Pharmacological Effects of Two Novel Bombesin-Like Peptides from the Skin Secretions of Chinese Piebald Odorous Frog (Odorrana schmackeri) and European Edible Frog (Pelophylax kl. esculentus) on Smooth Muscle
Abstract
:1. Introduction
2. Results
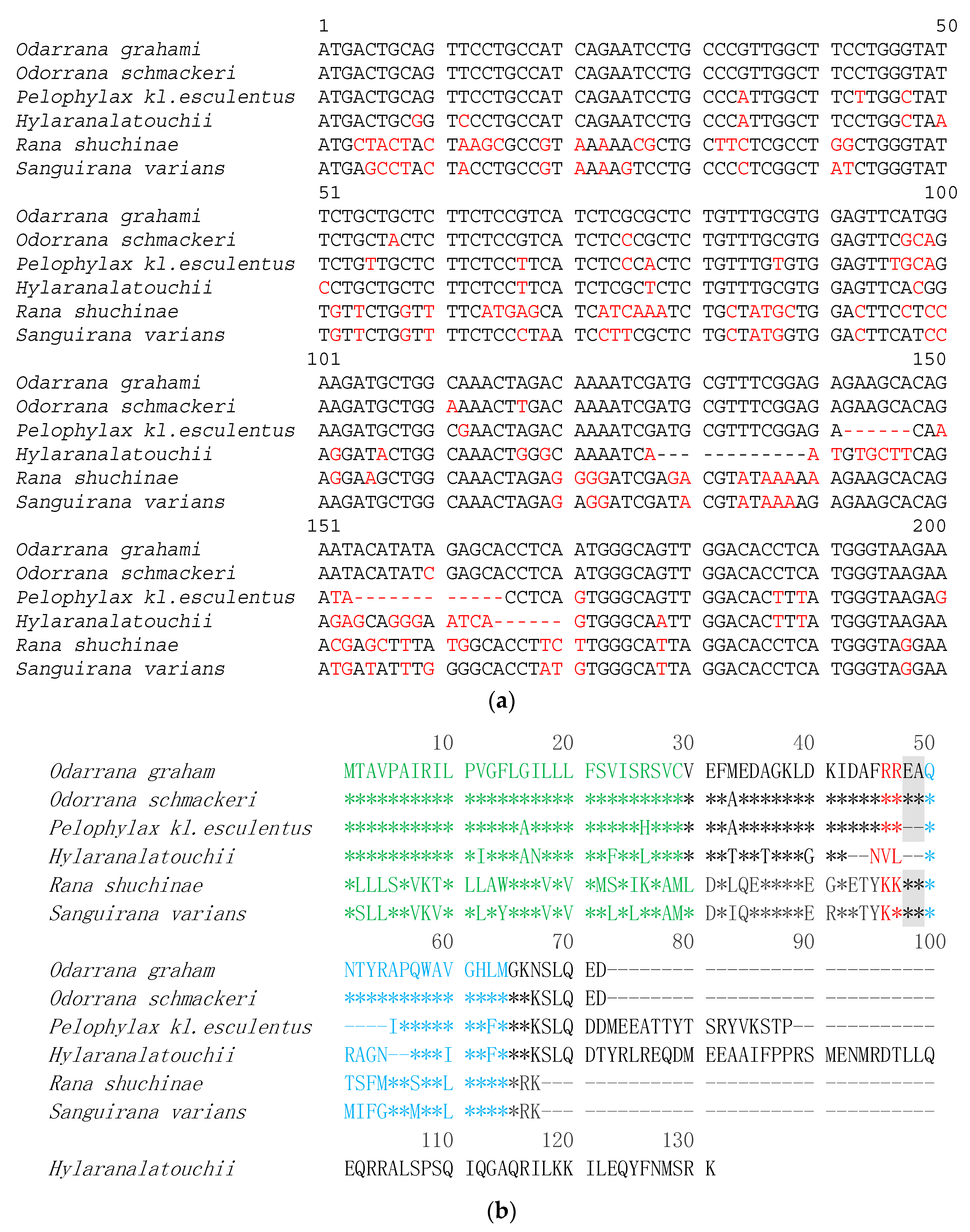
2.1. Molecular Cloning of Bombesin-OS and Bombesin-PE Precursor-Encoding cDNAs
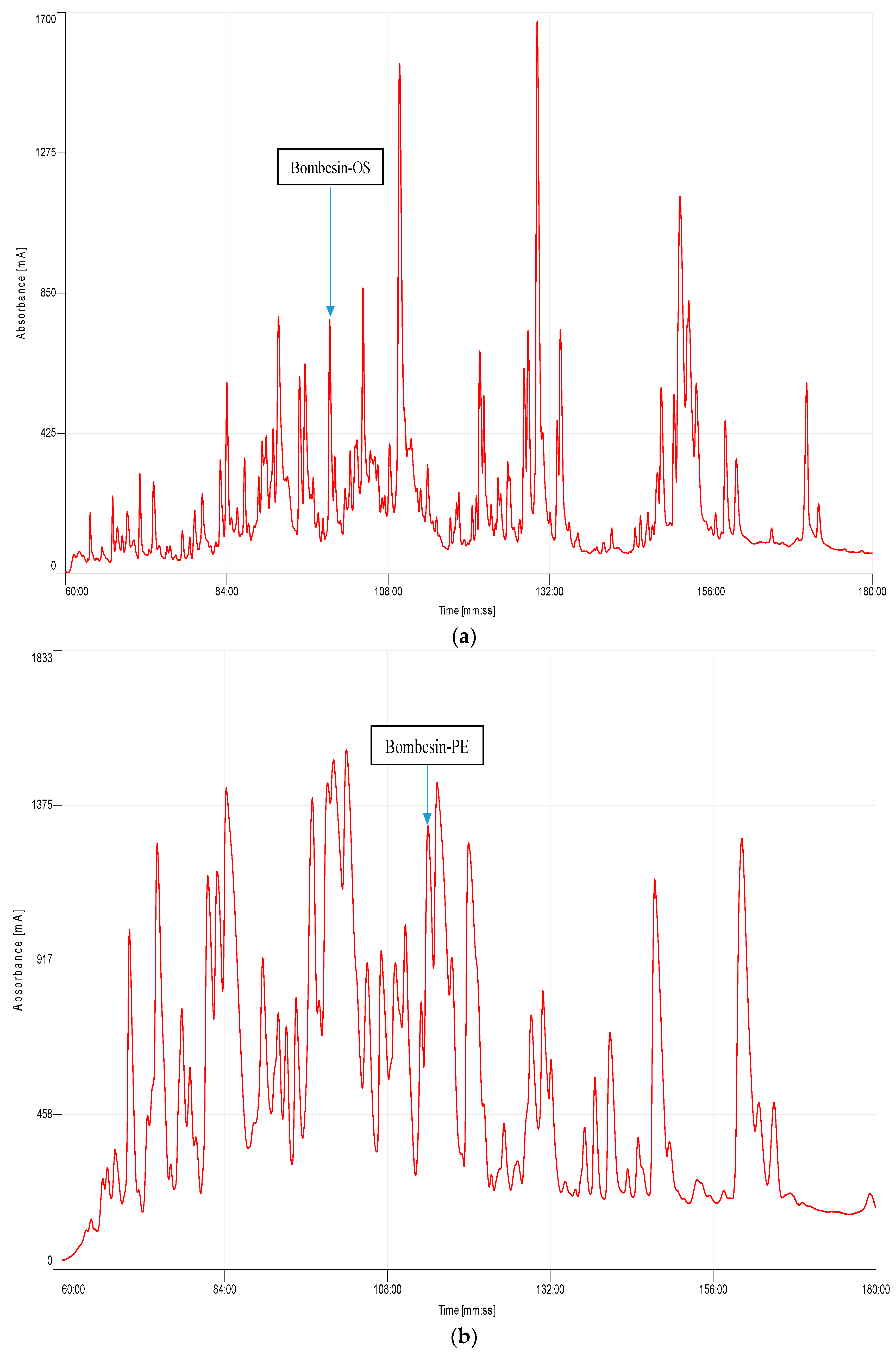
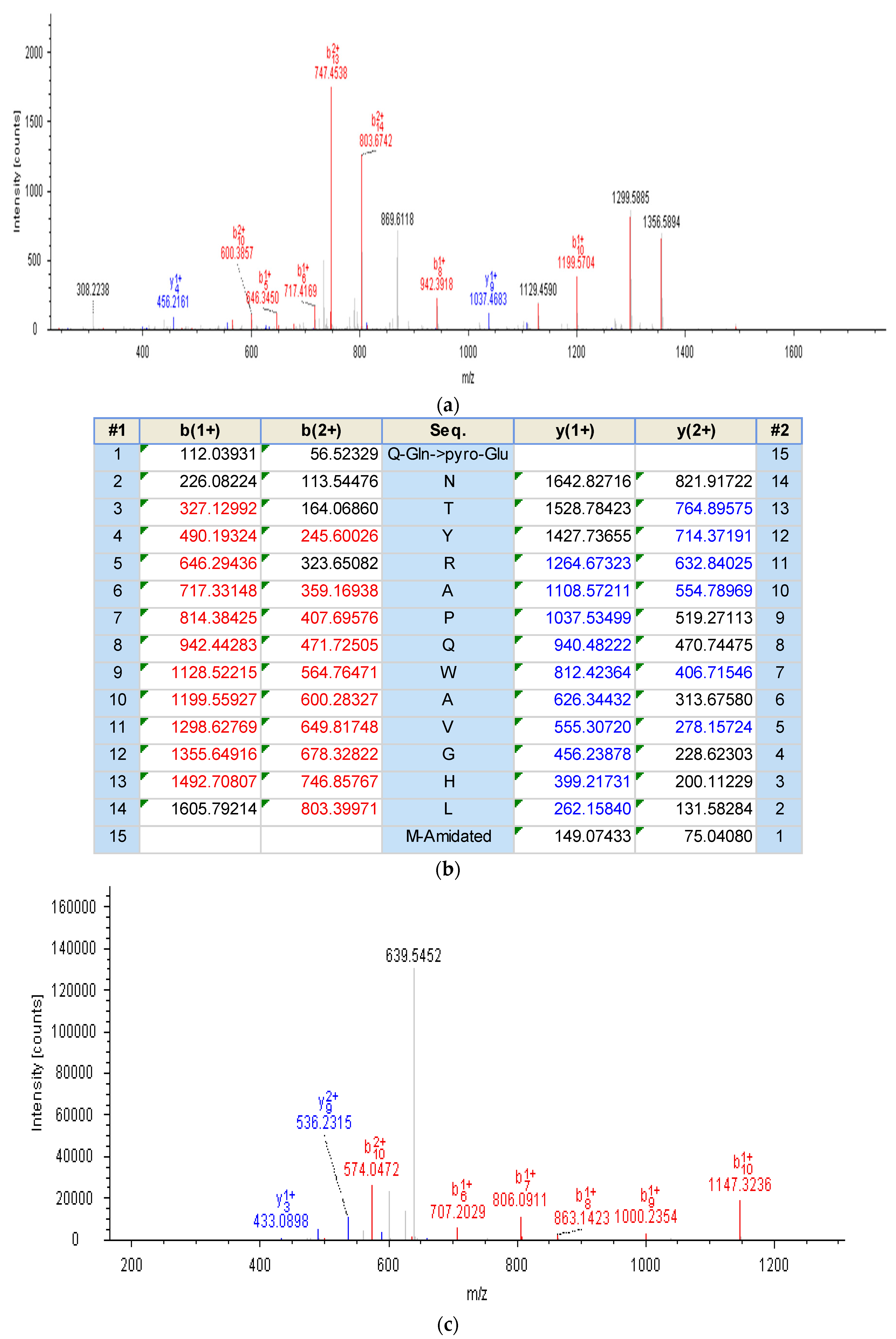
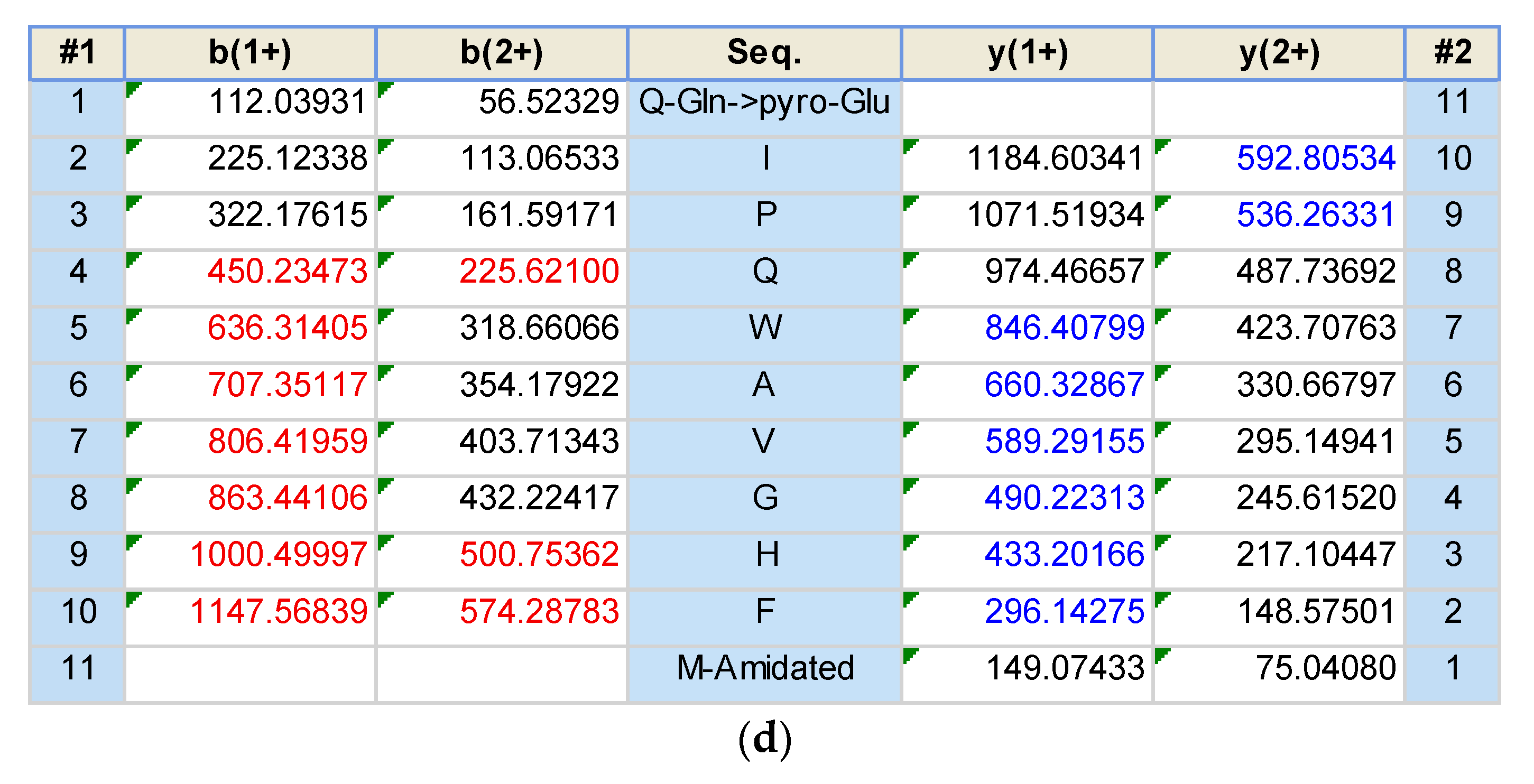
2.2. Identification and Structural Characterisation of Bombesin-OS and Bombesin-PE
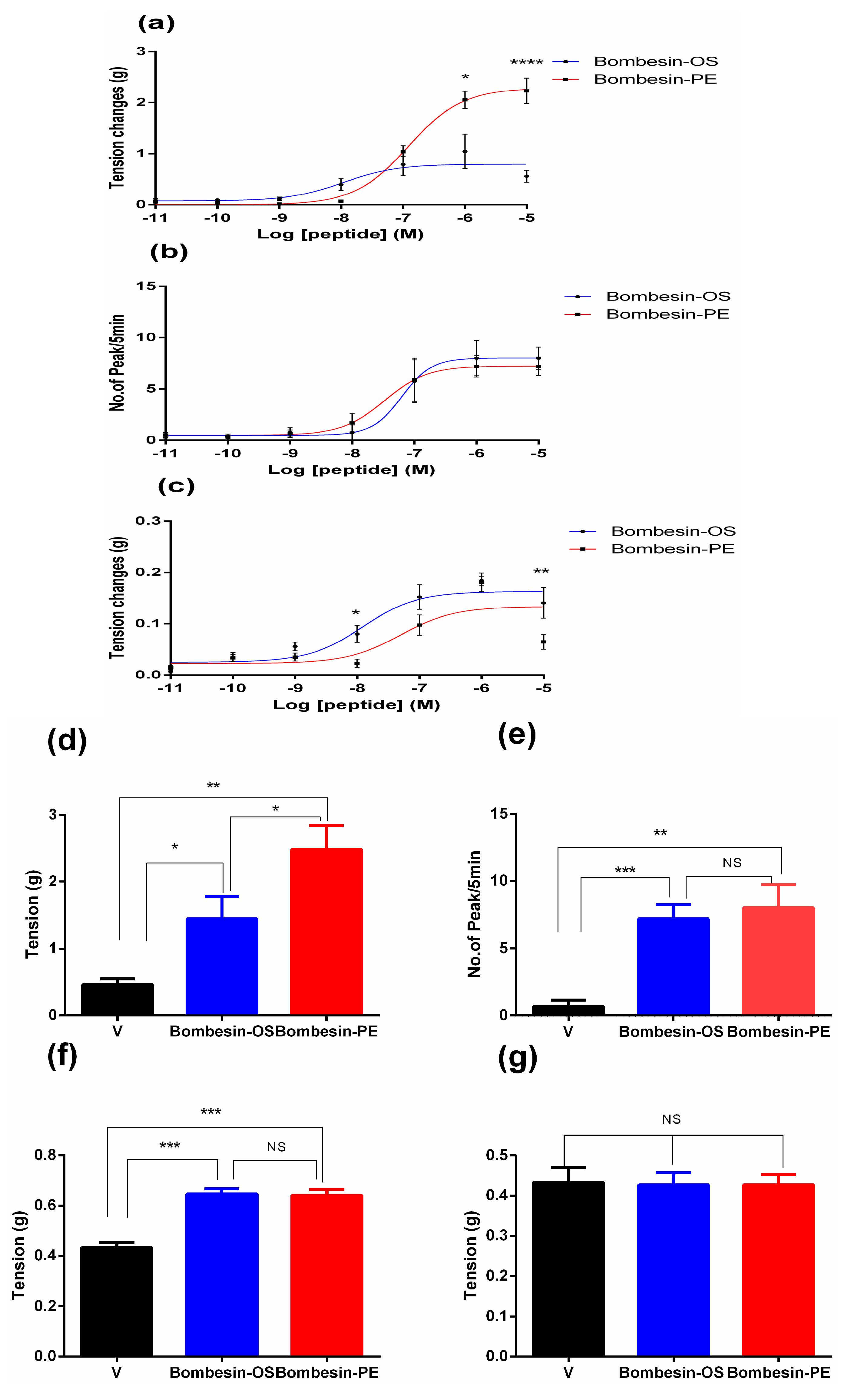
2.3. Pharmacological Effects of Bombesin-OS and Bombesin-PE on Smooth Muscle
3. Discussion
4. Materials and Methods
4.1. Specimen Biodata and Secretion Harvesting
4.2. “Shotgun” Cloning of Odorrana schmackeri and Pelophylax kl. esculentus Skin Secretion-Derived cDNA Library
4.3. Isolation and Structure Identification of Peptides
4.4. Solid-Phase Peptide Synthesis of Bombesin-OS and Bombesin-PE Peptides
4.5. The Effects of Bombesin-OS and Bombesin-PE on Rat Smooth Muscles Tension
4.6. Statistical Anaylysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cDNA | Complementary DNA |
| RACE | Rapid Amplification of cDNA Ends |
| Fmoc | 9-fluorenylmethyloxycarbonyl |
| α-CHCA | Alpha-cyano-4-hydroxycin-namic acid |
| HBTU | 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexauorophosphate |
| EDT | Ethanedithiol |
| NMM | N-methylmorpholine |
| DMF | Dimethylformamide |
| NUP | Nested Universal Primer |
References
- Anastasi, A.; Erspamer, V.; Bucci, M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the european amphibiansbombina andalytes. Experientia 1971, 27, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Minamino, N.; Kangawa, K.; Matsuo, H. Neuromedin b: A novel bombesin-like peptide identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 1983, 114, 541–548. [Google Scholar] [CrossRef]
- McDonald, T.J.; Jörnvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.R.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Minamino, N.; Kangawa, K.; Matsuo, H. Neuromedin c: A bombesin-like peptide identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 1984, 119, 14–20. [Google Scholar] [CrossRef]
- Bai, B.; Wang, H.; Xue, Y.; Wu, Y.; Zhou, M.; Wei, M.; Chen, T.; Wang, L.; Shaw, C. The structures of four bombesins and their cloned precursor-encoding cdnas from acid-solvated skin secretion of the european yellow-bellied toad, bombina variegata. Peptides 2012, 36, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Zhang, Y.; Wang, H.; Zhou, M.; Yu, Y.; Ding, S.; Chen, T.; Wang, L.; Shaw, C. Parallel peptidome and transcriptome analyses of amphibian skin secretions using archived frozen acid-solvated samples. Mol. Biotechnol. 