Rapid Classification and Identification of Chemical Components of Schisandra Chinensis by UPLC-Q-TOF/MS Combined with Data Post-Processing
Abstract
:1. Introduction
2. Results and Discussion
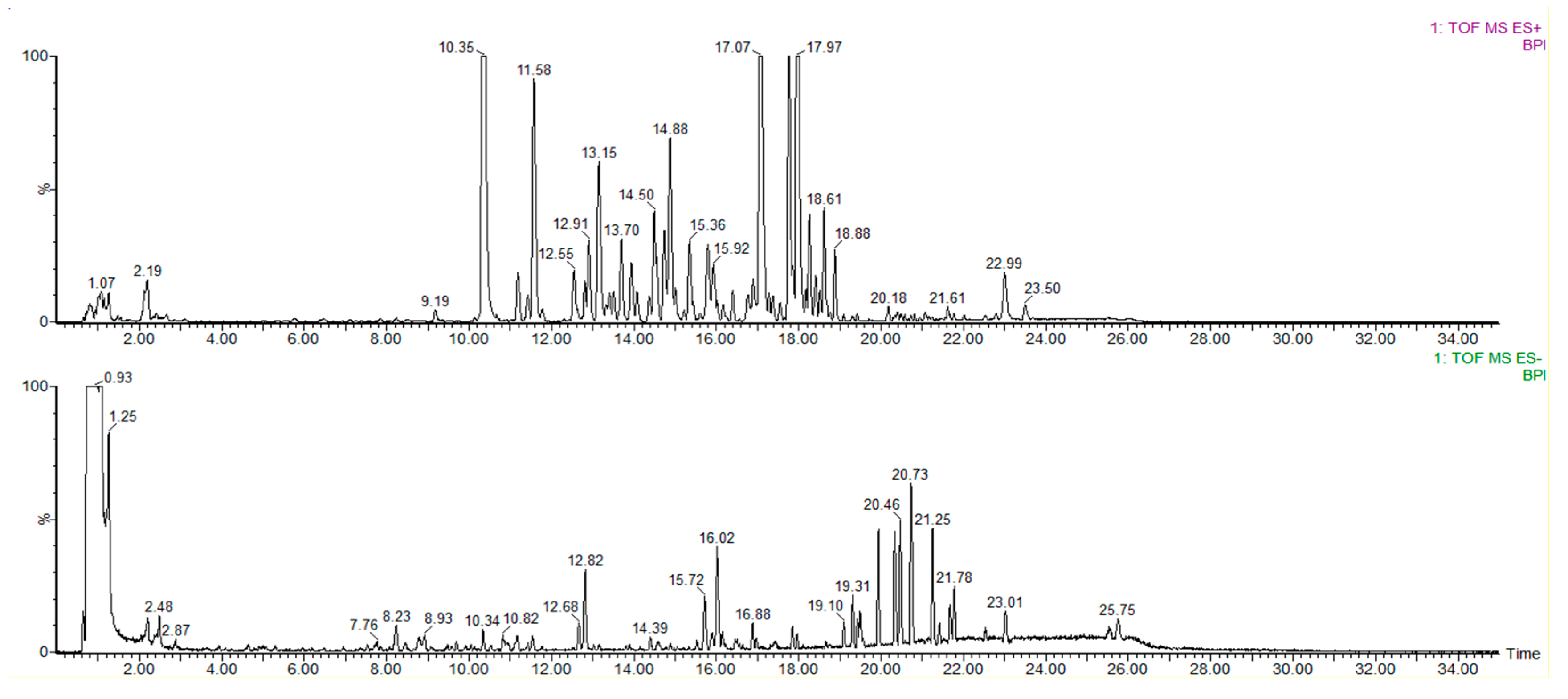
2.1. The Establishment of Data Post-Processing Technology Based on UPLC-Q-TOF/MS
2.2. Lignans
2.3. Triterpenoids
2.4. Fatty Acids
2.5. Organic Acids
3. Materials and Methods
3.1. Sample Preparation
3.2. UPLC and MS Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Lai, H.; Jia, X.; Liu, J.; Zhang, Z.; Qi, Y.; Zhang, J.; Song, J.; Wu, C.; Zhang, B. Comprehensive chemical analysis of Schisandra chinensis by HPLC–DAD–MS combined with chemometrics. Phytomedicine 2013, 20, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Marzec-Wróblewska, U.; Bucinski, A.; Luczkiewicz, M.; Ekiert, H. Accumulation of dibenzocyclooctadiene lignans in agarcultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl. Microbial. Biotechnol. 2016, 100, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, S.; Tong, S.; Li, X.; Yan, J. An efficient strategy for the extraction and purification of lignans from Schisandra chinensis by a combination of supercritical fluid extraction and high-speed counter-current chromatography. J. Sep. Sci. 2013, 36, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, B.; Liu, X.; Meng, X. Purification of two triterpenoids from Schisandra chinensis by macroporous resin combined with high-speed counter-current chromatography. J. Chromatogr. Sci. 2013, 52, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhang, H.; Gong, C.; Zhu, Z.; Zhao, L.; Xu, Y.; Wang, B.; Zhang, G. Analysis of lignans in Schisandra chinensis and rat plasma by high-performance liquid chromatography diodearray detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, G.Z.; Chen, Y.; Liao, M.C.; Liu, X.M.; Chen, S.; Liu, L.; Lei, X.X. Terpenes from Schisandra sphenanthera. Helv. Chim. Acta 2011, 94, 491–496. [Google Scholar] [CrossRef]
- Zhitnitsky, D.; Rose, J.; Lewinson, O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.F. A Comparison of the Cytotoxic Effects of Fatty Acids on In Vitro Breast and Prostate Cell Lines. Ph.D. thesis, University of Huddersfield, West Yorkshire, UK, 2016. [Google Scholar]
- Huang, X.; Song, F.; Liu, Z.; Liu, S. Studies on lignan constituents from Schinensis (Turcz.) Baill. fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J. Mass Spectrom. 