Peptide Nucleic Acid Based Molecular Authentication for Identification of Four Medicinal Paeonia Species Using Melting Array Analysis of the Internal Transcribed Spacer 2 Region
Abstract
:1. Introduction
2. Results
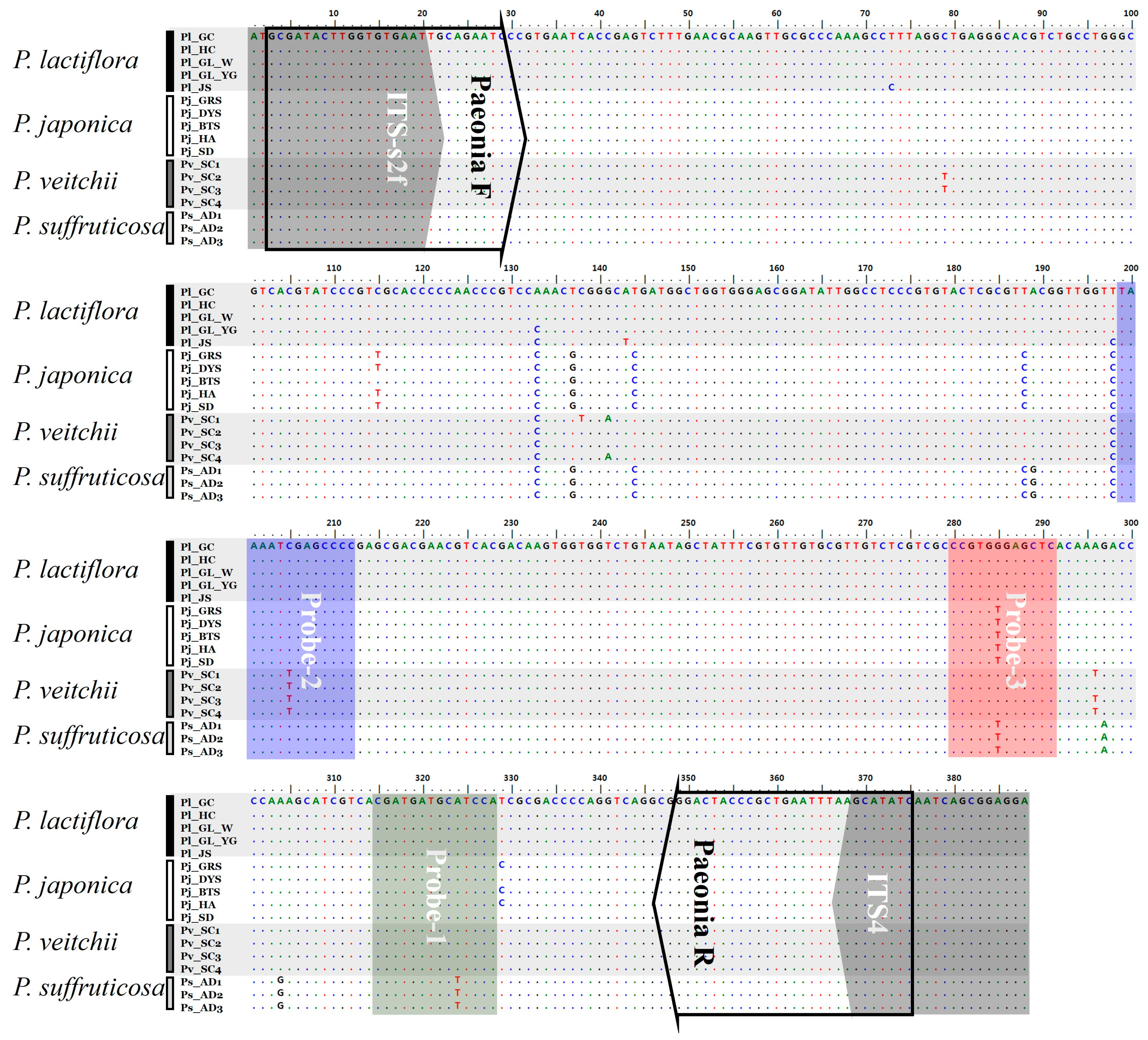
2.1. Analysis of ITS2 Sequences
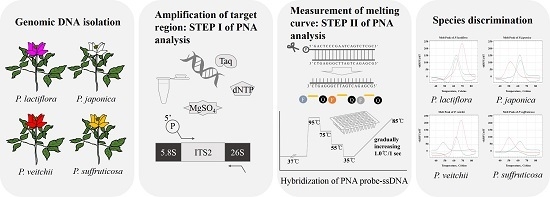
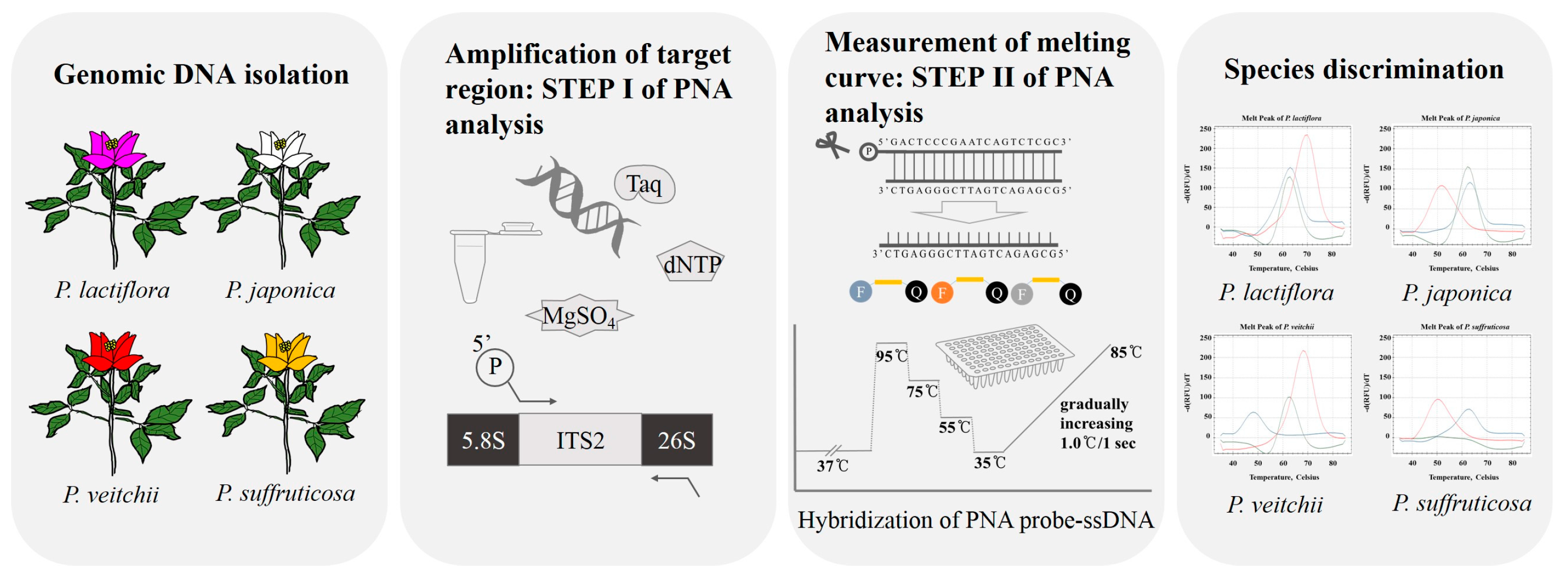
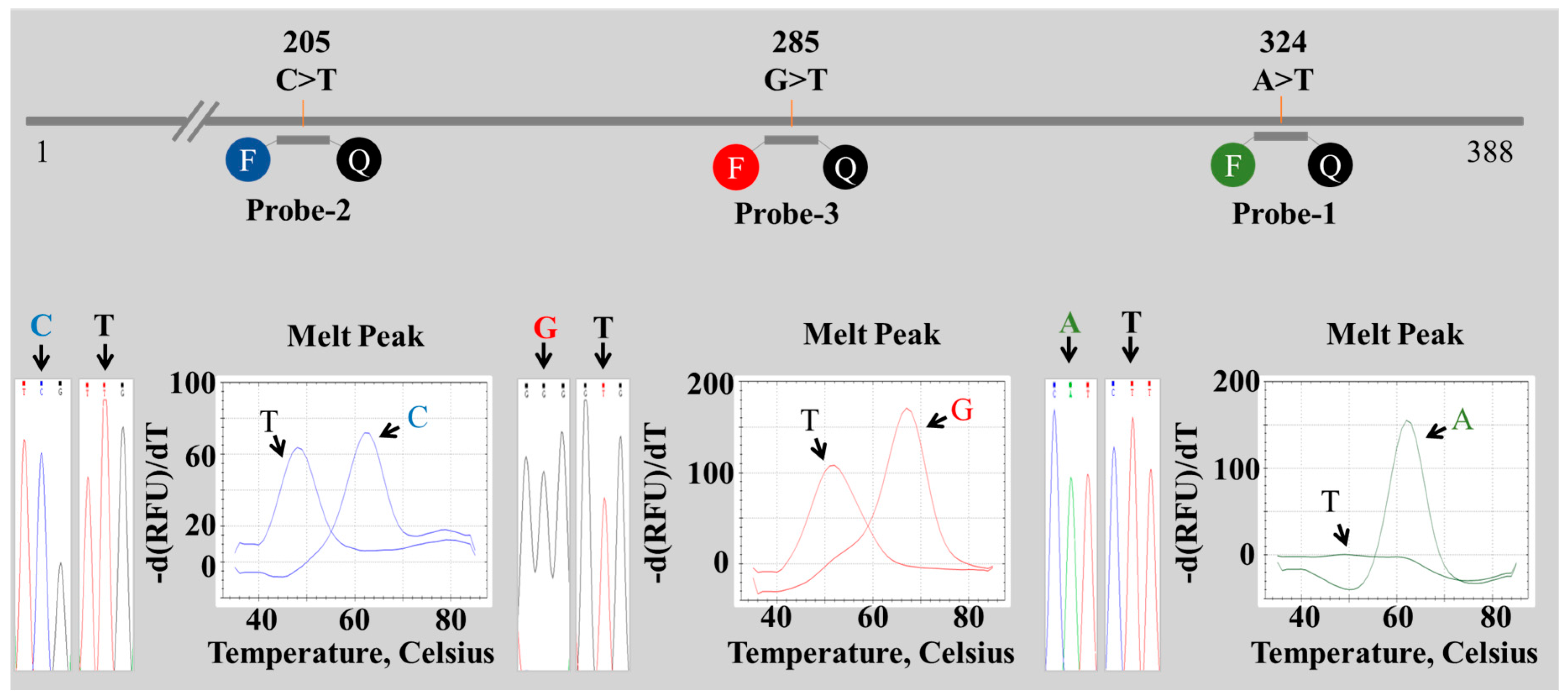
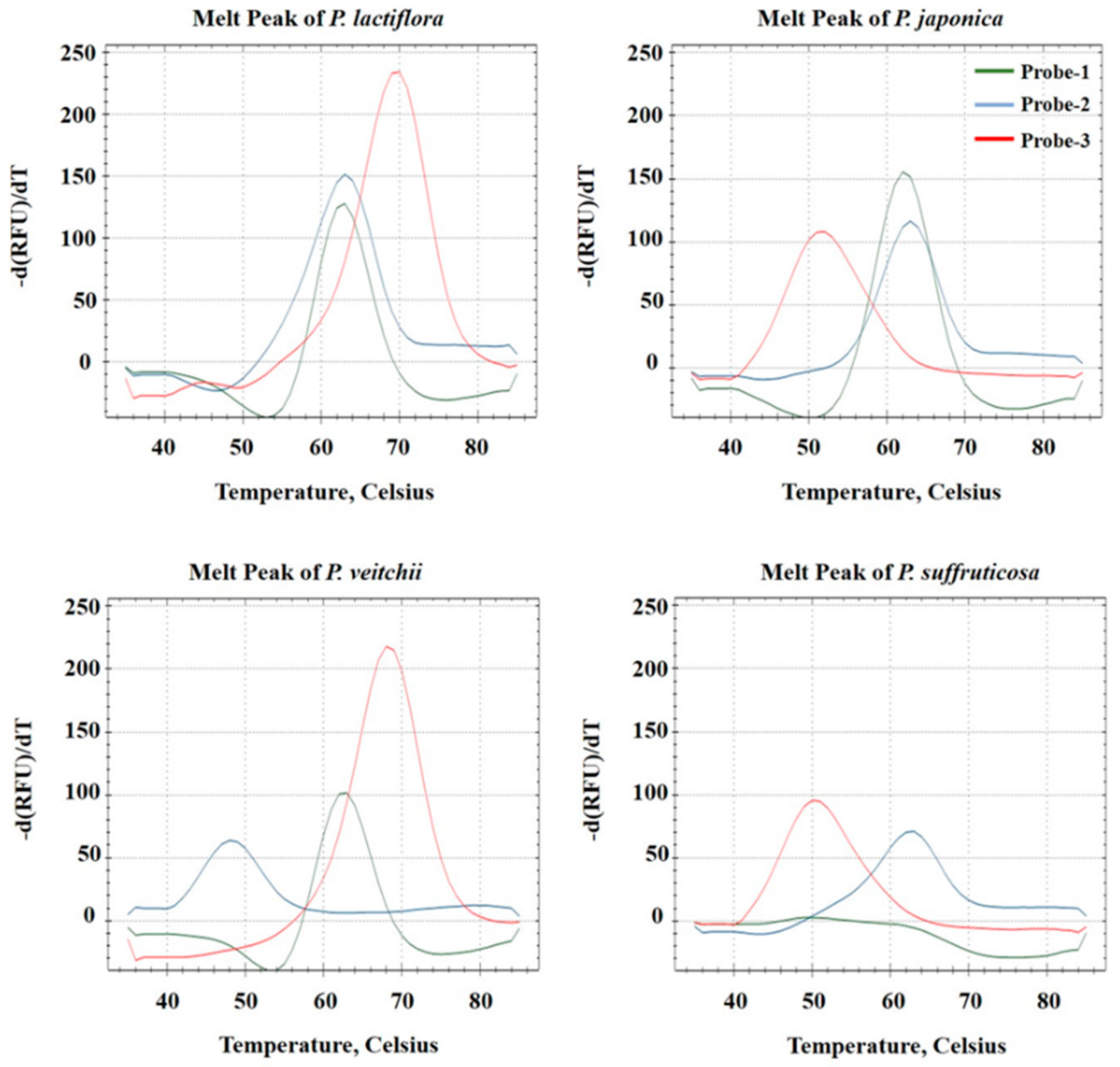
2.2. Establishment of a PNA Melting Array Method for Identification of Paeonia Species
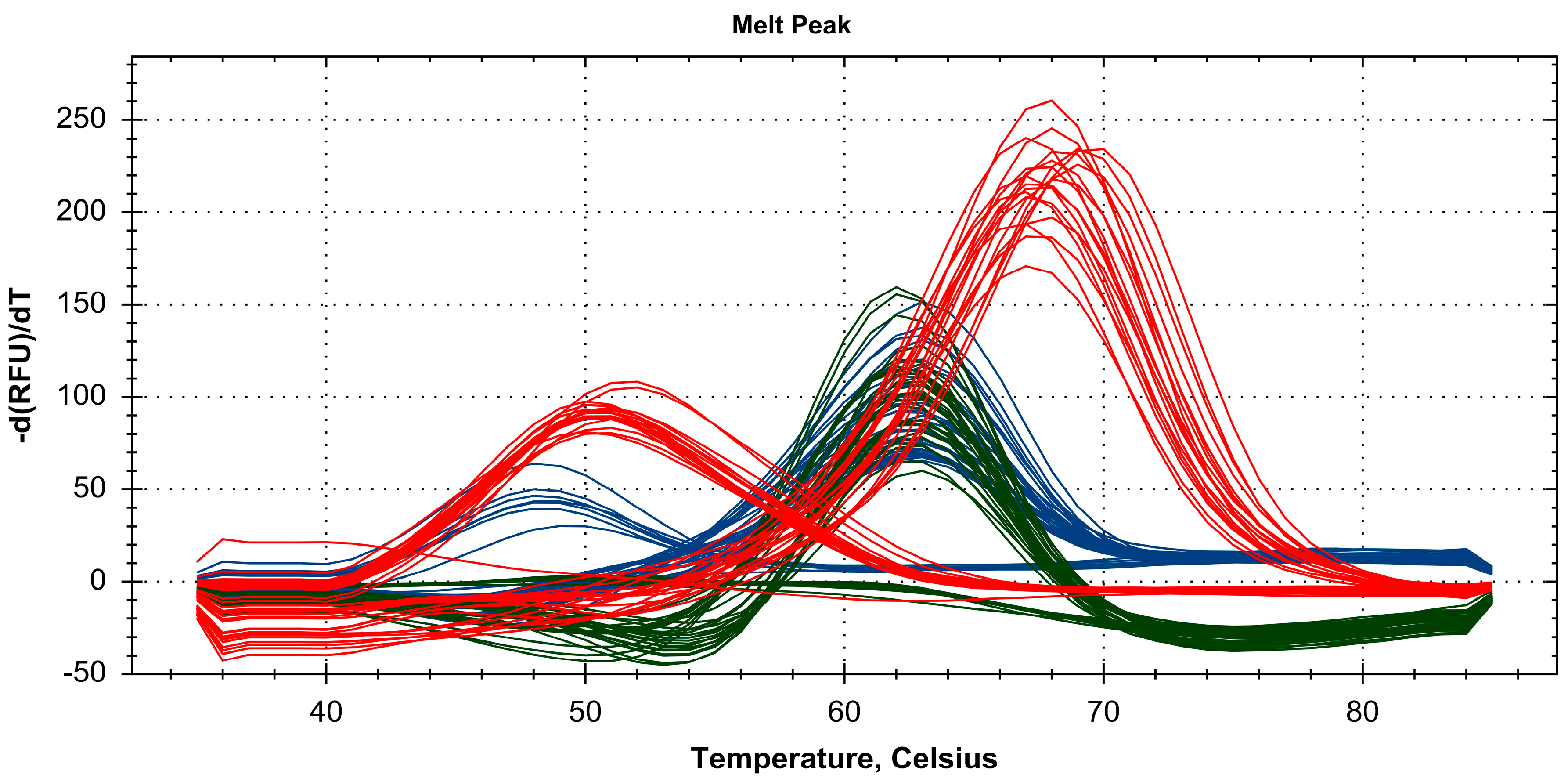
2.3. Discrimination of Four Paeonia Species Based on Melting Array Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and DNA Extraction
4.2. Amplification and Sequencing of ITS2 Regions
4.3. Comparative Analysis of ITS2 Sequences for the Design of PNA Probes
4.4. Establishment of PNA-Based Melting Array Method
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Defining Dictionary for Medicinal Herbs in Korea Institute of Oriental Medicine. Available online: http://boncho.kiom.re.kr/codex/ (accessed on 9 May 2017). (In Korean).
- Kim, W.J.; Moon, B.C.; Yang, S.; Han, K.S.; Choi, G.; Lee, A.Y. Rapid authentication of the herbal medicine plant species Aralia continentalis Kitag. and Angelica biserrata C.Q. Yuan and R.H. Shan using ITS2 sequences and multiplex-SCAR markers. Molecules 2016, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Ji, Y.; Choi, G.; Kang, Y.M.; Yang, S.; Moon, B.C. Molecular identification and phylogenetic analysis of important medicinal plant species in genus Paeonia based on rDNA-ITS, matK, and rbcL DNA barcode sequences. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lee, Y.; Oh, B.G.; Cho, C.; Kim, Y.; Shin, M.; Hong, M.; Jung, S.K.; Bae, H. Inhibition effects of Moutan Cortex Radicis on secretion of eotaxin in A549 human epithelial cells and eosinophil migration. J. Ethnopharmacol. 2007, 114, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Sun, Q.; Xu, B.; Yu, Y.; Wang, X. Molecularly imprinted solid phase extraction in a syringe filter for determination of triazine herbicides in Radix Paeoniae Alba by ultra-fast liquid chromatography. Talanta 2016, 148, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Bjørnstad, Å.; Ndjiondjop, M.N. An overview of molecular marker methods for plants. Afr. J. Biotechnol. 2006, 5, 2540–2568. [Google Scholar]
- Moon, B.C.; Ji, Y.; Lee, Y.M.; Kang, Y.M.; Kim, H.K. Authentication of Akebia quinata DECNE. from its common adulterant medicinal plant species based on the RAPD-derived SCAR markers and multiplex-PCR. Genes Genom. 2015, 37, 23–32. [Google Scholar] [CrossRef]
- Joshi, K.; Chavan, P.; Warude, D.; Patwardhan, B. Molecular markers in herbal drug technology. Curr. Sci. 2004, 87, 159–165. [Google Scholar]
- Li, J.; Cui, Y.; Jiang, J.; Yu, J.; Niu, L.; Deng, J.; Shen, F.; Zhang, L.; Yue, B.; Li, J. Applying DNA barcoding to conservation practice: A case study of endangered birds and large mammals in China. Biodivers. Conserv. 2017, 26, 653–668. [Google Scholar] [CrossRef]