2013, 54, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Melchiorri, P. Active polypeptides: From amphibian skin to gastrointestinal tract and brain of mammals. Trends Pharmacol. Sci. 1980, 1, 391–395. [Google Scholar] [CrossRef]
- Nakajima, T. Active peptides in amphibian skin. Trends Pharmacol. Sci. 1981, 2, 202–205. [Google Scholar] [CrossRef]
- Erspamer, V. Discovery, isolation, and characterization of bombesin-like peptides. Ann. N. Y. Acad. Sci. 1988, 547, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Wada, E.; Santo-Yamada, Y.; Wada, K. Bombesin and its family of peptides: Prospects for the treatment of obesity. Eur. J. Pharmacol. 2002, 440, 281–290. [Google Scholar] [CrossRef]
- Iwabuchi, M.; Ui-Tei, K.; Yamada, K.; Matsuda, Y.; Sakai, Y.; Tanaka, K.; Ohki-Hamazaki, H. Molecular cloning and characterization of avian bombesin-like peptide receptors: New tools for investigating molecular basis for ligand selectivity. Br. J. Pharmacol. 2003, 139, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nagalla, S.R.; Barry, B.J.; Creswick, K.C.; Eden, P.; Taylor, J.T.; Spindel, E.R. Cloning of a receptor for amphibian [phe13] bombesin distinct from the receptor for gastrin-releasing peptide: Identification of a fourth bombesin receptor subtype (bb4). Proc. Natl. Acad. Sci. USA 1995, 92, 6205–6209. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Moody, T.W.; Igarashi, H.; Ito, T.; Jensen, R.T. Bombesin-related peptides and their receptors: Recent advances in their role in physiology and disease states. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ladenheim, E.E.; Knipp, S. Capsaicin treatment differentially affects feeding suppression by bombesin-like peptides. Physiol. Behav. 2007, 91, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bédard, T.; Mountney, C.; Kent, P.; Anisman, H.; Merali, Z. Role of gastrin-releasing peptide and neuromedin b in anxiety and fear-related behavior. Behav. Brain Res. 2007, 179, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, H.; Mori, M.; Fujiwara, N.; Moriyama, A. Pharmacological characteristics of bombesin receptor mediating hypothermia in the central nervous system of rats. Brain Res. 2003, 969, 88–94. [Google Scholar] [CrossRef]
- Roesler, R.; Kent, P.; Luft, T.; Schwartsmann, G.; Merali, Z. Gastrin-releasing peptide receptor signaling in the integration of stress and memory. Neurobiol. Learn. Mem. 2014, 112, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Krane, I.; Naylor, S.; Helin-Davis, D.; Chin, W.W.; Spindel, E. Molecular cloning of cdnas encoding the human bombesin-like peptide neuromedin b. Chromosomal localization and comparison to cdnas encoding its amphibian homolog ranatensin. J. Biol. Chem. 1988, 263, 13317–13323. [Google Scholar] [PubMed]