2007, 42, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; He, Q.; Zhang, Y.; Qu, H.; Cheng, Y. Characterisation and identification of isomeric dibenzocyclooctadiene lignans from Schisandra Chinensis by high-performance liquid chromatography combined with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2009, 20, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Lu, Y.; Chen, D. Authentication of Schisandra chinensis and Schisandra sphenanthera in Chinese patent medicines. J. Pharm. Biomed. Anal. 2016, 131, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Da-shuai, C. Methodological Establishment of HPLC-DAD for Determination of 10 Active Ingredients in Wuweizi. J. Int. Transl. Med. 2015, 3, 171–179. [Google Scholar]
- Štěrbová, H.; Ševčíková, P.; Kvasničková, L.; Glatz, Z.; Slanina, J. Determination of lignans in Schisandra chinensis using micellar electrokinetic capillary chromatography. Electrophoresis 2002, 23, 253–258. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, M.; Huang, J.Y.; Yu, X.X.; Zhao, M.Q.; Liang, Z.K.; Xie, Z.S.; Xu, X.J. Preparative separation and purification of deoxyschizandrin from Schisandrae Sphenantherae Fructus by high-speed counter-current chromatography. J. Pharm. Anal. 2013, 3, 429–433. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yang, B.Y.; Liang, J.; Yang, Q.; Wang, D.; Kuang, H.X. Quantitative analysis and fingerprint profiles for quality control of Fructus Schisandrae by gas chromatography: Mass spectrometry. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Li, Y.D.; Shi, Y.P.; Jin, L.; Chen, J. Quality control of traditional Chinese medicines: A review. Chin. J. Nat. Med. 2013, 11, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Tak, V.; Purohit, A.; Pardasani, D.; Goud, D.R.; Jain, R.; Dubey, D. Simultaneous detection and identification of precursors, degradation and co-products of chemical warfare agents in drinking water by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1370, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, Q.; Chen, C.; Song, C.; Xu, Y.; Xiang, Y.; Feng, Y.; Ouyang, H.; Zhang, Y.; Jiang, H. The rapid discovery and identification of physalins in the calyx of Physalis alkekengi L. var. franchetii (Mast.) Makino using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J. Chromatogr. A 2014, 1361, 139–152. [Google Scholar] [PubMed]
- Xu, L.; Mu, L.H.; Peng, J.; Liu, W.W.; Tan, X.; Li, Z.L.; Wang, D.X.; Liu, P. UPLC-Q-TOF-MS E analysis of the constituents of Ding-Zhi-Xiao-Wan, a traditional Chinese antidepressant, in normal and depressive rats. J. Chromatogr. B 2016, 1026, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Guo, T.; Xu, X.D.; Yang, J.S. Rapid identification and simultaneous analysis of multiple constituents from Rheum tanguticum Maxim. Ex Balf. by UPLC/Q-TOF-MS. Nat. Prod. Res. 2017, 31, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.D.; Zhao, Y.W.; Li, Y.J.; Wang, X.J.; Si, H.H.; Huang, W.Z.; Wang, Z.Z.; Ma, S.P.; Xiao, W. Qualitative and quantitative analysis of major constituents from DazhuHongjingtian capsule by UPLC/Q-TOF-MS/MS combined with UPLC/QQQ-MS/MS. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liang, Y.; Jiye, A.; Hao, H.; Liu, L.; Zheng, X.; Dai, C.; Zhou, Y.; Guan, T.; Liu, Y. Post acquisition data processing techniques for lipid analysis by quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2012, 905, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Z.; Hou, Z.; Yang, B.; Li, A.Z.; Guo, X.J.; Wang, Y.M.; Li, Y.B. Rapid classification and identification of complex chemical compositionss in TCM based on UPLC-Q-TOF/MS coupled with data processing techniques using the KuDieZi injection as an example. Anal. Methods 2015, 7, 5210–5217. [Google Scholar] [CrossRef]
- Yuan, L.; Yin, J.; Tian, M.; Xie, J.; Wang, Y.; Hou, Z.; Li, Y.; Zhang, Y. The classification and identification of complex chemical compositionss in yanhusuo herb using UPLC-Q-TOF/MS. Anal. Methods 2016, 8, 2274–2281. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Wang, Z.; Wang, Y.; Liu, T.; Li, S.; Wang, C.; Wang, H.; Tu, P. Identification of metabolites of deoxyschizandrin in rats by UPLC–Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques. J. Chromatogr. B 2014, 949, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Shen, P.; Shen, Y. Preparative separation and purification of deoxyschisandrin and γ-schisandrin from Schinensis (Turcz.) Baill by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1066, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, S.X.; Pu, J.X.; Li, J.R.; Ding, L.S.; Chen, D.F.; Sun, H.D.; Xu, H.X. Ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometric procedure for qualitative and quantitative analyses of nortriterpenoids and lignans in the genus Schisandra. J. Pharm. Biomed. Anal. 2011, 56, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.X. Analysis of lignans in Schisandra by HPLC, UPLC/Q-TOF-MS. Master′s Thesis, Heilongjiang University Of Chinese Medicine, Harbin, China, June 2012. [Google Scholar]
- Wang, Q.; Liu, Y.; Zheng, X.W.; Yu, J.D.; Dai, Z.; Lu, J.; Lin, R.C. Analysis of Lignans in Shengmai Injection by HPLC-MS/MS. Chin. Pharm. J. 2013, 48, 132–135. [Google Scholar]
- Ji, G. Studies on the Chemical Constituents of Kadsuracoccinea (Lem.) A. C. Smith. Master′s Thesis, Soochow University, Suzhou, China, May 2011. [Google Scholar]
- Shi, P.Y. Studies on masss Dectrometric fragmentation patterns of four categories of compounds in TCMs and pharmacokinetics of QI-SHEN-YI-QI Formula. Ph.D. Thesis, Zhejiang University, Hangzhou, China, June 2010. [Google Scholar]
