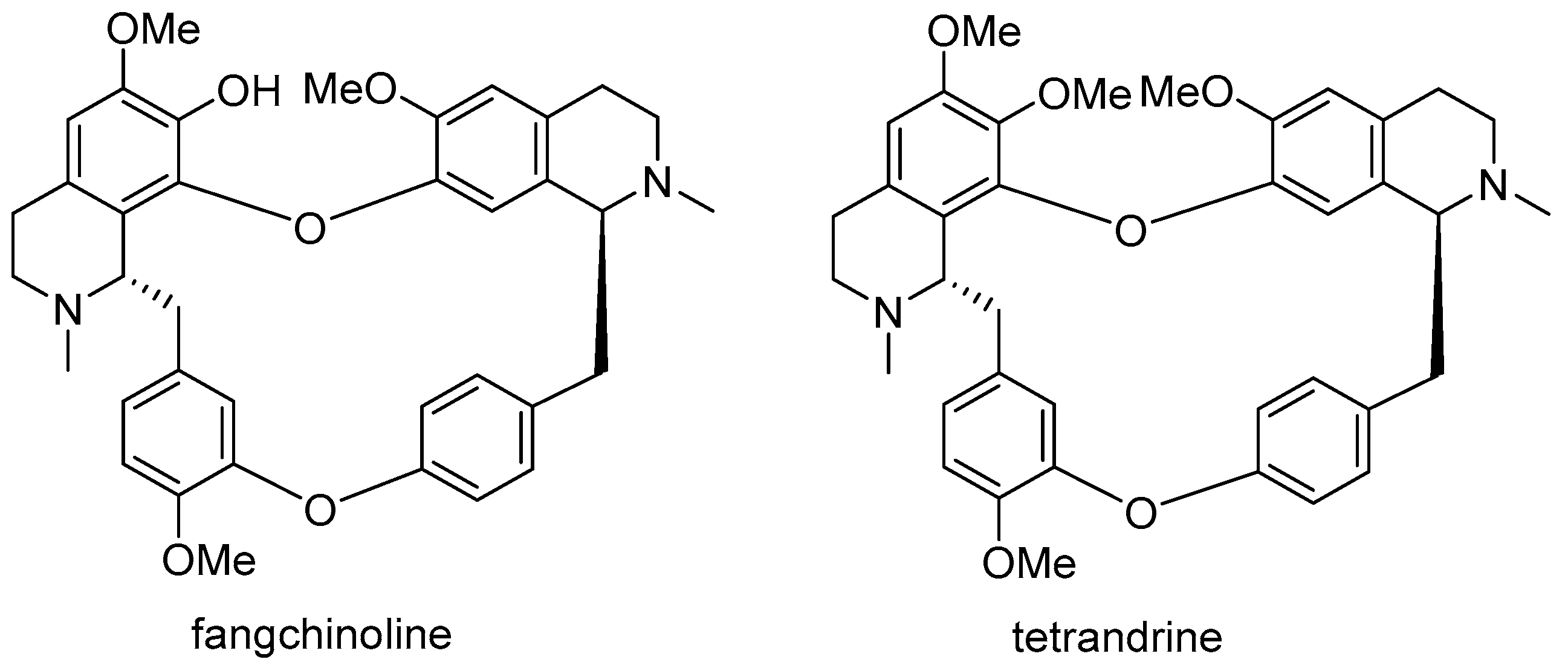
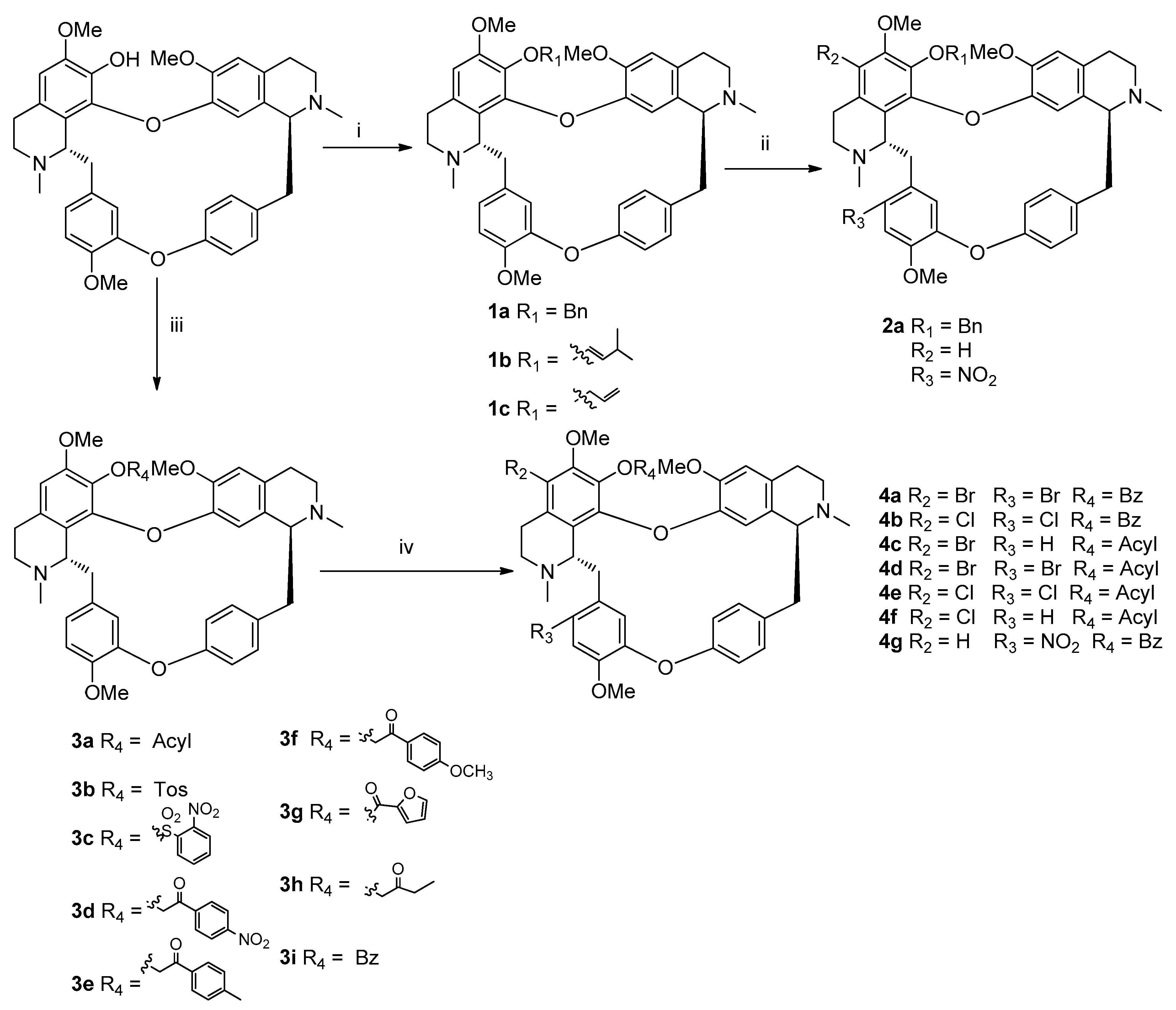
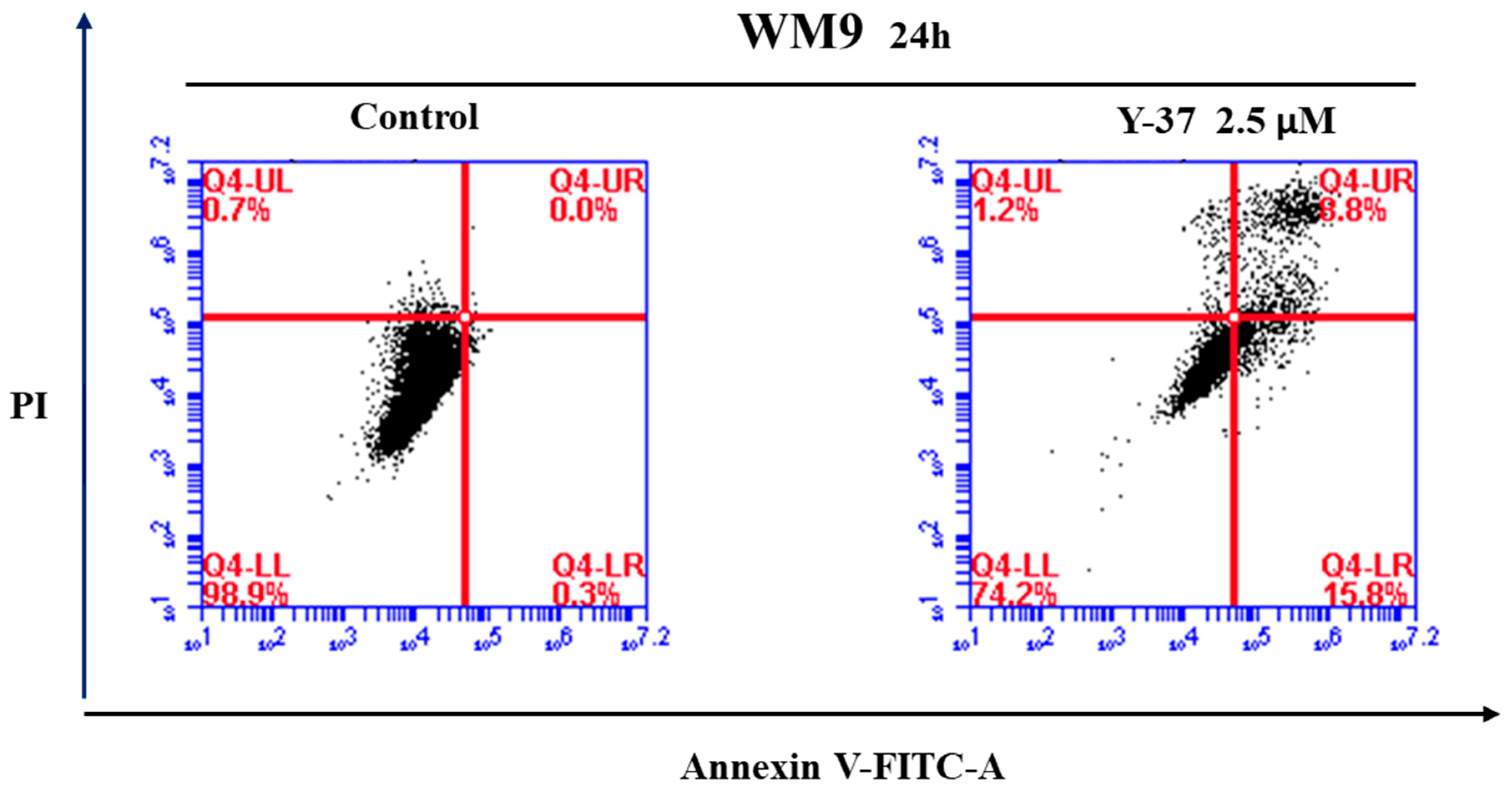
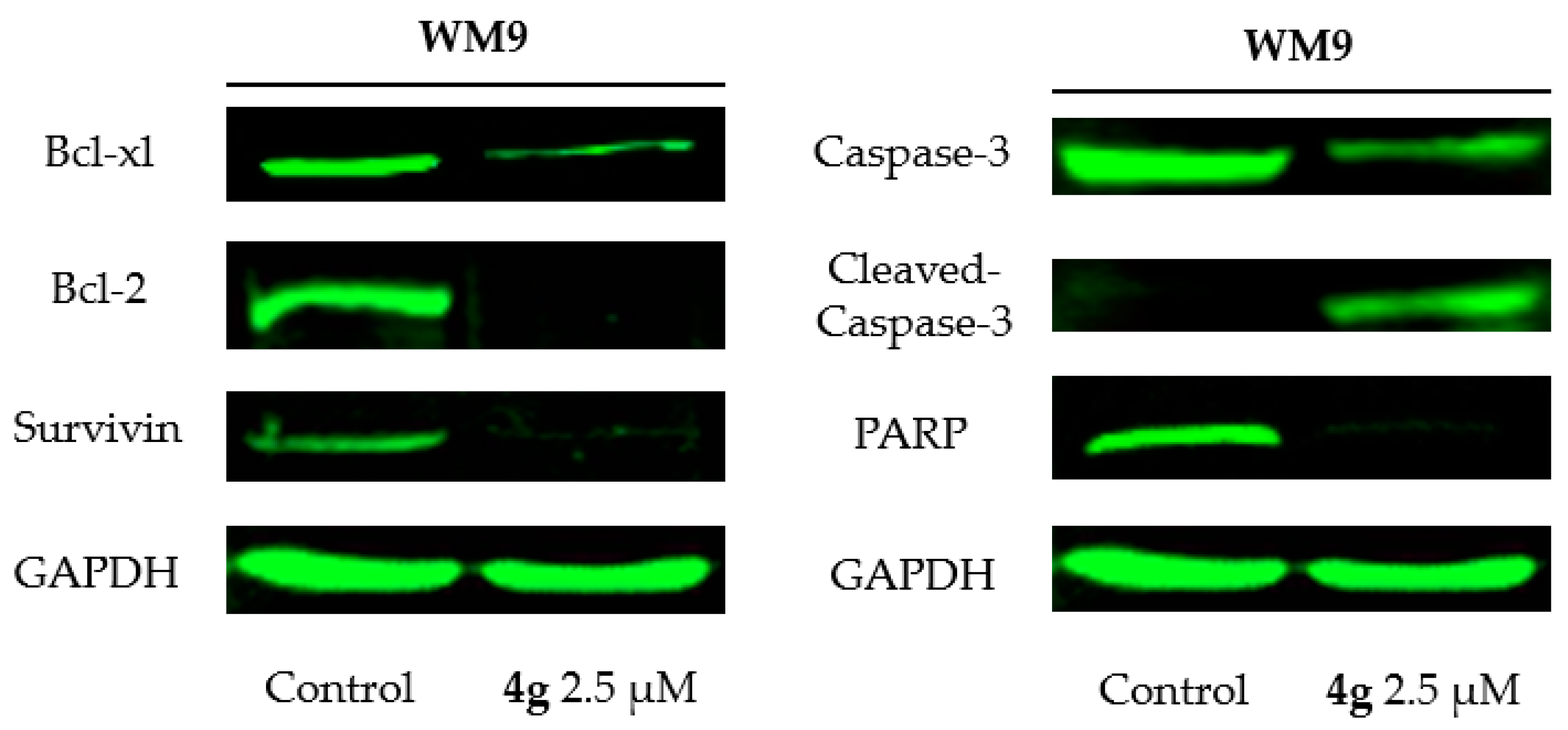
3.1. Method of Synthesis
The reagents and solvents were purchased commercially from Adamas and J&K chemical and were used without further purification unless otherwise noted. The solvents were purified according to the Guidelines in Purification of Laboratory Chemicals. Column chromatography was performed on silica gel (200–300 mesh, Qingdao, Shandong, China) using the indicated eluents. Thin-layer (0.25 mm, GF254) chromatography was carried out on silica gel plates (Qingdao, Shandong, China). NMR spectra were recorded on 400 MHz (Varian, Palo Alto, CA, USA) or spectrometers in appropriate solvents using TMS as internal standard or the solvent signals as secondary standards and the chemical shifts are shown in δ scales. Multiplicities of NMR signals were designated as s (singlet), d (doublet), t (triplet), br (broad), and m (multiplet, for unresolved lines). 13C-NMR spectra were recorded on 100 MHz or spectrometers. High-resolution mass spectra were obtained by using ESI-QTOF (Bruker, Billerica, MA, USA) mass spectrometry.