- Miao, Y.; Li, W.; Duan, L.; Xiao, Y. A bombesin-like peptide from skin of sanguirana varians. Comp. Biochem. Physiol. Part B 2010, 155, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, J.; Chen, Z.; Miao, Y.; Li, W. A novel bombesin-like peptide from skin of rana shuchinae. Mol. Biol. Rep. 2011, 38, 3599–3603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Xu, X.; Wang, X.; Liu, D.; Lai, R. Multiple bombesin-like peptides with opposite functions from skin of odorrana grahami. Genomics 2007, 89, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, T.; Zhou, M.; Wang, L.; Su, S.; Shaw, C. Ranatensin-hl: A bombesin-related tridecapeptide from the skin secretion of the broad-folded frog, hylarana latouchii. Molecules 2017, 22, 1110. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Chang, L.-C.; Lin, K.-J.; Tey, S.-L.; Su, Y.-T.; Liu, C.-W.; Tsai, T.-R.; Huang, S.-C. Mechanism of bombesin-induced tonic contraction of the porcine lower esophageal sphincter. Sci. Rep. 2015, 5, 15879. [Google Scholar] [CrossRef] [PubMed]
- Samgina, T.Y.; Artemenko, K.A.; Gorshkov, V.A.; Ogourtsov, S.V.; Zubarev, R.A.; Lebedev, A.T. De novo sequencing of peptides secreted by the skin glands of the caucasian green frog rana ridibunda. Rapid Commun. Mass Spectrom. 