An Efficient Approach for Lipase-Catalyzed Synthesis of Retinyl Laurate Nutraceutical by Combining Ultrasound Assistance and Artificial Neural Network Optimization
Abstract
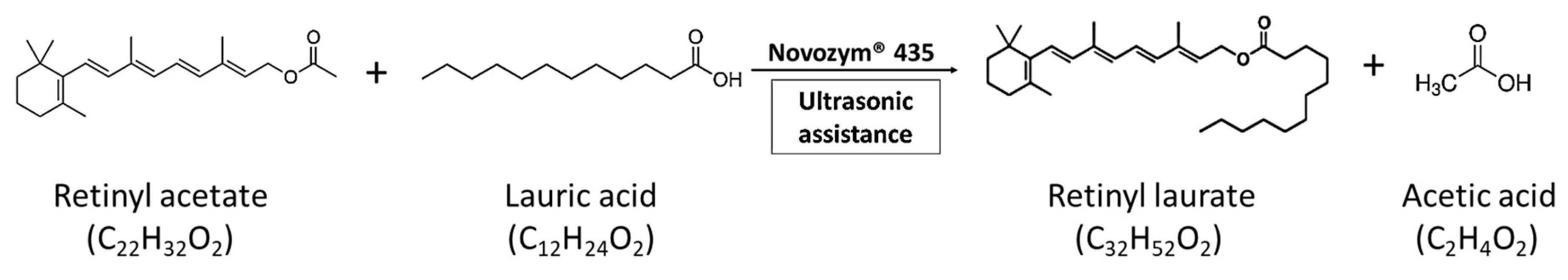
:1. Introduction
2. Results and Discussion
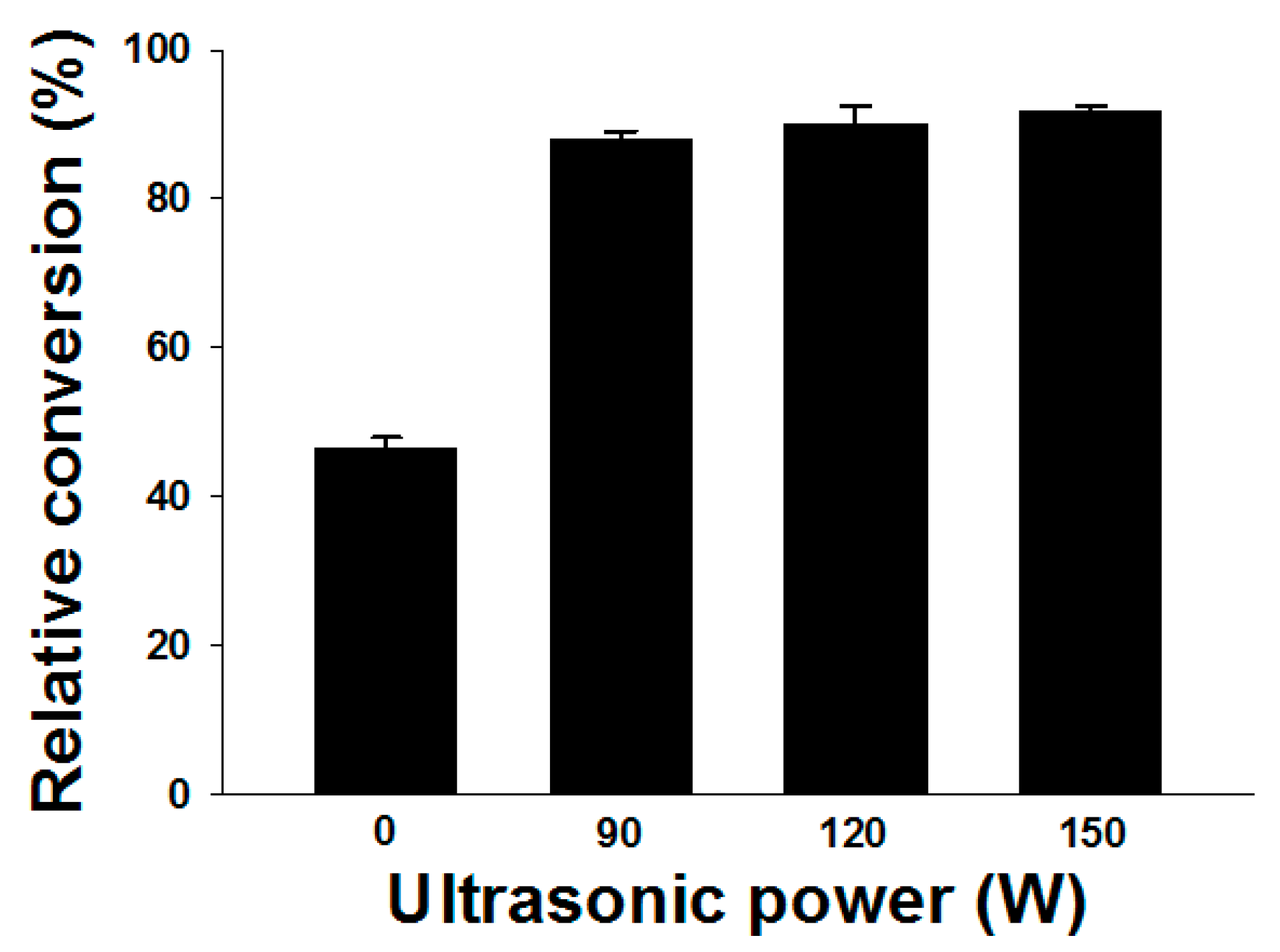
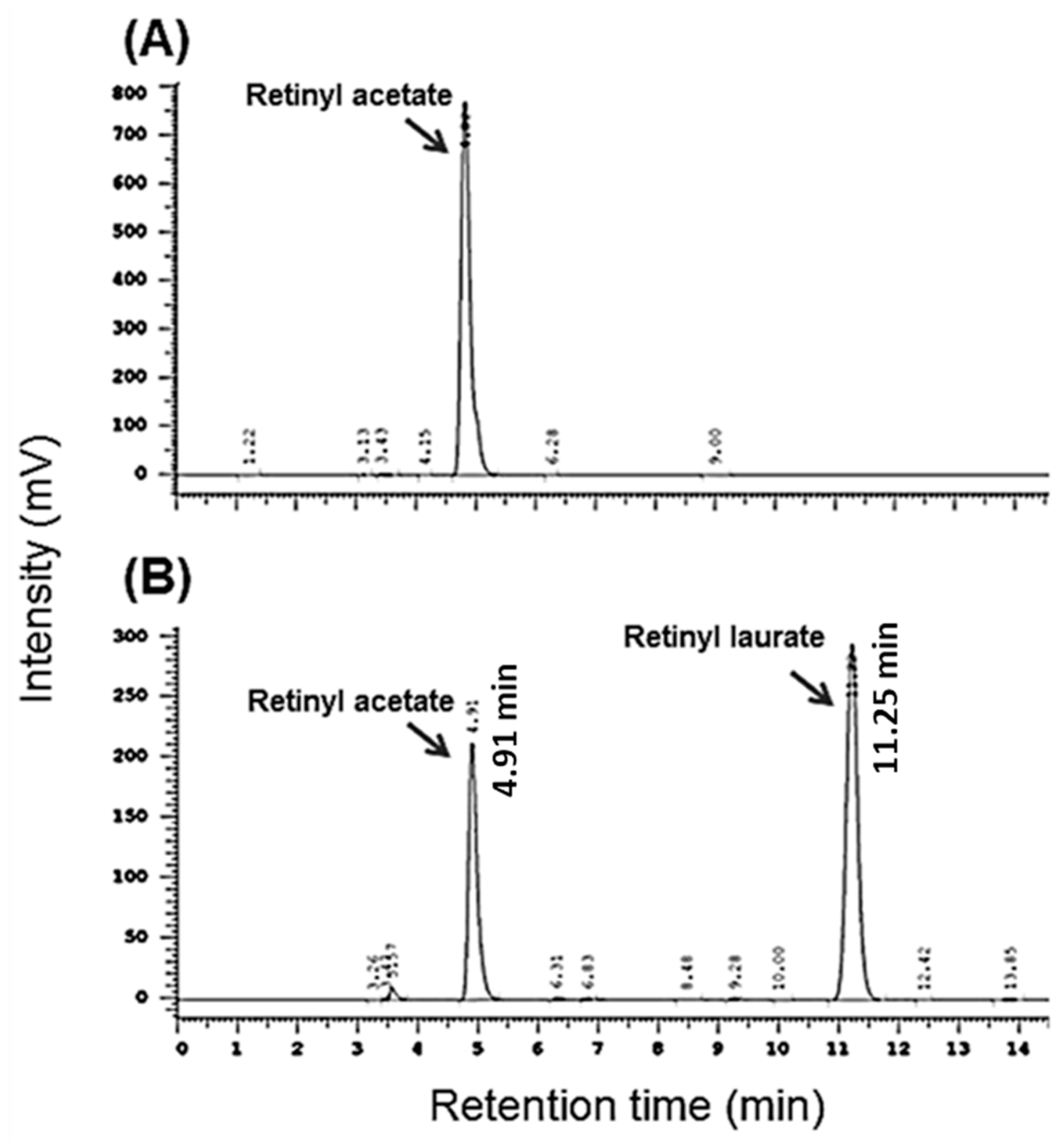
2.1. Effect of Ultrasound
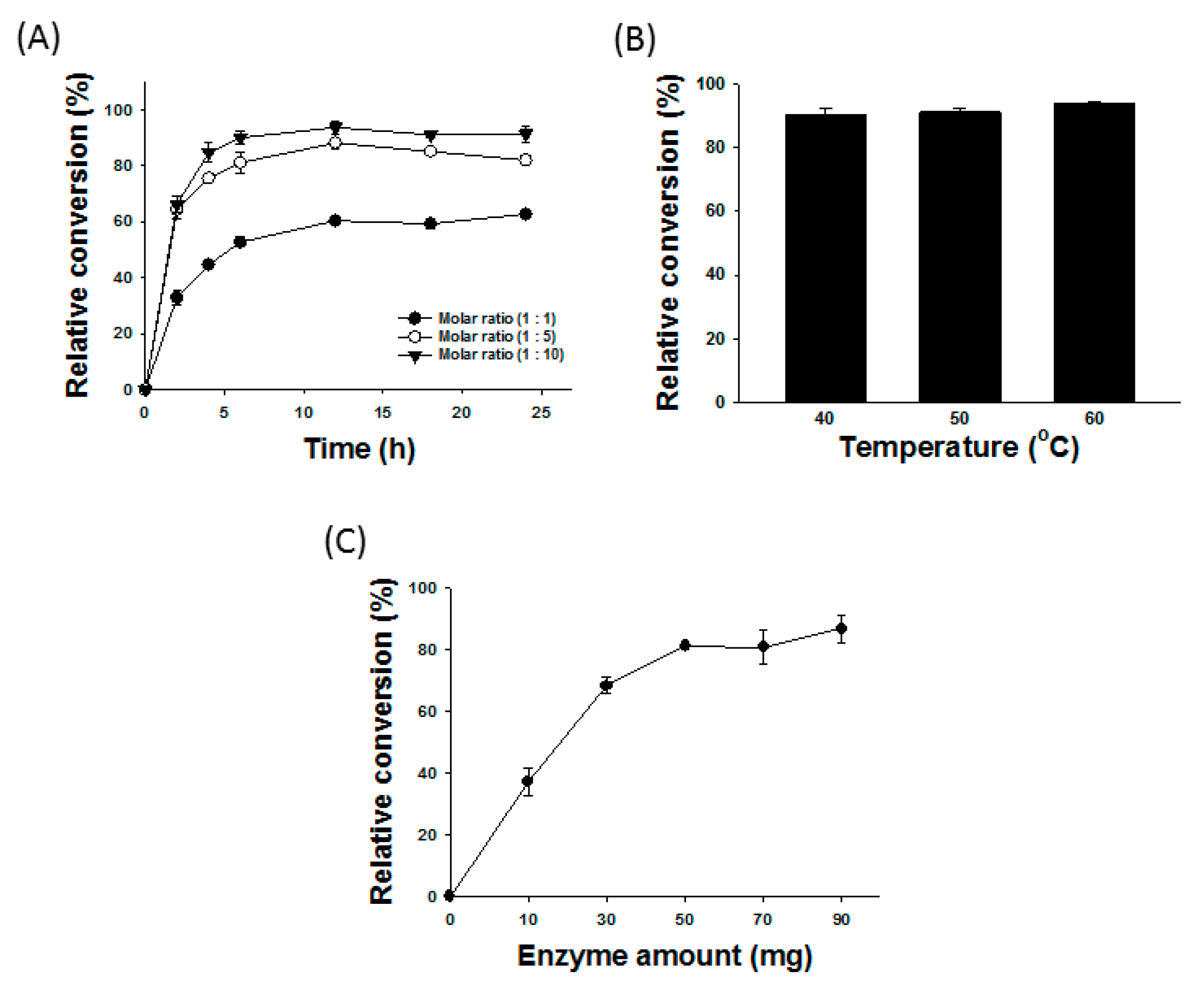
2.2. Preliminary Test
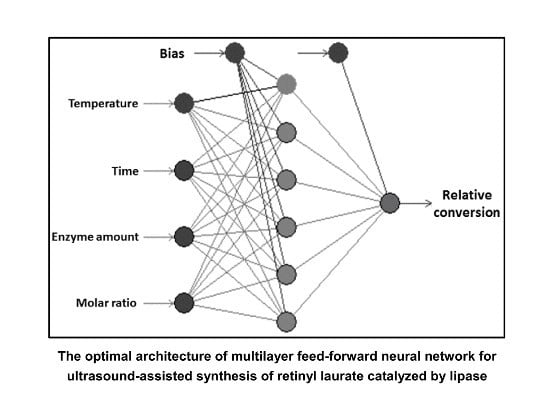
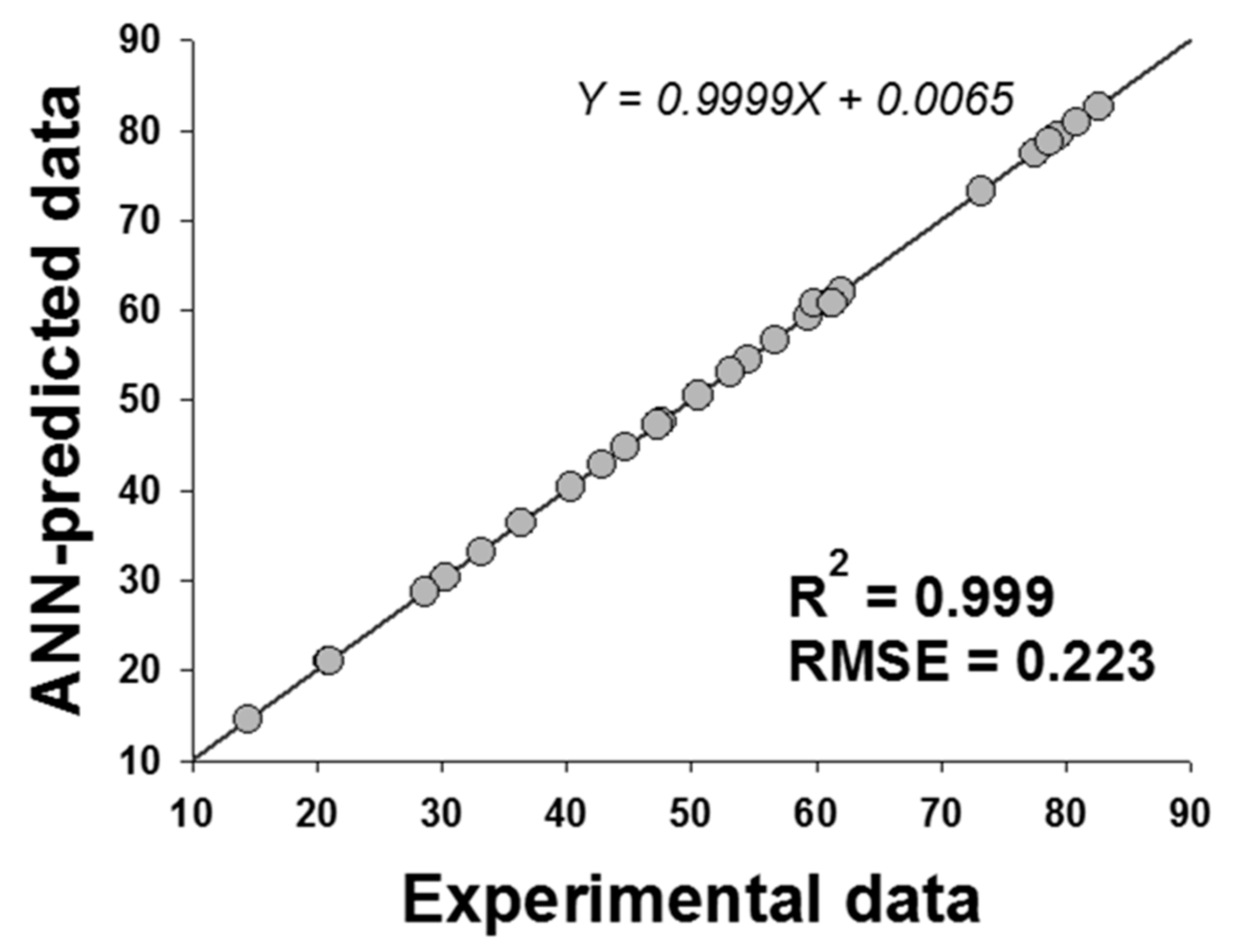
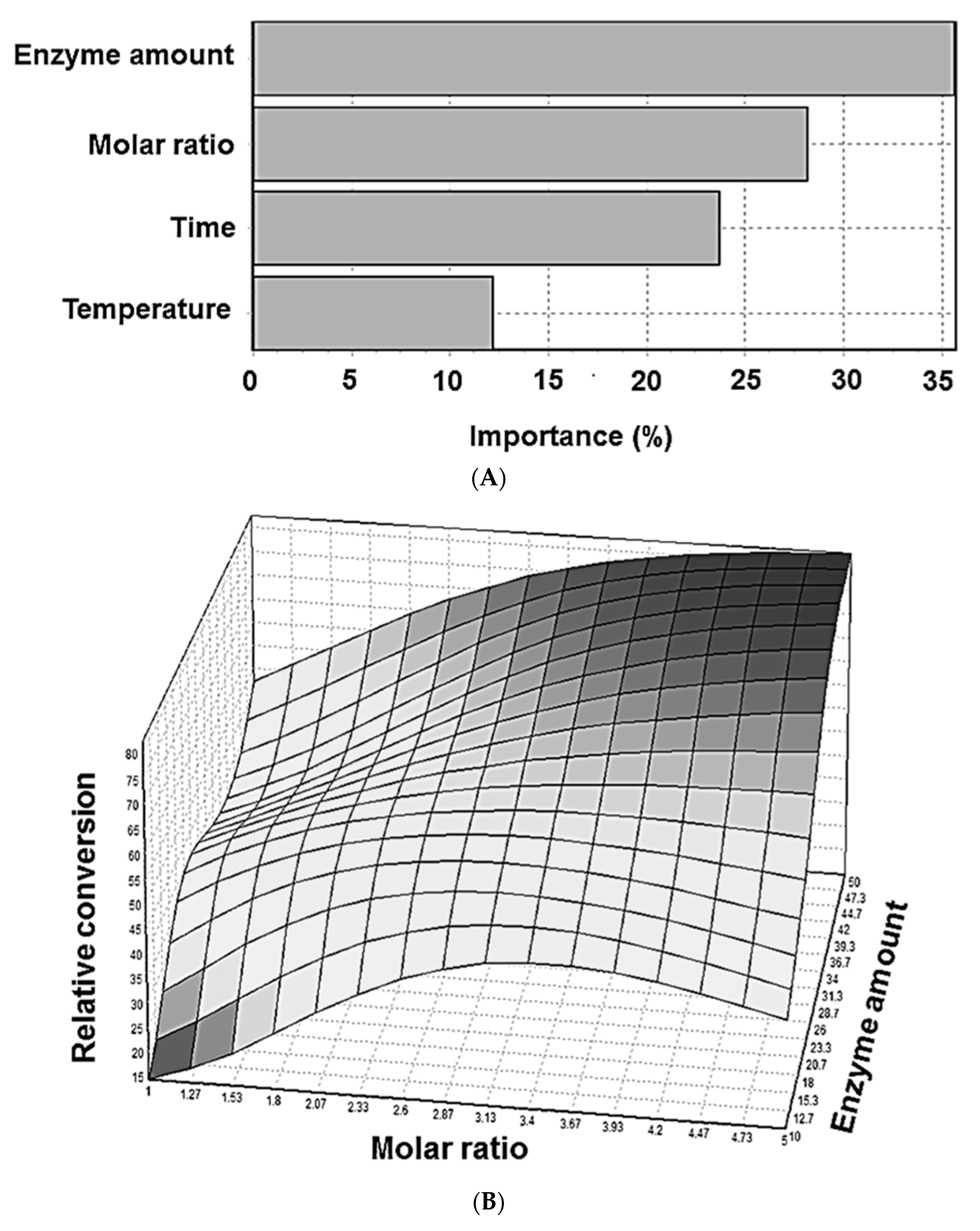
2.3. Artificial Neural Network (ANN)
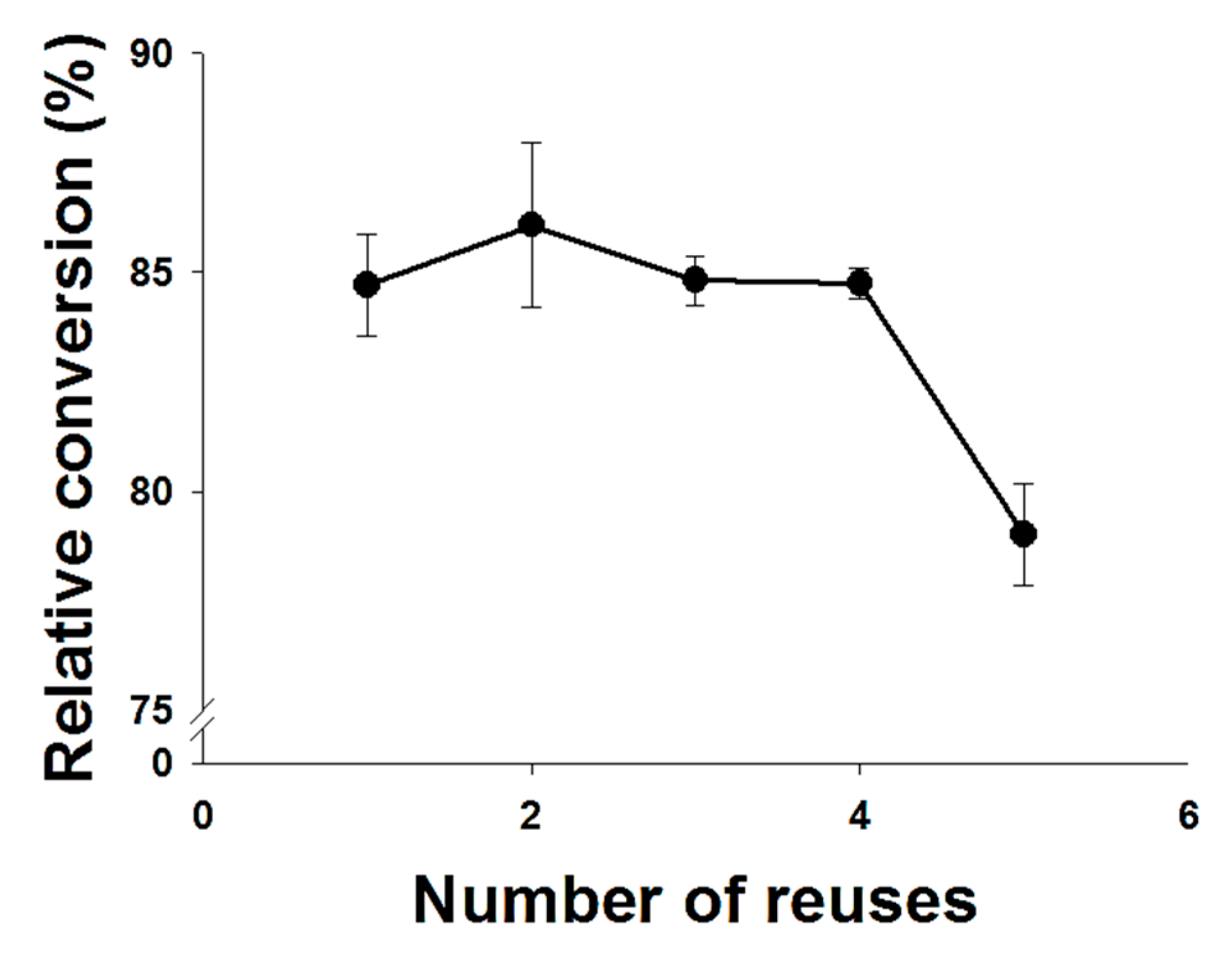
2.4. Enzyme Reusability
3. Materials and Methods
3.1. Materials
3.2. Lipase-Catalyzed Retinyl Laurate Synthesis by Ultrasound Assistance
3.3. Central Composite Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammerling, U. Retinol as electron carrier in redox signaling, a new frontier in vitamin A research. Hepatobiliary Surg. Nutr. 2016, 5, 15–28. [Google Scholar] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajed, K.; Heard, C.M. Topical delivery of retinyl ascorbate co-drug. 1. Synthesis, penetration into and permeation across human skin. Int. J. Pharm. 2004, 280, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, B.; Kim, H.; Um, S.; Lee, J.; Ryoo, H.; Jung, H. Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg. Med. Chem. 2008, 16, 6387–6393. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Kong, Y.; Kim, H.; Kang, J. Synthesis and in vitro biological activity of retinyl polyhydroxybenzoates, novel hybrid retinoid derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.A.; Ryan, K.S.; Moore, B.S.; Gulder, T.A. Total (bio)synthesis: Strategies of nature and of chemists. Top. Curr. Chem. 2010, 297, 149–203. [Google Scholar] [PubMed]
- Maugard, T.; Tudella, J.; Legoy, M.D. Study of vitamin ester synthesis by lipase-catalyzed transesterification in organic media. Biotechnol. Prog. 2000, 16, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Maugard, T.; Rejasse, B.; Legoy, M.D. Synthesis of water-soluble retinol derivatives by enzymatic method. Biotechnol. Prog. 2002, 18, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.H.; Liu, T.; Tan, T.W. Synthesis of vitamin A esters by immobilized Candida sp. lipase in organic media. Chin. J. Chem. Eng. 2006, 14, 81–86. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, J.Q.; Wang, Y.-H.; Liu, H.; Han, P.-F.; Wei, P. Synthesis of retinyl palmitate catalyzed by Candida sp. 99–125 lipase immobilized on fiber-like SBA-15 mesoporous material. J. Nanosci. Nanotechnol. 2011, 11, 7593–7602. [Google Scholar]
