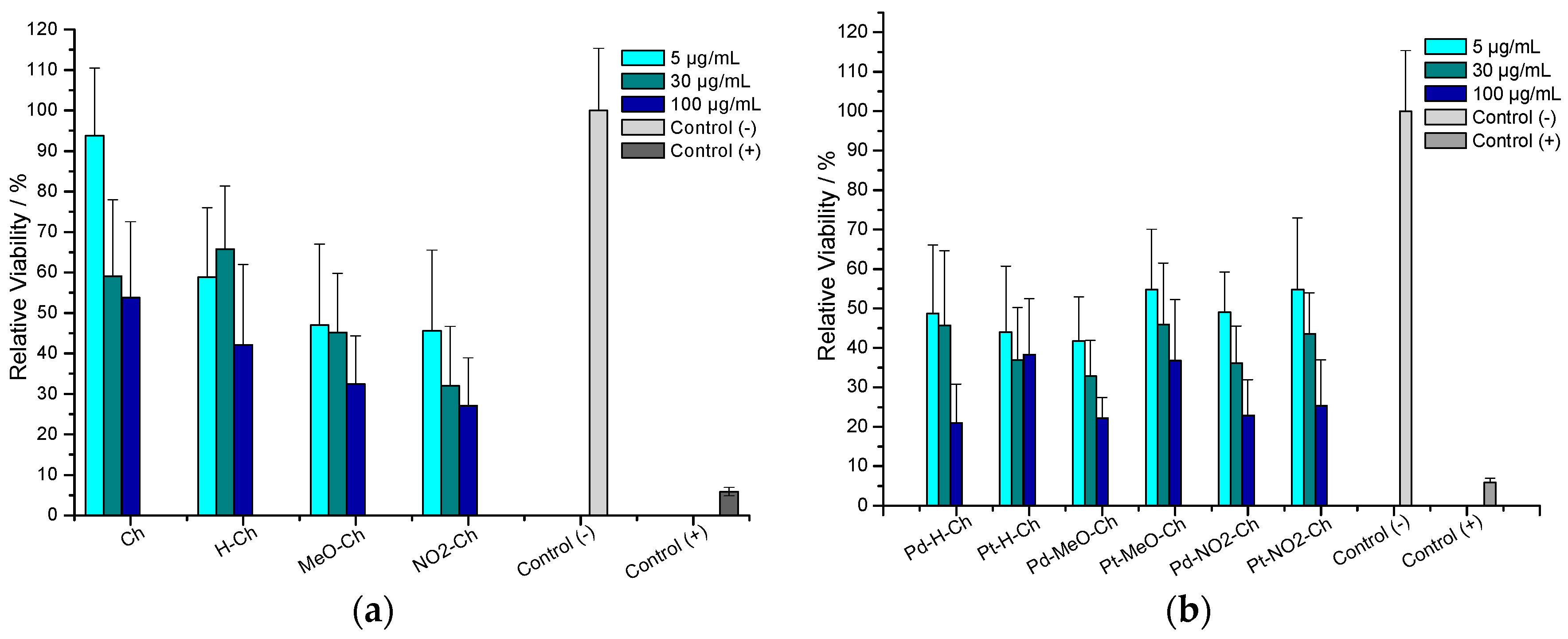
2.8. Antitumor Assay
3. Materials and Methods
3.1. Materials
3.2. Chitosan Purification
3.3. Synthesis of Biopolymeric Schiff Bases
3.4. Synthesis of Pd(II) and Pt(II) Complexes
3.5. Characterization
3.5.1. 1H-NMR Spectroscopy
3.5.2. Size Exclusion Chromatography
3.5.3. Infrared Spectroscopy
3.5.4. Thermal Analysis
3.5.5. X-ray Powder Diffraction (XPRD)
3.5.6. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDAX)
3.5.7. Electronic Absorption Spectra
3.5.8. Antibacterial Assay
3.5.9. Antifungal Assay
3.5.10. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Choi, B.-K.; Kim, K.-Y.; Yoo, Y.-J.; Oh, S.-J.; Choi, J.-H.; Kim, C.-Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against actinobacillus actinomycetemcomitans and streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in ige–antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohyd. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Anan, N.A.; Hassan, S.M.; Saad, E.M.; Butler, I.S.; Mostafa, S.I. Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidenechitosan schiff-base complexes of Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Ru(III), Rh(III), Pd(II) and Au(III). Carbohyd. Res. 2011, 346, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.; Inanan, T.; Menteş, A. Synthesis, characterization, and catalytic activity in suzuki coupling and catalase-like reactions of new chitosan supported Pd catalyst. Carbohyd. Polym. 2016, 145, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Elshaarawy, R.F.M.; Refaee, A.A.; El-Sawi, E.A. Pharmacological performance of novel poly-(ionic liquid)-grafted chitosan-N-salicylidene schiff bases and their complexes. Carbohyd. Polym. 2016, 146, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Selvan, A.; Sudharsan, S. Metallation of ethylenediamine based schiff base with biologically active Cu(II), Ni(II) and Zn(II) ions: Synthesis, spectroscopic characterization, electrochemical behaviour, DNA binding, photonuclease activity and in vitro antimicrobial efficacy. Spectrochim. Acta A 2011, 79, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and biological activity of schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-M.; He, N.-P.; Song, P.-F.; He, Y.-F.; Ding, L.; Lei, Z.-Q. Preparation of nano-chitosan Schiff-base copper complexes and their anticancer activity. Polym. Adv. Technol. 2009, 20, 959–964. [Google Scholar] [CrossRef]
- De Araújo, E.L.; Barbosa, H.F.G.; Dockal, E.R.; Cavalheiro, É.T.G. Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int. J. Biol. Macromol. 2017, 95, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.E.; Dockal, E.R.; Cavalheiro, É.T.G. Synthesis and characterization of Schiff bases from chitosan and salicylaldehyde derivatives. Carbohyd. Polym. 2005, 60, 277–282. [Google Scholar] [CrossRef]