2008, 22, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Merali, Z. Bombesin-like peptides and associated receptors within the brain: Distribution and behavioral implications. Peptides 2004, 25, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Identification of miscellaneous peptides from the skin secretion of the european edible frog, Pelophylax kl. Esculentus. Protein J. 2016, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, D.; Xi, X.; Wu, Y.; Ma, C.; Zhou, M.; Wang, L.; Yang, M.; Chen, T.; Shaw, C. Identification and characterisation of the antimicrobial peptide, phylloseptin-pt, from the skin secretion of phyllomedusa tarsius, and comparison of activity with designed, cationicity-enhanced analogues and diastereomers. Molecules 2016, 21, 1667. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, W.; Mantyh, P.; Mantyh, C.; McVey, D.; Vigna, S. Bombesin/grp-preferring and neuromedin b-preferring receptors in the rat urogenital system. Neuropeptides 1993, 24, 43–52. [Google Scholar] [CrossRef]
- Kortezova, N.; Mizhorkova, Z.; Milusheva, E.; Coy, D.H.; Vizi, E.S.; Varga, G. Grp-preferring bombesin receptor subtype mediates contractile activity in cat terminal ileum. Peptides 1994, 15, 1331–1333. [Google Scholar] [CrossRef]
- Erspamer, G.F.; Severini, C.; Erspamer, V.; Melchiorri, P.; Delle Fave, G.; Nakajima, T. Parallel bioassay of 27 bombesin-like peptides on 9 smooth muscle preparations. Structure-activity relationships and bombesin receptor subtypes. Regul. Pept. 1988, 21, 1–11. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds bombesin-OS and bombesin-PE are available from the authors. |