- Chen, H.C.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimization of ultrasound-accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrason. Sonochem. 2011, 18, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Hsiao, F.W.; Chen, J.H.; Hsieh, C.W.; Liu, Y.C.; Shieh, C.J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4’-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Paludo, N.; Alves, J.S.; Altmann, C.; Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem. 2015, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, A.L.; Subhedar, P.B.; Gogate, P.R. Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution. Ultrason. Sonochem. 2016, 29, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.S.; Rathod, V.K. Ultrasound assisted enzyme catalyzed degradation of Cetirizine dihydrochloride. Ultrason. Sonochem. 2015, 24, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.J.; Zhao, H.X.; Sun, W.J.; Wei, Z.; Yu, S.L.; Zhou, Q.; Dong, Y. Ultrasound-assisted lipase-catalyzed synthesis of d-isoascorbyl palmitate: Process optimization and Kinetic evaluation. Chem. Cent. J. 2013, 7, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.T.; Bernshteyn, M.A.; Kabiri-Bamoradian, K. Constructing meta-models for computer experiments. J. Qual. Technol. 2003, 35, 264–274. [Google Scholar]
- Wang, F.; Chen, L.; Jiang, S.; He, J.; Zhang, X.; Peng, J.; Xu, Q.; Li, R. Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box-Behnken design. J. Liposome Res. 2014, 24, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Edwards, K.L. The use of artificial neural networks in materials science based research. Mater. Des. 2007, 28, 1747–1752. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Comparison of response surface methodology and artificial neural network approach towards efficient ultrasound-assisted biodiesel production from muskmelon oil. Ultrason. Sonochem. 2015, 23, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nelofer, R.; Ramanan, R.N.; Rahman, R.N.; Basri, M.; Ariff, A.B. Comparison of the estimation capabilities of response surface methodology and artificial neural network for the optimization of recombinant lipase production by E. coli BL21. J. Ind. Microbiol. Biotechnol. 2012, 39, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kumar, D.; Johari, R.; Singh, C.P. Enzymatic transesterification of Jatropha curcas oil assisted by ultrasonication. Ultrason. Sonochem. 2011, 18, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Gogate, P.R.; Pandit, A.B. Ultrasound assisted synthesis of isopropyl esters from palm fatty acid distillate. Ultrason. Sonochem. 2009, 16, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Batistella, L.; Lerin, L.A.; Brugnerotto, P.; Danielli, A.J.; Trentin, C.M.; Popiolski, A.; Treichel, H.; Oliveira, J.V.; de Oliveira, D. Ultrasound-assisted lipase-catalyzed transesterification of soybean oil in organic solvent system. Ultrason. Sonochem. 2012, 19, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lerin, L.A.; Feiten, M.C.; Richetti, A.; Toniazzo, G.; Treichel, H.; Mazutt, M.A.; Oliveira, J.V.; Oestreicher, E.G.; de Oliveira, D. Enzymatic synthesis of ascorbyl palmitate in ultrasound-assisted system: Process optimization and kinetic evaluation. Ultrason. Sonochem. 2011, 18, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Aouf, C.; Durand, E.; Lecomte, J.; Figuerola-Espinoza, M.C.; Dubreucq, E.; Fulcrand, H.; Villeneuve, P. The use of lipases as biocatalysts for the epoxidation of fatty acids and phenolic compounds. Green Chem. 2014, 16, 1740–1754. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Coca, C.; Camocho, S.; Adlercreutz, D.; Mattiasson, B.; Hatti-Kaul, R. Chemo-enzymatic epoxidation of linoleic acid: Parameters influencing the reaction. Eur. J. Lipid Sci. Technol. 2005, 107, 864–870. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Su, D.; Huang, Y.; Wang, Y.; Li, Y.H. Ultrasound-assisted aqueous two-phase system for extraction and enrichment of Zanthoxylum armatum lignans. Molecules 2015, 20, 15273–15286. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Li, Y.; Kuo, C.H.; Twu, Y.K.; Shieh, C.J. Highly efficient synthesis of an emerging lipophilic antioxidant: 2-ethylhexyl ferulate. Molecules 2016, 21, 478. [Google Scholar] [CrossRef] [PubMed]