- Benqtsson-Palme, J.; Hartmann, M.; Eriksson, K.M.; Pal, C.; Thorell, K.; Larsson, D.G.; Nilsson, R.H. METAXA2: Improved identification and taxonomic classification of small and large subunit rRNA in metagenomic data. Mol. Ecol. 2015, 15, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.; Abarenkov, K.; Kõljalg, U.; Öpik, M.; Chai, B.; Cole, J.; Wang, Q.; Crous, P.; Robert, V.; Helgason, T.; et al. Sequence-based classification and identification of Fungi. Mycologia 2016, 108, 1049–1068. [Google Scholar] [PubMed]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Tripathi, A.M.; Tyagi, A.; Kumar, A.; Singh, A.; Singh, S.; Chaudhary, L.B.; Roy, S. The Internal Transcribed Spacer (ITS) region and trnhH-psbA are suitable candidate loci for DNA barcoding of tropical tree species of India. PLoS ONE 2013, 8, e57934. [Google Scholar] [CrossRef] [PubMed]
- Mattia, F.D.; Bruni, I.; Galimberti, A.; Cattaneo, F.; Casiraghi, M.; Labra, M. A comparative study of different DNA barcoding markers for the identification of some members of Lamiacaea. Food Res. Int. 2011, 44, 693–702. [Google Scholar] [CrossRef]
- Ganie, S.H.; Upadhyay, P.; Das, S.; Sharma, M.P. Authentication of medicinal plants by DNA markers. Plant Gene 2015, 4, 83–99. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, X.; Liu, Y.; Yang, L.; Cai, Y.; Quan, S.; Pan, L.; Chen, S. A novel quantitative real-time PCR method for identification and quantification of mammalian and poultry species in foods. Food Control 2017, 76, 42–51. [Google Scholar] [CrossRef]