| (I) Sample | (J) Sample | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Bladder | Bombesin-OS | Bombesin-PE | −1.107636 * | 0.3938992 | 0.025 | −2.088772 | −0.126500 |
| V | 1.027333 * | 0.4119152 | 0.050 | 0.001323 | 2.053344 | ||
| Bombesin-PE | Bombesin-OS | 1.107636 * | 0.3938992 | 0.025 | 0.126500 | 2.088772 | |
| V | 2.134970 * | 0.3810193 | 0.003 | 1.185915 | 3.084024 | ||
| V | Bombesin-OS | −1.027333 * | 0.4119152 | 0.050 | −2.053344 | −0.001323 | |
| Bombesin-PE | −2.134970 * | 0.3810193 | 0.000 | −3.084024 | −1.185915 | ||
| Uterus | Bombesin-OS | Bombesin-PE | 0.244444 | 1.1355882 | 0.975 | −2.584113 | 3.073002 |
| V | 7.333333 * | 1.1650889 | 0.001 | 4.431295 | 10.235372 | ||
| Bombesin-PE | Bombesin-OS | −0.244444 | 1.1355882 | 0.975 | −3.073002 | 2.584113 | |
| V | 7.088889 * | 1.1355882 | 0.000 | 4.260332 | 9.917446 | ||
| V | Bombesin-OS | −7.333333 * | 1.1650889 | 0.000 | −10.235372 | −4.431295 | |
| Bombesin-PE | −7.088889 * | 1.1355882 | 0.000 | −9.917446 | −4.260332 | ||
| ileum | Bombesin-OS | Bombesin-PE | −0.010141 | 0.0207680 | 0.877 | −0.061748 | 0.041465 |
| V | 0.182444 * | 0.0217817 | 0.000 | 0.128319 | 0.236570 | ||
| Bombesin-PE | Bombesin-OS | 0.010141 | 0.0207680 | 0.877 | −0.041465 | 0.061748 | |