- Abdelwahab, H.; Hassan, S.; Yacout, G.; Mostafa, M.; El Sadek, M. Synthesis and biological evaluation of new imine- and amino-chitosan derivatives. Polymers 2015, 7, 2690–2700. [Google Scholar] [CrossRef]
- Antony, R.; Theodore David Manickam, S.; Saravanan, K.; Karuppasamy, K.; Balakumar, S. Synthesis, spectroscopic and catalytic studies of Cu(II), Co(II) and Ni(II) complexes immobilized on Schiff base modified chitosan. J. Mol. Struct. 2013, 1050, 53–60. [Google Scholar] [CrossRef]
- Baran, T. A new chitosan Schiff base supported Pd(II) complex for microwave-assisted synthesis of biaryls compounds. J. Mol. Struct. 2017, 1141, 535–541. [Google Scholar] [CrossRef]
- Nagesh, G.Y.; Mahendra Raj, K.; Mruthyunjayaswamy, B.H.M. Synthesis, characterization, thermal study and biological evaluation of Cu(II), Co(II), Ni(II) and Zn(II) complexes of Schiff base ligand containing thiazole moiety. J. Mol. Struct. 2015, 1079, 423–432. [Google Scholar] [CrossRef]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009, 253, 1384–1397. [Google Scholar] [CrossRef]
- Han, T.Y.; Guan, T.S.; Iqbal, M.A.; Haque, R.A.; Sharmila Rajeswari, K.; Khadeer Ahamed, M.B.; Abdul Majid, A.M.S. Synthesis of water soluble copper(II) complexes: Crystal structures, DNA binding, oxidative DNA cleavage, and in vitro anticancer studies. Med. Chem. Res. 2014, 23, 2347–2359. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H-NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Krátký, M.; Dzurková, M.; Janoušek, J.; Konečná, K.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. Sulfadiazine salicylaldehyde-based Schiff bases: Synthesis, antimicrobial activity and cytotoxicity. Molecules 2017, 22, 1573. [Google Scholar] [CrossRef] [PubMed]
- Majerz, I.; Pawlukojć, A.; Sobczyk, L.; Dziembowska, T.; Grech, E.; Szady-Chełmieniecka, A. The infrared, raman and inelastic neutron scattering studies on 5-nitro-N-salicylideneethylamine. J. Mol. Struct. 2000, 552, 243–247. [Google Scholar] [CrossRef]
- Pajak, J.; Maes, G.; De Borggraeve, W.M.; Boens, N.; Filarowski, A. Matrix-isolation FT-IR and theoretical investigation of the vibrational properties of the sterically hindered ortho-hydroxy acylaromatic schiff bases. J. Mol. Struct. 2007, 844, 83–93. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Yuan, W.; Lin, Q.; Ji, L.; Liu, F. Preparation and antibacterial activity of Schiff bases from O-carboxymethyl chitosan and para-substituted benzaldehydes. Polym. Bull. 2012, 68, 1215–1226. [Google Scholar] [CrossRef]
- Ali, O.A.M. Synthesis, spectroscopic, fluorescence properties and biological evaluation of novel Pd(II) and Cd(II) complexes of noon tetradentate Schiff bases. Spectrochim. Acta A 2014, 121, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Fu, B.; Qiao, Y.; Wang, C.; Huang, Y.-Y.; Liu, C.-C.; Tian, C.; Du, J.-L. Synthesis, characterization and cytotoxicity studies of platinum(II) complexes with reduced amino acid ester Schiff-bases as ligands. Inorg. Chim. Acta 2014, 419, 135–140. [Google Scholar] [CrossRef]