- Huang, S.X.; Han, Q.B.; Lei, C.; Pu, J.X.; Xiao, W.L.; Yu, J.L.; Yang, L.M.; Xu, H.X.; Zheng, Y.T.; Sun, H.D. Isolation and characterization of miscellaneous terpenoids of Schisandrachinensis. Tetrahedron 2008, 64, 4260–4267. [Google Scholar] [CrossRef]
- Li, B. Study on Separation and Purification Methods Structural Identification and the Activity of Inhibiting Liver Cancer of Triterpenoid from SchisandraChinensis(Turcz.)Baill. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 1 June 2011. [Google Scholar]
- Liu, J.S.; Huang, M.F. Study on the structures of schisanlactone C and schisanlactone D, Two new triterpene lactones from schisandra SP. Acta Chim. Sin. 1984, 42, 464–469. [Google Scholar]
- Wu, H.Q.; Huang, X.L.; Lin, X.S.; Huang, F.; Zhu, Z.X.; Ma, Y.F. Gas Chromatographic Retention Time Rule and Mass Spectrometric Fragmentation Rule of Fatty Acids and Their Application in Food Analysis. Chin. J. Anal. Chem. 2007, 35, 998–1003. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Q.; Li, N.; Wang, Z.J.; Lu, J.Q.; Qiao, Y.J. Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MS n. Talanta 2013, 104, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, L.; Shan, M.Q.; Qian, Y.; Ding, A.W. Simultaneous determination of eight organic acids in Chaenomelis Fructus by UFLC-MS. Chin. Tradit. Herb. Drugs 2016, 47, 2465–2469. [Google Scholar]
- Dou, Z.H.; Luo, L.; An, L.P.; Shi, Z.; Yang, A.H. UPLC-MS/MS analysis of ethanol extracts from Schisandra chinensis. Herald Med. 2013, 32, 239–241. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |




| Classification | Subclass | Neutral Loss | Characteristic Fragments |
|---|---|---|---|
| Lignans | Type 1 biphenyl cyclooctene lignans (without OH) | 70 Da (C5H10) 56 Da (C4H8) | 415 [C24H31O6]+ 401 [C23H29O6]+ 331 [C18H19O6]+ 330 [C18H18O6]+ 301 [C17H17O5]+ |
| Type 2 biphenyl cyclooctene lignans (with OH) | 18 Da (H2O) 54 Da (C4H6) | ||
| Type 3 biphenyl cyclooctene lignans (with OH and benzoyl), | 18 Da (H2O) 122 Da (C6H5COOH) 30 Da (CH2O) | ||
| Type 4 biphenyl cyclooctene lignans (with OH and angeloyl or tigloyl) | 18 Da (H2O) 100 Da (C4H7COOH) 30 Da (CH2O) | ||
| Open-loop lignans | 182 [C9H10O4]+ 122 [C7H6O2]+ | ||
| Triterpenoids | Lanostane-type | 46 Da (HCOOH) 45 Da (HCOO−) | |
| Cycloartane-type | 312 [C22H32O]+ 271 [C19H27O]+ | ||
| Schisanra-type | 60 Da (CH2C(OH)2) 74 Da (CH3CH=C(OH)2) | ||
| Fatty acids | 55 [C4H7]+ or 54[C4H6]− 67 [C5H7]+ or 66[C4H6]− 79 [C6H7]+ or 78[C4H6]− | ||
| Organic acids | 44 Da (CO2) 18 Da (H2O) |