- Basri, M.; Rahman, R.N.; Ebrahimpour, A.; Salleh, A.B.; Gunawan, E.R.; Rahman, M.B. Comparison of estimation capabilities of response surface methodology (RSM) with artificial neural network (ANN) in lipase-catalyzed synthesis of palm-based wax ester. BMC Biotechnol. 2007, 7, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.L.R.; Pena, F.P.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica. J. Chem. Tecnol. Biotechnol. 2013, 88, 1089–1095. [Google Scholar] [CrossRef]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: Reaction optimization by response surface methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Xun, E.N.; Lv, X.L.; Kang, W.; Wang, J.X.; Zhang, H.; Wang, L.; Wang, Z. Immobilization of Pseudomonas fluorescens lipase onto magnetic nanoparticles for resolution of 2-octanol. Appl. Biochem. Biotechnol. 2012, 168, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Lerin, L.; Ceni, G.; Richett, A.; Kubiak, G.; Vladimir Oliveira, J.; Toniazzo, G.; Treichel, H.; Oestreicher, E.G.; Oliveira, D. Successive cycles of utilization of novozym 435 in three different reaction systems. Braz. J. Chem. Eng. 2011, 28, 181–188. [Google Scholar] [CrossRef]
Sample Availability: Samples of retinyl laurate are available from the authors. |


| Treatment No. a | Experimental Parameters | Relative Conversion (%) b | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||