- Ferhat-Hamida, Z.; Barbier, J.; Labruquere, S.; Duprez, D. The chemical state of palladium in alkene and acetylene oxidation. Appl. Catal. B Environ. 2001, 29, 195–205. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Aydın, C.; Abd El-sadek, M.S.; Zheng, K.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol–gel calcination technique. Opt. Laser Technol. 2013, 48, 447–452. [Google Scholar] [CrossRef]
- Mostafa, S.I. Synthesis, characterization and antineoplastic activity of 5-chloro-2,3-dihydroxypyridine transition metal complexes. J. Coord. Chem. 2008, 61, 1553–1567. [Google Scholar] [CrossRef]
- Mostafa, S.I.; Badria, F.A. Synthesis, spectroscopic, and anticancerous properties of mixed ligand Palladium(II) and Silver(I) complexes with 4,6-Diamino-5-hydroxy-2-mercaptopyrimidine and 2,2′-Bipyridyl. Met. Based Drugs 2008, 723634. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, R.; Geetha, A.; Thilagavathi, M.; Karvembu, R.; Krishnan, V.; Bertagnolli, H.; Natarajan, K. Synthesis, characterization, exafs investigation and antibacterial activities of new ruthenium(III) complexes containing tetradentate Schiff base. J. Inorg. Biochem. 2004, 98, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, T.; Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(III) complexes derived from chitosan Schiff base. Int. J. Biol. Macromol. 2016, 90, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Facchi, S.; Follmann, H.; Pereira, A.; Rubira, A.; Muniz, E. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, A.; Souza, A.L.; Delezuk, J.A.M.; Pavinatto, F.J.; Campana-Filho, S.P.; Oliveira, O.N. Interaction of O-acylated chitosans with biomembrane models: Probing the effects from hydrophobic interactions and hydrogen bonding. Colloid Surf. B 2014, 114, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, P.; Laxma Reddy, K. Synthesis, spectral characterisation, morphology, biological activity and DNA cleavage studies of metal complexes with chromone Schiff base. Arab. J. Chem. 2016, 9, 596–605. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Pérez, J.M.; López-Solera, I.; Masaguer, J.R.; Luque, A.; Román, P.; Edwards, A.; Alonso, C.; Navarro-Ranninger, C. Novel tetranuclear orthometalated complexes of Pd(II) and Pt(II) derived from p-isopropylbenzaldehyde thiosemicarbazone with cytotoxic activity in cis-DDP resistant tumor cell lines. Interaction of these complexes with DNA. J. Med. Chem. 1998, 41, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Jaividhya, P.; Dhivya, R.; Akbarsha, M.A.; Palaniandavar, M. Efficient DNA cleavage mediated by mononuclear mixed ligand copper(II) phenolate complexes: The role of co-ligand planarity on DNA binding and cleavage and anticancer activity. J. Inorg. Biochem. 2012, 114, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Shilpa, A.; Raju, N.; Raghavaiah, P. Synthesis, structure, DNA binding and cleavage properties of ternary amino acid Schiff base-phen/bipy Cu(II) complexes. J. Inorg. Biochem. 