| V | 0.192586 * | 0.0207680 | 0.000 | 0.140980 | 0.244192 | ||
| V | Bombesin-OS | −0.182444 * | 0.0217817 | 0.000 | −0.236570 | −0.128319 | |
| Bombesin-PE | −0.192586 * | 0.0207680 | 0.000 | −0.244192 | −0.140980 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Ma, C.; Zhou, M.; Zhang, Y.; Xi, X.; Zhong, R.; Chen, T.; Shaw, C.; Wang, L. Pharmacological Effects of Two Novel Bombesin-Like Peptides from the Skin Secretions of Chinese Piebald Odorous Frog (Odorrana schmackeri) and European Edible Frog (Pelophylax kl. esculentus) on Smooth Muscle. Molecules 2017, 22, 1798. https://doi.org/10.3390/molecules22101798
Zhou X, Ma C, Zhou M, Zhang Y, Xi X, Zhong R, Chen T, Shaw C, Wang L. Pharmacological Effects of Two Novel Bombesin-Like Peptides from the Skin Secretions of Chinese Piebald Odorous Frog (Odorrana schmackeri) and European Edible Frog (Pelophylax kl. esculentus) on Smooth Muscle. Molecules. 2017; 22(10):1798. https://doi.org/10.3390/molecules22101798
Chicago/Turabian StyleZhou, Xiaowei, Chengbang Ma, Mei Zhou, Yuning Zhang, Xinping Xi, Ruimin Zhong, Tianbao Chen, Chris Shaw, and Lei Wang. 2017. "Pharmacological Effects of Two Novel Bombesin-Like Peptides from the Skin Secretions of Chinese Piebald Odorous Frog (Odorrana schmackeri) and European Edible Frog (Pelophylax kl. esculentus) on Smooth Muscle" Molecules 22, no. 10: 1798. https://doi.org/10.3390/molecules22101798