| 1 | 2 | 40 | 30 | 3 | 33.16 ± 1.21 |
| 2 | 4 | 40 | 10 | 3 | 20.97 ± 1.17 |
| 3 | 4 | 40 | 50 | 3 | 61.99 ± 3.33 |
| 4 | 4 | 40 | 30 | 1 | 30.27 ± 2.67 |
| 5 | 4 | 40 | 30 | 5 | 56.69 ± 3.56 |
| 6 | 6 | 40 | 30 | 3 | 54.56 ± 1.93 |
| 7 | 2 | 60 | 30 | 3 | 47.59 ± 1.14 |
| 8 | 6 | 60 | 30 | 3 | 73.19 ± 0.74 |
| 9 | 4 | 60 | 10 | 3 | 50.55 ± 4.50 |
| 10 | 4 | 60 | 30 | 5 | 79.36 ± 0.94 |
| 11 | 4 | 60 | 50 | 3 | 80.85 ± 0.57 |
| 12 | 4 | 60 | 30 | 1 | 42.87 ± 1.41 |
| 13 | 2 | 50 | 10 | 3 | 21.04±1.50 |
| 14 | 2 | 50 | 30 | 5 | 53.10 ± 1.83 |
| 15 | 2 | 50 | 50 | 3 | 59.30 ± 4.06 |
| 16 | 2 | 50 | 30 | 1 | 28.63 ± 1.16 |
| 17 | 4 | 50 | 50 | 1 | 47.27 ± 0.42 |
| 18 | 6 | 50 | 30 | 5 | 77.47 ± 2.49 |
| 19 | 6 | 50 | 30 | 1 | 40.33±1.72 |
| 20 | 6 | 50 | 10 | 3 | 44.71 ± 1.32 |
| 21 | 6 | 50 | 50 | 3 | 78.69 ± 0.31 |
| 22 | 4 | 50 | 30 | 3 | 61.06±1.82 |
| 23 | 4 | 50 | 50 | 5 | 82.64 ± 1.94 |
| 24 | 4 | 50 | 30 | 3 | 59.80 ± 2.46 |
| 25 | 4 | 50 | 10 | 1 | 14.52 ± 1.85 |
| 26 | 4 | 50 | 30 | 3 | 61.30 ± 1.27 |
| 27 | 4 | 50 | 10 | 5 | 36.35 ± 3.77 |
| Run | Independent Variable | Relative Conversion (%) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental a | ANN-Predicted | |
| 1 | 4.5 | 46 | 20 | 4.5 | 60.98 ± 3.55 | 58.75 |
| 2 | 2.25 | 54 | 40 | 1.5 | 50.37 ± 2.29 | 46.70 |
| 3 | 3.25 | 54 | 15 | 4.5 | 54.91 ± 3.97 | 50.93 |
| R2 | 0.992 | |||||