2011, 105, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Signini, R.; Campana Filho, S.P. On the preparation and characterization of chitosan hydrochloride. Polym. Bull. 1999, 42, 159–166. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, É.T.G. Influence of some reactional parameters on the substitution degree of biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Carbohyd. Polym. 2006, 65, 557–561. [Google Scholar] [CrossRef]
- Oliveira Junior, E.N.; Gueddari, N.E.E.; Moerschbacher, B.M.; Franco, T.T. Growth rate inhibition of phytopathogenic fungi by characterized chitosans. Braz. J. Microbiol. 2012, 43, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci. Rep. 2015, 5, 10048. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds chitosan, Schiff bases and complexes are available from the authors. |


| Polymers | /% | /% | /kDa | /g mol−1 |
|---|---|---|---|---|
| CCh | 90.1 | - | 334.3 | 165.4 |
| Ch | 90.4 | - | 222.9 | 165.3 |
| H-Ch | - | 51.2 | 294.7 * | 218.2 |
| MeO-Ch | - | 54.3 | 321.0 * | 237.7 |
| NO2-Ch | - | 48.0 | 319.3 * | 236.4 |
| Sample | Process (Air Atmosphere) | TGA/DTG | DTA | ||
|---|---|---|---|---|---|
| Temperature Range/°C | Mass Loss/% | a Ratio | Temperature Peak/°C * | ||
| CCh | Dehydration | 22.2–171.5 | 7.2 | 71.3 (endo) | |
| 1st step | 171.5–382.3 | 51.7 | 1.27 | 303.9 (exo) | |
| 2nd step | 382.3–707.5 | 40.6 | 481.6 (exo) | ||
| Ch | Dehydration | 22.3–182.9 | 8.1 | 76.9 (endo) | |
| 1st step | 182.9–402.2 | 47.5 | 1.07 | 303.9 (exo) | |
| 2nd step | 402.2–713.2 | 44.5 | 541.1 (exo) | ||
| H-Ch | Dehydration | 22.2–128.0 | 3.8 | 60.9 (endo) | |
| 1st step | 128.0–390.8 | 41.5 | 0.76 | 313.3 (exo) | |
| 2nd step | 390.8–713.2 | 54.6 | 543.0 (exo) | ||
| MeO-Ch | Dehydration | 23.3–107.2 | 2.4 | 188.5 (endo) | |
| 1st step | 107.2–358.7 | 43.6 | 0.80 | 313.3 (exo) | |
| 2nd step | 358.7–727.3 | 54.3 | 500.5 (exo) | ||
| NO2-Ch | Dehydration | 22.2–146.0 | 3.2 | 78.9 (endo) | |
| 1st step | 146.0–368.1 | 39.6 | 0.69 | 312.4 (exo) | |
| 2nd step | 368.1–733.0 | 57.1 | 496.7 (exo) | ||
| Sample | Process (Air Atmosphere) | TGA/DTG | DTA | |||
|---|---|---|---|---|---|---|
| a ΔT/°C | Mass Loss/% | b Residue Exp/% | c Residue Calc/% | Peaks/°C * | ||
| Pd-Ch | Pd-Ch·nH2O → Pd-Ch + nH2O | 24.1–181.9 | 12.0 | - | - | 65.6 (endo) |
| Pd-Ch → Res-Pd | 181.9–357.7 | 43.5 | - | - | 221.6 (endo) | |
| decomposition of Res-Pd | 357.7–648.9 | 35.2 | - | - | 426.8 (exo) | |
| residue PdO | - | - | 8.0 | 40.7 | 820.0 (endo) | |
| Pd-H-Ch | Pd-H-Ch·nH2O → Pd-H-Ch + nH2O | 23.1–172.4 | 8.7 | - | - | 58.1 (endo) |
| Pd-H-Ch → Res-Pd | 172.4–367.5 | 39.2 | - | - | 224.1 (endo) | |
| decomposition of Res-Pd | 367.5–560.7 | 36.6 | - | - | 426.8 (exo) | |
| residue PdO | - | - | 10.8 | 19.3 | 809.4 (endo) | |
| Pd-MeO-Ch | Pd-MeO-Ch·nH2O → Pd-MeO-Ch + nH2O | 23.6–165.8 | 7.5 | - | - | 73.8 (endo) |