- Kitpipit, T.; Penchart, K.; Ouithavon, K.; Satasook, C.; Linacre, A.; Thanakiatkrai, P. A novel real time PCR assay using melt curve analysis for ivory identification. Forensic Sci. Int. 2016, 267, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, H.S.; Tsuge, K.; Kubota, S.; Aragane, M.; Ohta, H.; Sugita, R. Species identification of white false hellebore (Veratrum album subsp. oxysepalum) using real-time PCR. Forensic Sci. Int. 2017, 275, 160–166. [Google Scholar] [PubMed]
- Iwobi, A.; Sebah, D.; Spielmann, G.; Maggipinto, M.; Schrempp, M.; Kraemer, I.; Gerdes, L.; Busch, U.; Huber, I. A multiplex real-time PCR method for the quantitative determination of equine (horse) fractions in meat products. Food Control 2017, 74, 89–97. [Google Scholar] [CrossRef]
- Hur, D.; Kim, M.S.; Song, M.; Jung, J.; Park, H. Detection of genetic variation using dual-labeled peptide nucleic acid (PNA) probe-based melting point analysis. Biol. Proced. Online 2015, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Choi, J.J.; Kim, J.Y.; Han, Y.L.; Lee, G.K. PNA clamping-assisted fluorescence melting curve analysis for detecting EGFR and KRAS mutations in the circulating tumor DNA of patients with advanced non-small cell lung cancer. BMC Cancer 2016, 16, 627. [Google Scholar]
- Kim, Y.T.; Kim, J.W.; Kim, S.K.; Joe, G.H.; Hong, I.S. Simultaneous genotyping of multiple somatic mutations by using a clamping PNA and PNA detection probes. Chembiochem 2015, 16, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pang, X.; Song, J.; Shi, L.; Yao, H.; Han, J.; Leon, C. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnol. Adv. 2014, 32, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.C.; Kim, W.J.; Han, K.S.; Yang, S.; Kang, Y.; Park, I.; Piao, R. Differentiating authentic Adenophorae Radix from its adulterants in commercially-processed samples using multiplexed ITS sequence-based SCAR markers. Appl. Sci. 2017, 7, 660. [Google Scholar] [CrossRef]
- Monteros, M.J.; Ha, B.K.; Phillips, D.V.; Boerma, H.R. SNP assay to detect the ‘Hyuuga’ red-brown lesion resistance gene for Asian soybean rust. Theor. Appl. Genet. 2010, 121, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Chu, Z.Z.; Liu, X.G.; Jing, H.C.; Liu, Y.G.; Hao, D.Y. A Cost-effective high-resolution melting approach using the EvaGreen dye for DNA polymorphism detection and genotyping in plants. J. Integr. Plant Biol. 2010, 52, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yan, S.; Li, J.; Xiong, C.; Shi, Y.; Wu, L.; Xiang, L.; Deng, B.; Ma, W.; Chen, S. Study of commercially available Lobelia chinensis products using bar-HRM technology. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Nilssson, R.H.; Tedersoo, L.; Abarenkov, K.; Ryberg, M.; Kristiansson, E.; Hartmann, M.; Schoch, C.L.; Nylander, J.A.A.; Bergsten, J.; Porter, T.M.; et al. Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys 2012, 4, 37–63. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
Sample Availability: Samples of the four Paeonia species are available from the authors and the herbarium KIOM. |
| Scientific Name | Amplicon Length (bp) | Intra-Species Distance (%) | Inter-Species Distance (%) | G + C (%) |