3.1.1. General Procedure for the Synthesis of 1a to 1c
To a solution of fangchinoline (0.16 mmol, 1 eq.) in 5 mL dimethylformamide under protection of argon at ambient temperature, sodium hydride (0.2 mmol, 55%, 1.2 eq.) was added and stirred for 10 min. Then the halide (0.2 mmol, 1.2 eq.) was added dropwise and stirred for 8 to 12 h. The mixture was poured into ammonium chloride solution with ice water bath. The aqueous phase was extracted with 2 × 30 mL of dichloromethane. The combined organic phase was washed with brine and dried on magnesium sulfonate, followed by removal of the solvent by rotavapor. The crude mixture was purified over by column chromatography (silica, eluent: DCM:MeOH = 50:1) to afford the pure product.
7-O-Benzyl-fangchinoline (1a) white amorphous solid: yield 83%. C44H46N2O6 ESI-MS: m/z 698.86 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.34 (1H, dd, J = 2.0, 8.0 Hz), 7.22 (3H, m), 7.15 (1H, dd, J = 2.4, 8.0 Hz), 7.00 (2H, m), 6.88 (2H, m), 6.80 (1H, dd, J = 2.4, 8.0 Hz), 6.52 (1H, s), 6.51 (1H, s), 6.33 (1H, s), 6.31 (1H, d, J = 8.0 Hz), 5.86 (1H, s), 4.61 (1H, d, J = 10.4 Hz), 4.26 (1H, d, J = 10.4 Hz), 3.94 (3H, s), 3.76 (1H, m), 3.73 (3H, s), 3.51 (3H, m), 3.37 (3H, s), 3.31–2.55 (8H, m), 2.53 (2H, m), 2.50 (3H, s), 2.35 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 154.0, 151.6, 149.5, 148.9, 148.8, 147.2, 144.2, 137.6, 136.7, 134.7, 132.7, 130.3, 128.4, 128.4, 128.1, 128.1, 128.1, 128.1, 128.1, 127.7, 127.7, 127.7, 123.0, 122.1, 122.1, 120.5, 116.2, 112.9, 111.6, 106.1, 74.5, 64.2, 61.7, 56.2, 56.0, 55.9, 45.5, 42.5, 42.3, 42.0, 40.3, 37.2, 24.5, 22.8.
7-O-(3-Methylbut-1-ene)-fangchinoline (1b) off-yellow amorphous solid: yield 57%. C42H48NO6 ESI-MS: m/z 676.85 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.37 (1H, dd, J = 2.0, 8.0 Hz), 7.15 (1H, dd, J = 2.4, 8.0 Hz), 6.92 (1H, d, J = 8.0 Hz, H-14), 6.88 (1H, s), 6.86 (1H, s), 6.83 (1H, dd, J = 2.4, 8.4 Hz), 6.54 (1H, d, J = 1.6 Hz), 6.51 (1H, s), 6.33 (1H, d, J = 2.4 Hz), 6.31 (1H, s), 5.96 (1H, s), 4.83 (1H, dd, J = 6.4, 7.6 Hz), 4.00 (1H, m), 3.94 (3H, s), 3.84 (1H, m), 3.75 (3H, s), 3.55 (2H, m), 3.37 (3H, s), 3.33 (1H, m), 2.97-2.70 (8H, m), 2.64 (3H, s), 2.52 (2H, m), 2.35 (3H, s), 1.60 (3H, s, –CH3–), 1.52 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 153.8, 151.7, 149.4, 148.8, 148.7, 147.1, 143.9, 136.8, 136.6, 135.2, 134.8, 132.8, 130.3, 128.1, 128.1, 127.3, 122.8, 122.1, 122.0, 120.6, 120.3, 116.1, 112.7, 111.5, 105.6, 69.3, 64.5, 61.6, 56.2, 55.9, 55.8, 45.7, 44.3, 42.7, 42.4, 42.0, 41.0, 29.8, 25.8, 24.4, 22.8, 22.2.
7-O-(Prop-1-ene)-fangchinoline (1c) off-yellow amorphous solid: yield 71%. C40H44N2O6 ESI-MS: m/z 648.80 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.37 (1H, d, J = 8.4 Hz, H-14′), 7.15 (1H, dd, J = 2.4, 8.4 Hz), 6.87 (2H, s, H-13), 6.82 (1H, dd, J = 2.4, 8.4 Hz), 6.54 (1H, s), 6.51 (1H, s), 6.33 (1H, dd, J = 2.4, 8.4 Hz), 6.31 (1H, s), 5.96 (1H, s), 5.45 (1H, m), 4.95 (2H, m), 4.05 (1H, dd, J = 5.2, 11.6 Hz), 3.93 (3H, s), 3.86 (1H, dd, J = 6.0, 11.2 Hz), 3.78 (2H, m), 3.74 (3H, s), 3.53 (2H, m), 3.37 (3H, s), 3.01–2.68 (8H, m), 2.60 (3H, s), 2.48 (2H, m), 2.33 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 153.6, 151.4, 149.3, 148.5, 148.4, 146.9, 143.7, 136.3, 135.0, 134.6, 133.9, 132.6, 130.1, 128.2, 128.0, 127.4, 122.8, 122.7, 121.9, 121.8, 120.3, 116.8, 115.9, 112.6, 111.3, 105.6, 73.4, 64.2, 61.3, 56.0, 55.7, 55.6, 45.4, 44.0, 42.5, 42.2, 41.8, 40.7, 24.1, 22.0.