| RMSE | 3.380 | |||||
| Optimal Condition | Relative Conversion (%) | ||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental a | ANN-Predicted |
| 4.4 | 58 | 50 | 5 | 88.31 ± 0.30 | 84.80 |
| Parameters | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Reaction time (h) | X1 | 2 | 4 | 6 |
| Reaction temperature (°C) | X2 | 40 | 50 | 60 |
| Enzyme amount (mg) | X3 | 10 | 30 | 50 |
| Molar ratio a | X4 | 1 | 3 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Li, H.-J.; Liu, Y.-C.; Kuo, C.-H.; Shieh, C.-J. An Efficient Approach for Lipase-Catalyzed Synthesis of Retinyl Laurate Nutraceutical by Combining Ultrasound Assistance and Artificial Neural Network Optimization. Molecules 2017, 22, 1972. https://doi.org/10.3390/molecules22111972
Huang S-M, Li H-J, Liu Y-C, Kuo C-H, Shieh C-J. An Efficient Approach for Lipase-Catalyzed Synthesis of Retinyl Laurate Nutraceutical by Combining Ultrasound Assistance and Artificial Neural Network Optimization. Molecules. 2017; 22(11):1972. https://doi.org/10.3390/molecules22111972
Chicago/Turabian StyleHuang, Shang-Ming, Hsin-Ju Li, Yung-Chuan Liu, Chia-Hung Kuo, and Chwen-Jen Shieh. 2017. "An Efficient Approach for Lipase-Catalyzed Synthesis of Retinyl Laurate Nutraceutical by Combining Ultrasound Assistance and Artificial Neural Network Optimization" Molecules 22, no. 11: 1972. https://doi.org/10.3390/molecules22111972