|---|---|---|---|---|
| P. lactiflora | 388 | 0.47 ± 0.45 | 1.84 ± 0.53 | 56.1 |
| P. japonica | 388 | 0.26 ± 0.17 | 1.87 ± 0.35 | 56.9 |
| P. veitchii | 388 | 0.61 ± 0.32 | 2.00 ± 0.69 | 55.9 |
| P. suffruticosa | 388 | 0.00 ± 0.00 | 2.25 ± 0.66 | 57.2 |
| Primer Name | Primer Sequence (5′ to 3′) | PCR Parameter | Reaction Component |
|---|---|---|---|
| ITS-s2f ITS4 | ATG CGA TAC TTG GTG TGA AT TCC TCC GCT TAT TGA TAT GC | Step 1: 95 °C, 2 min Step 2: 95 °C, 40 s 53 °C, 30 s 72 °C, 40 s (35 cycles) Step 3: 72 °C 5 min | gDNA: 1 µL (15 ng/µL) Primer: each 2 µL (10 pmol/µL) 2 × Pre-mix: 20 µL Final vol.: 40 µL |
| Paeonia F Paeonia R | GCG ATA CTT GGT GTG AAT TGC AGA ATC GAT ATG CTT AAA TTC AGC GGG TAG TCC | Step 1: 95 °C, 10 min Step 2: 95 °C, 30 s 60 °C, 40 s 72 °C, 40 s (33 cycles) Step 3: 72 °C, 1 min 20 °C, 1 min | gDNA: 1 µL (15 ng/µL) Primer: mixture 1 µL 2 × qPCR Pre-mix: 10 µL Final vol.: 20 µL |
| PNA Probe-1 PNA Probe-2 PNA Probe-3 | CGA TGA TGC ATC CA TAA ATC GAG CCC CG CCG TGG GAG CTC | Step 1: 37 °C, 1 h Step 2: 95 °C, 2 min 75 °C, 1 min 55 °C, 1 min 35 °C, 3 min Step 3: 35–85 °C, increasing 1 °C/s | Amplicon DNA: 9 µL 2 × melting array Buffer A: 10 µL Melting array Buffer B: 0.5 µL PNA probe mixture: 0.5 µL Final vol.: 20 µL |
| Species | Aligned Nucleic Acid Position | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 137 | 144 | 188 | 189 | 205 | 285 | 297 | 304 | 324 | |
| P. lactiflora | T | T | T | A | C | G | G | A | A |
| P. japonica | G | C | C | A | C | T | G | A | A |
| P. veitchii | T | T | T | A | T | G | G | A | A |