3.1.2. General Procedure for the Synthesis of 3a to 3i
To a solution of fangchinoline (0.16 mmol, 1 eq.) in 5 mL dichloromethane with the protection of argon at ambient temperature, acyl halide (0.18 mmol, 1.1 eq.) and DMAP (0.04 mmol, 0.25 eq.) were added and stirred for 1.5 h. The reaction was poured into 10 mL saturated sodium bicarbonate solution and exacted with 2 × 30 mL of dichloromethane. The combined organic phase was washed with brine and dried by magnesium sulfonate, followed by removal of the solvent by rotavapor. The residue was purified by column chromatography (silica, eluent: DCM:MeOH = 50:1) to afford the pure product.
7-O-Acetyl-fangchinoline (3a) off-white amorphous solid: yield 96%. C39H42N2O7 ESI-MS: m/z 650.77 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.4 Hz), 7.13 (1H, dd, J = 2.8, 8.4 Hz), 6.86 (1H, s), 6.85 (1H, s), 6.80 (1H, dd, J = 2.4, 8.4 Hz), 6.57 (1H, s), 6.52 (1H, s), 6.32 (1H, dd, J = 2.0, 8.4 Hz), 6.28 (1H, s), 6.05 (1H, s), 3.92 (3H, s), 3.88 (1H, dd, J = 5.6, 11.2 Hz), 3.76 (3H, s), 3.74–3.36 (3H, m), 3.35 (3H, s), 3.23 (1H, dd, J = 6.0, 12.4 Hz), 3.00–2.69 (7H, m), 2.61 (3H, s), 2.55–2.34 (2H, m), 2.28 (3H, s), 2.32 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.9, 154.3, 151.9, 148.7, 147.6, 147.2, 146.5, 141.3, 140.1, 135.7, 133.5, 132.5, 130.8, 129.1, 127.8, 122.1, 121.9, 121.8, 121.5, 120.1, 115.8, 111.6, 104.3, 63.6, 61.9, 56.2, 55.4, 50.8, 45.6, 44.1, 42.6, 42.1, 41.8, 39.7, 29.7, 25.5, 23.3.
7-O-Tosyl-fangchinoline (3b) yellow amorphous solid: yield 90%. C44H46N2O8S ESI-MS: m/z 762.29 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.75 (2H, s), 7.50 (2H, s), 7.47 (2H, t, J = 15.2 Hz), 7.35 (2H, d, J = 6.6 Hz), 7.15 (2H, d, J = 6.4 Hz), 7.10 (1H, s), 6.92 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 155.0, 149.9, 148.5, 148.0, 147.1, 144.2, 144.0, 138.8, 134.9, 132.7, 130.4, 130.0, 130.0, 128.9, 128.9, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-(o-Nirtobenzessulfonyl)-fangchinoline (3c) yellow amorphous solid: yield 90%. C44H46N3O10S ESI-MS: m/z 793.89 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.50 (1H, dd, J = 2.0, 8.0 Hz), 8.01–8.05 (3H, m), 7.35 (2H, dd, J = 2.6, 8.6 Hz), 7.15 (2H, dd, J = 2.4, 8.4 Hz), 7.10 (1H, s), 6.92 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 155.0, 149.9, 148.5, 148.0, 148.0, 147.1, 144.2, 144.0, 138.8, 136.3, 134.9, 133.4, 130.4, 128.9, 128.9, 128.3, 128.3, 128.3, 128.0, 125.2, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-(p-Nitro-benzoyl)-fangchinoline (3d) yellow amorphous solid: 90%. C44H43N3O9 ESI-MS: m/z 757.84 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.30–8.38 (4H, m), 7.35 (2H, dd, J = 2.2, 8.2 Hz), 7.15 (2H, dd, J = 2.4, 8.4 Hz), 7.10 (1H, s), 6.90 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 2.36–3.41 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ 165.2, 154.2, 153.1, 149.9, 149.6, 148.3, 148.1, 147.2, 144.9, 133.6, 132.7, 132.7, 132.3, 131.3, 131.3, 128.5, 128.5, 128.2 127.8, 126.1, 123.8, 123.8, 123.0, 123.0, 121.6, 121.6, 119.5, 117.8, 110.2, 110.1, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 22.8.
7-O-(p-Methyl-benzoyl)-fangchinoline (3e) brown amorphous solid: yield 95%. C45H46N2O7 ESI-MS: m/z 726.86 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.03 (1H, dd, J = 2.4, 8.4 Hz), 7.42 (2H, dd, J = 2.0, 8.0 Hz), 7.35 (2H, dd, J = 2.0, 8.0 Hz), 7.15 (2H, dd, J = 2.0, 8.0 Hz), 7.10 (1H, s), 6.90 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.41 (3H, s), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3,100 MHz) δ (ppm): 165.2, 154.2, 153.1, 149.9, 149.6, 148.3, 148.1, 147.2, 144.9, 133.6, 132.7, 132.7, 132.3, 130.2, 130.2, 128.8, 128.8, 128.2 127.8, 126.1, 123.8, 123.8, 123.0, 123.0, 121.6, 121.6, 119.5, 117.8, 110.2, 110.1, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 22.8.