| Pd-MeO-Ch → Res-Pd | 165.8–307.1 | 32.8 | - | - | 236.0 (exo) | |
| decomposition of Res-Pd | 307.1–626.6 | 48.9 | - | - | 361.1 (exo) | |
| residue PdO | - | - | 9.6 | 19.3 | 813.3 (endo) | |
| Pd-NO2-Ch | Pd-NO2-Ch·nH2O → Pd-NO2-Ch + nH2O | 24.1–173.4 | 10.5 | - | - | 73.2 (endo) |
| Pd-NO2-Ch → Res-Pd | 173.4–352.1 | 38.3 | - | - | 233.9 (exo) | |
| decomposition of Res-Pd | 352.1–631.9 | 34.3 | - | - | 399.3 (exo) | |
| residue PdO | - | - | 15.8 | 17.1 | 813.4 (endo) | |
| Pt-Ch | Pt-Ch·nH2O → Pt-Ch + nH2O | 20.8–144.0 | 7.2 | - | - | 61.5 (endo) |
| Pt-Ch → Res-Pt | 144.0–360.2 | 42.7 | - | - | 239.8 (endo) | |
| decomposition of Res-Pd | 360.21–720.4 | 41.9 | - | - | 415.2 (exo) | |
| residue PdO | - | - | 8.5 | 53.0 | 512.9 (exo) | |
| Pt-H-Ch | Pt-H-Ch·nH2O → Pt-H-Ch + nH2O | 19.8–167.7 | 11.3 | - | - | 58.1 (endo) |
| Pt-H-Ch → Res-Pt | 167.7–319.4 | 31.0 | - | - | 234.6 (endo) | |
| decomposition of Res-Pt | 319.4–583.0 | 36.5 | - | - | 444.0 (exo) | |
| residue PtO | - | - | 20.2 | 26.2 | - | |
| Pt-MeO-Ch | Pt-MeO-Ch·nH2O → Pt-MeO-Ch + nH2O | 19.8–163.9 | 7.2 | - | - | 68.5 (endo) |
| Pt-MeO-Ch → Res-Pt | 163.9–371.5 | 38.2 | - | - | 239.6 (endo) | |
| decomposition of Res-Pt | 371.5–587.4 | 34.5 | - | - | 426.7 (exo) | |
| residue PtO | - | - | 20.3 | 26.5 | - | |
| Pt-NO2-Ch | Pd-NO2-Ch·nH2O → Pd-NO2-Ch + nH2O | 21.3–157.3 | 8.9 | - | - | - |
| Pd-NO2-Ch → Res-Pd | 157.3–350.7 | 36.5 | - | - | 226.9 (endo) | |
| decomposition of Res-Pd | 350.7–539.3 | 34.5 | - | - | 444.0 (exo) | |
| residue PdO | - | - | 20.2 | 23.5 | - | |
| P. syringae | F. graminearum | |||
|---|---|---|---|---|
| Sample | MIC μg mL−1 | IC50 μg mL−1 | MIC μg mL−1 | IC50 μg mL−1 |
| Chitosan Schiff bases | ||||
| Ch | >50 | 42 | 30 | 24 |
| H–Ch | 25 | 13 | 50 | 44 |
| MeO–Ch | >50 | 14 | 60 | 54 |
| NO2–Ch | >50 | 9 | 40 | 34 |
| Complexes | ||||
| Pd–H–Ch | 25 | 7 | 60 | 55 |
| Pd–MeO–Ch | >50 | 14 | >60 | 56 |
| Pd–NO2–Ch | >50 | 14 | >60 | >60 |
| Pt–H–Ch | 25 | 10 | 60 | 55 |
| Pt–MeO–Ch | >50 | 15 | >60 | >60 |
| Pt–NO2–Ch | >50 | 15 | 60 | 53 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, H.F.G.; Attjioui, M.; Ferreira, A.P.G.; Dockal, E.R.; El Gueddari, N.E.; Moerschbacher, B.M.; Cavalheiro, É.T.G. Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes. Molecules 2017, 22, 1987. https://doi.org/10.3390/molecules22111987
Barbosa HFG, Attjioui M, Ferreira APG, Dockal ER, El Gueddari NE, Moerschbacher BM, Cavalheiro ÉTG. Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes. Molecules. 2017; 22(11):1987. https://doi.org/10.3390/molecules22111987
Chicago/Turabian StyleBarbosa, Hellen Franciane Gonçalves, Maha Attjioui, Ana Paula Garcia Ferreira, Edward Ralph Dockal, Nour Eddine El Gueddari, Bruno M. Moerschbacher, and Éder Tadeu Gomes Cavalheiro. 2017. "Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes" Molecules 22, no. 11: 1987. https://doi.org/10.3390/molecules22111987