| P. suffruticosa | G | C | C | G | C | T | A | G | T |
| Species | Probe 1 | Probe 2 | Probe 3 | Barcode | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NT 1 | Tm 2 | MT 3 | NT 1 | Tm 2 | MT 3 | NT 1 | Tm 2 | MT 3 | ||
| P. lactiflora | A | 63 °C | PM | C | 63 °C | PM | G | 70 °C | PM | 1 1 1 |
| P. japonica | A | 63 °C | PM | C | 63 °C | PM | T | 52 °C | MM | 1 1 0 |
| P. veitchii | A | 63 °C | PM | T | 48 °C | MM | G | 70 °C | PM | 1 0 1 |
| P. suffruticosa | T | ND | MM | C | 63 °C | PM | T | 52 °C | MM | 0 1 0 |
| No. | Collection Site | Sample Name | Barcode | Discrimination Result |
|---|---|---|---|---|
| 1 | Tongyoung, Gyeongnam, Korea | P. lactiflora | 1 1 1 | P. lactiflora |
| 2 | Jeongseon, Gangwon, Korea | P. lactiflora | 1 1 1 | P. lactiflora |
| 3 | Jinju, Gyeongnam, Korea | P. lactiflora | 1 1 1 | P. lactiflora |
| 4 | Sejong, Sejong, Korea | P. lactiflora | 1 1 1 | P. lactiflora |
| 5 | Inje, Gangwon, Korea | P. lactiflora | 1 1 1 | P. lactiflora |
| 6 | Yanji, Jilin, China | P. lactiflora | 1 1 1 | P. lactiflora |
| 7 | Andong, Gyeongbuk, Korea | P. japonica | 1 1 0 | P. japonica |
| 8 | Geochang, Gyeongnam, Korea | P. japonica | 1 1 0 | P. japonica |
| 9 | Sejong, Sejong, Korea | P. japonica * | 1 1 1 | P. lactiflora * |
| 10 | Geumsan, Chungnam, Korea | P. japonica | 1 1 0 | P. japonica |
| 11 | Hamyang, Gyeongnam, Korea | P. japonica | 1 1 0 | P. japonica |
| 12 | Jeongseon, Gangwon, Korea | P. japonica | 1 1 1 | P. japonica |
| 13 | Sancheong, Gyeongnam, Korea | P. japonica | 1 1 1 | P. japonica |