7-O-(p-Methoxy-benzoyl)-fangchinoline (3f) yellow amorphous solid: yield 91%. C45H46N2O8 ESI-MS: m/z 742.87 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.13 (2H, dd, J = 2.0, 8.0 Hz), 7.42 (2H, dd, J = 2.2, 8.2 Hz), 7.35 (2H, dd, J = 2.0, 8.0 Hz), 7.15 (2H, dd, J = 2.0, 8.0 Hz), 7.10 (1H, s), 6.90 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.83 (3H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.41 (3H, s), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.8, 153.7, 149.5, 147.0, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 131.2, 131.2, 130.4, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 114.2, 114.2, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-(Furan-m-formyl)-fangchinoline (3g) yellow amorphous solid: yield 90%. C44H44N2O7 ESI-MS: m/z 702.80 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.97 (1H, m), 7.63 (1H, m), 7.35 (2H, dd, J = 2.0, 8.0 Hz), 7.15 (2H, dd, J = 2.0, 8.0 Hz), 7.10 (1H, s), 6.90 (1H, s), 6.83 (3H, m), 6.52 (1H, s), 4.57 (2H, m), 3.92 (3H, s), 3.90 (6H, s), 3.83 (3H, s), 3.81(3H, s), 3.00 (4H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.41 (3H, s), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 177.5, 153.7, 149.5, 147.0, 145.6, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 130.4, 130.0, 130.0, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 118.6, 116.0, 112.8, 112.0, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-Propionyl-fangchinoline (3h) light yellow amorphous solid: yield 88%. C40H44N2O7, ESI-MS: m/z 664.80 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.0 Hz), 7.13 (1H, dd, J = 2.0, 8.0 Hz), 6.86 (1H, s), 6.80 (1H, dd, J = 2.0, 8.0 Hz), 6.52 (1H, s), 6.48 (1H, s), 6.43 (1H, s), 6.24 (1H, d, J = 8.0 Hz), 5.91 (1H, s), 5.68 (1H, s), 3.93 (3H, s), 3.72 (1H, m), 3.61 (1H, dd, J = 5.6, 11.2 Hz), 3.00 (3H, s), 2.85 (1H, m), 2.73 (3H, m), 2.64 (3H, m), 2.43 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 172.6, 153.7, 149.5, 147.0, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 130.4, 130.0, 130.0, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 27.8, 24.6, 22.8, 11.5.
7-O-Benzoyl-fangchinoline (3i) light yellow amorphous solid: yield 97%. C44H44N2O7 ESI-MS: m/z 712.84 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.16 (2H, dd, J = 2.0, 8.0 Hz), 7.78 (1H, s), 7.47 (2H, t, J = 15.2 Hz), 7.34 (2H, m), 7.10 (1H, s), 6.87 (1H, s), 6.77 (1H, m), 6.60 (1H, s), 6.44 (1H, s), 6.39 (1H, s), 6.24 (1H, m), 5.57 (1H, s), 3.92 (3H, s), 3.69 (3H, s), 3.55 (3H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.8, 153.7, 149.5, 147.0, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 130.4, 130.0, 130.0, 128.9, 128.9, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
3.1.3. General Procedure for the Synthesis of 4a to 4h
7-O-Benzoyl-fangchinoline (3i, 0.16 mmol, 1 eq.) or 7-O-Acetyl-fangchinoline (3a, 0.16 mmol, 1 eq.) was added to 5 mL of trifluoracetic acid at 0 °C, and N-bromosuccinimide (0.18 mmol, 1.1 eq.) was added all at once and stirred for 10 min. Then, the reaction mixture was warmed up to room temperature and stirred for another 3 h. The mixture was then poured into ice-water and aqueous sodium bicarbonate was added dropwise until pH was about 8. The aqueous phase was exacted with 2 × 30 mL of dichloromethane. The combined organic phase was washed with brine and dried by magnesium sulfonate, followed by removal of the solvent by rotavapor. The crude mixture was purified by column chromatography (silica, eluent: DCM:MeOH = 30:1) to afford compound 4c.
For compound 4f, 1.1 eq. of N-chlorosuccinimide was added, while the other procedures were the same.
For compounds 4a, 4b, 4d and 4e, 2.2 eq. of N-bromosuccinimide or N-chlorosuccinimide was added, while the other procedures were the same.
7-O-Benzoyl-5,14-dibromo-fangchinoline (4a) brown amorphous solid: yield 63%. C44H42Br2N2O7 ESI-MS: m/z 870.64 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.16 (2H, dd, J = 2.0, 8.0 Hz), 7.78 (1H, s), 7.47 (2H, t, J = 15.2 Hz), 7.34 (2H, m), 7.10 (1H, s), 6.60 (1H, s), 6.44 (1H, s), 6.39 (1H, s), 6.24 (1H, m), 5.57 (1H, s), 3.92 (3H, s), 3.69 (3H, s), 3.55 (3H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.8, 153.7, 149.5, 147.0, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 130.4, 130.0, 130.0, 128.9, 128.9, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 115.7, 112.8, 112.5, 111.5, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-Benzoyl-5,14-dichloro-fangchinoline (4b) dark yellow amorphous solid: yield 79%. C44H42Cl2N2O7 ESI-MS: m/z 781.73 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.16 (2H, dd, J = 2.0, 8.0 Hz), 7.78 (1H, s), 7.47 (2H, t, J = 15.2 Hz), 7.34 (2H, m), 7.10 (1H, s), 6.60 (1H, s), 6.44 (1H, s), 6.39 (1H, s), 6.24 (1H, m), 5.57 (1H, s), 3.92 (3H, s), 3.69 (3H, s), 3.55 (3H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.8, 153.7, 149.5, 147.0, 142.8, 134.9, 134.8, 133.1, 132.7, 132.5, 130.4, 130.0, 130.0, 128.9, 128.9, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 114.7, 114.1, 112.8, 111.5, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.
7-O-Acetyl-5-bromo-fangchinoline (4c) light yellow amorphous solid: yield 75%. C39H41BrN2O7 ESI-MS: m/z 729.67 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.4 Hz), 7.13 (1H, dd, J = 2.8, 8.4 Hz), 6.86 (1H, s), 6.85 (1H, s), 6.80 (1H, dd, J = 2.4, 8.4 Hz), 6.52 (1H, s), 6.32 (1H, dd, J = 2.0, 8.4 Hz), 6.28 (1H, s), 6.05 (1H, s), 3.92 (3H, s), 3.88 (1H, dd, J = 5.6, 11.2 Hz), 3.76 (3H, s), 3.74–3.36 (3H, m), 3.35 (3H, s), 3.23 (1H, dd, J = 6.0, 12.4 Hz), 3.00–2.69 (7H, m), 2.61 (3H, s), 2.55–2.34 (2H, m), 2.28 (3H, s), 2.32 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.9, 154.3, 151.9, 148.7, 147.6, 147.2, 146.5, 141.3, 140.1, 135.7, 133.5, 132.5, 130.8, 129.1, 127.8, 122.1, 121.9, 121.8, 121.5, 120.1, 115.8, 112.5, 111.6, 63.6, 61.9, 56.2, 55.4, 50.8, 45.6, 44.1, 42.6, 42.1, 41.8, 39.7, 29.7, 25.5, 23.3.
7-O-Acetyl-5,14-dibromo-fangchinoline (4d) yellow amorphous solid: yield 50%. C39H40Br2N2O7 ESI-MS: m/z 808.56 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.4 Hz), 7.13 (1H, dd, J = 2.8, 8.4 Hz), 6.86 (1H, s), 6.80 (1H, dd, J = 2.4, 8.4 Hz), 6.52 (1H, s), 6.32 (1H, dd, J = 2.0, 8.4 Hz), 6.28 (1H, s), 6.05 (1H, s), 3.92 (3H, s), 3.88 (1H, dd, J = 5.6, 11.2 Hz), 3.76 (3H, s), 3.74–3.36 (3H, m), 3.35 (3H, s), 3.23 (1H, dd, J = 6.0, 12.4 Hz), 3.00–2.69 (7H, m), 2.61 (3H, s), 2.55–2.34 (2H, m), 2.28 (3H, s), 2.32 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.9, 154.3, 151.9, 148.7, 147.6, 147.2, 146.5, 141.3, 140.1, 135.7, 133.5, 132.5, 130.8, 129.1, 127.8, 122.1, 121.8, 121.5, 120.1, 115.8, 115.0, 112.5, 111.6, 63.6, 61.9, 56.2, 55.4, 50.8, 45.6, 44.1, 42.6, 42.1, 41.8, 39.7, 29.7, 25.5, 23.3.
7-O-Acetyl-5,14-dichloro-fangchinoline (4e) yellow amorphous solid: yield 43%. C39H40Cl2N2O7 ESI-MS: m/z 719.66 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.4 Hz), 7.13 (1H, dd, J = 2.8, 8.4 Hz), 6.85 (1H, s), 6.80 (1H, dd, J = 2.4, 8.4 Hz), 6.52 (1H, s), 6.32 (1H, dd, J = 2.0, 8.4 Hz), 6.28 (1H, s), 6.05 (1H, s), 3.92 (3H, s), 3.88 (1H, dd, J = 5.6, 11.2 Hz), 3.76 (3H, s), 3.74–3.36 (3H, m), 3.35 (3H, s), 3.23 (1H, dd, J = 6.0, 12.4 Hz), 3.00–2.69 (7H, m), 2.61 (3H, s), 2.55–2.34 (2H, m), 2.28 (3H, s), 2.32 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.9, 154.3, 151.9, 148.7, 147.6, 147.2, 146.5, 141.3, 140.1, 135.7, 133.5, 132.5, 130.8, 129.1, 127.7, 122.1, 121.8, 121.5, 120.1, 115.8, 114.1, 112.5, 111.6, 63.6, 61.9, 56.2, 55.4, 50.8, 45.6, 44.1, 42.6, 42.1, 41.8, 39.7, 29.7, 25.5, 23.3.