| 14 | Ngawa, Sichuan, China | P. veitchii | 1 0 1 | P. veitchii |
| 15 | Ngawa, Sichuan, China | P. veitchii | 1 0 1 | P. veitchii |
| 16 | Gannan, Gansu, China | P. veitchii | 1 0 1 | P. veitchii |
| 17 | Jinju, Gyeongnam, Korea | P. suffruticosa | 0 1 0 | P. suffruticosa |
| 18 | Jinju, Gyeongnam, Korea | P. suffruticosa | 0 1 0 | P. suffruticosa |
| 19 | Jinju, Gyeongnam, Korea | P. suffruticosa | 0 1 0 | P. suffruticosa |
| 20 | Jinju, Gyeongnam, Korea | P. suffruticosa | 0 1 0 | P. suffruticosa |
| 21 | Jinju, Gyeongnam, Korea | P. suffruticosa | 0 1 0 | P. suffruticosa |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.J.; Yang, S.; Choi, G.; Moon, B.C. Peptide Nucleic Acid Based Molecular Authentication for Identification of Four Medicinal Paeonia Species Using Melting Array Analysis of the Internal Transcribed Spacer 2 Region. Molecules 2017, 22, 1922. https://doi.org/10.3390/molecules22111922
Kim WJ, Yang S, Choi G, Moon BC. Peptide Nucleic Acid Based Molecular Authentication for Identification of Four Medicinal Paeonia Species Using Melting Array Analysis of the Internal Transcribed Spacer 2 Region. Molecules. 2017; 22(11):1922. https://doi.org/10.3390/molecules22111922
Chicago/Turabian StyleKim, Wook Jin, Sungyu Yang, Goya Choi, and Byeong Cheol Moon. 2017. "Peptide Nucleic Acid Based Molecular Authentication for Identification of Four Medicinal Paeonia Species Using Melting Array Analysis of the Internal Transcribed Spacer 2 Region" Molecules 22, no. 11: 1922. https://doi.org/10.3390/molecules22111922