7-O-Acetyl-5-chloro-fangchinoline (4f) yellow amorphous solid: yield 69%. C39H41BrN2O7 ESI-MS: m/z 729.67 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.33 (1H, dd, J = 2.0, 8.4 Hz), 7.13 (1H, dd, J = 2.8, 8.4 Hz), 6.86 (1H, s), 6.85 (1H, s), 6.80 (1H, dd, J = 2.4, 8.4 Hz), 6.52 (1H, s), 6.32 (1H, dd, J = 2.0, 8.4 Hz), 6.28 (1H, s), 6.05 (1H, s), 3.92 (3H, s), 3.88 (1H, dd, J = 5.6, 11.2 Hz), 3.76 (3H, s), 3.74–3.36 (3H, m), 3.35 (3H, s), 3.23 (1H, dd, J = 6.0, 12.4 Hz), 3.00–2.69 (7H, m), 2.61 (3H, s), 2.55–2.34 (2H, m), 2.28 (3H, s), 2.32 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.9, 154.3, 151.9, 148.7, 147.6, 147.2, 146.5, 141.3, 140.1, 135.7, 133.5, 132.5, 130.8, 129.1, 127.8, 122.1, 121.9, 121.8, 121.5, 120.1, 115.8, 112.5, 111.6, 63.6, 61.9, 56.2, 55.4, 50.8, 45.6, 44.1, 42.6, 42.1, 41.8, 39.7, 29.7, 25.5, 23.3.
3.1.4. General Procedure for the Synthesis of Compounds 2a and 4g
Under the protection of argon, concentrated nitric acid (0.6 mL) was carefully dropped into the acetic anhydride (1.5 mL) in a pre-dried 10 mL round flask and stirred for 5 min at −10 °C to obtain the mixed acid. 7-Benzyl-fangchinoline (1a, 0.80 mmol, 1 eq.) or 7-O-Benzoyl-fangchinoline (3i, 0.80 mmol, 1eq.) dissolved in 3 mL dichloromethane was added into the mixed acid dropwise. The reaction was allowed to stir at room temperature for 70 min before 10 mL of water was added. The aqueous phase was exacted with 2 × 10 mL of dichloromethane. The combined organic phase was washed with brine and dried over magnesium sulfonate. The solvents were removed by rotavapor. The crude mixture was purified by column chromatography (silica, eluent: DCM:MeOH = 40:1) to afford the pure product.
7-Benzyl-14-nitro-fangchinoline (2a) yellow amorphous solid: yield 68%. C44H45N3O8 ESI-MS: m/z 743.86 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 7.42 (1H, s), 7.37 (1H, dd, J = 2.0, 8.0 Hz), 7.22 (1H, m), 7.21 (2H, d, J = 2.0 Hz), 7.13 (1H, dd, J = 2.4, 8.0 Hz), 6.99 (2H, m), 6.75 (1H, dd, J = 2.4, 8.0 Hz), 6.52 (1H, s), 6.51 (1H, s), 6.33 (1H, s), 6.29 (1H, dd, J = 2.0, 8.4 Hz), 5.85 (1H, s), 4.58 (1H, d, J = 10.8 Hz), 4.28 (1H, d, J = 10.8 Hz), 3.99 (3H, s), 3.81 (2H, m), 3.72 (3H, s), 3.59 (2H, m), 3.38 (3H, s), 3.32–2.69 (8H, m), 2.54 (1H, d, J = 13.6 Hz), 2.49 (3H, s), 2.35 (1H, dd, J = 4.0, 16.8 Hz), 2.22 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 154.0, 153.3, 151.1, 148.6, 147.8, 144.2, 142.2, 138.6, 136.7, 132.5, 129.0, 129.0, 128.5, 128.5, 128.0, 127.9, 127.9, 127.8, 127.8, 127.7, 127.7, 127.7, 123.0, 122.1, 122.1, 120.5, 116.2, 112.9, 111.6, 106.1, 74.5, 64.2, 61.7, 56.2, 56.0, 55.9, 45.5, 42.5, 42.3, 42.0, 40.3, 37.2, 24.5, 22.8.
7-O-Benzoyl-14-nitro-fangchinoline (4g) light brown amorphous solid: yield 85%. C44H42Br2N2O7 ESI-MS: m/z 757.84 [M + H]+; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.16 (2H, dd, J = 2.0, 8.0Hz), 7.78 (1H, s), 7.47 (2H, t, J = 15.2 Hz), 7.34 (2H, m), 6.66 (1H, s), 6.60 (1H, s), 6.44 (1H, s), 6.39 (1H, s), 6.24 (1H, m), 5.57 (1H, s), 3.92 (3H, s), 3.69 (3H, s), 3.55 (3H, m), 3.48 (3H, s), 3.41–2.36 (9H, m), 2.17 (3H, s), 1.89 (2H, m), 1.26 (3H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 163.6, 154.1, 152.9, 151.0, 149.4, 149.1, 148.8, 144.1, 142.7, 133.5, 132.4, 130.0, 130.0, 128.6, 128.6, 128.3, 128.3, 128.3, 128.0, 128.0, 122.8, 122.8, 122.3, 122.2, 121.8, 120.7, 116.0, 112.8, 111.5, 105.7, 64.0, 61.4, 56.2, 56.2, 56.1, 45.6, 44.0, 42.4, 41.9, 41.6, 41.3, 29.8, 24.6, 22.8.