| No. | Classification | Name | Formula | m/z [M + H]+ | Time (min) | Experimental m/z [M + H]+ | ppm | Fragment Ions | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Type 1 biphenyl cyclooctene lignans (without OH) | Deoxyschisandrin | C24H32O6 | 417.2277 | 17.07 | 417.2289 | 2.88 | 417 [M + H]+ 439 [M + Na]+ 440 [M + H + Na]+ 402 [M + H − CH3]+ 347 [M + H − C5H10]+ 370 [M + H − CH3 − CH3OH]+ 316 [M + H − C5H10 − OCH3]+ 301 [M + H − C5H10 − OCH3 − CH3]+ 361 [M+H − C4H8]+ | [10,27,28] |
| 2 | Type 1 biphenyl cyclooctene lignans (without OH) | SchisandrinB | C23H28O6 | 401.1964 | 17.97 | 401.1962 | 0.50 | 401 [M + H]+ 423 [M + Na]+ 386 [M + H − CH3]+ 331 [M + H − C5H10]+ 301 [M + H − C5H10− OCH3]+ 371 [M + H − CH3− CH3]+ | [10,11,28] |
| 3 | Type 1 biphenyl cyclooctene lignans (without OH) | Schisandrin C | C22H24O6 | 385.1651 | 18.41 | 385.1642 | 2.08 | 385 [M + H]+ 407 [M + Na]+ 370 [M + H − CH3]+ 315 [M + H − C5H10]+ 300 [M + H − C5H10 − CH3]+ | [10] |
| 4 | Other lignans | Gomisin J | C22H28O6 | 389.1964 | 11.43 | 389.1953 | 2.83 | 389 [M + H]+ 411 [M + Na]+ 319 [M + H − C5H10]+ 342 [M + H − CH3OH − CH3]+ 358 [M + H − OCH3]+ 374 [M + H − CH3]+ | [10] |
| 5 | Type 2 biphenyl cyclooctene lignans (with OH) | Schisandrol A | C24H32O7 | 433.2226 | 10.35 | 433.2226 | 0.00 | 433 [M + H]+ 455 [M + Na]+ 415 [M + H − H2O]+ 384 [M + H − H2O − OCH3]+ 361 [M + H − H2O − C4H6]+ | [11,29,30] |
| 6 | Type 2 biphenyl cyclooctene lignans (with OH) | Schisandrol B | C23H28O7 | 417.1913 | 11.58 | 417.1889 | 5.75 | 417 [M + H]+ 439 [M + Na]+ 399 [M + H − H2O]+ 345 [M + H − H2O − C4H6]+ 367 [M + H − H2O − OCH3]+ | [10] |
| 7 | Type 2 biphenyl cyclooctene lignans (with OH) | Schisanhenol | C23H30O6 | 403.212 | 14.50 | 403.2109 | 2.73 | 403 [M + H]+ 425 [M + Na]+ 385 [M + H − H2O]+ 331 [M + H − H2O − C4H6]+ 354 [M + H − H2O − OCH3]+ | [10] |
| 8 | Type 3 biphenyl cyclooctene lignans (with OH and benzoyl) | Schisantherin A | C30H32O9 | 537.2124 | 14.90 | 537.2104 | 3.72 | 537 [M + H]+ 560 [M + H + Na]+ 519 [M + H − H2O]+ 415 [M + H − C6H5COOH]+ 385 [M + H − C6H5COOH − CH2O]+ 371 [M + H − C6H5COOH − C2H4O]+ | [10,11,29] |
| 9 | Type 3 biphenyl cyclooctene lignans(with OHand benzoyl) | Gomisin G | C30H32O9 | 537.2124 | 14.55 | 537.2114 | 1.86 | 537 [M + H]+ 559 [M + Na]+ 519 [M + H − H2O]+ 415 [M + H − C6H5COOH]+ 385 [M + H − C6H5COOH − CH2O]+ 371 [M + H − C6H5COOH − C2H4O]+ 340 [M + H − C6H5COOH − C2H4O − OCH3]+ | [10] |
| 10 | Type 3 biphenyl cyclooctene lignans (with OH and benzoyl) | Schisantherin D | C29H28O9 | 521.1811 | 15.09 | 521.1818 | 1.34 | 521 [M + H]+ 543 [M + Na]+ 559 [M + K]+ 399 [M + H − C6H5COOH]+ 369 [M + H − C6H5COOH − CH2O]+ | [10] |
| 11 | Type 3 biphenyl cyclooctene lignans (with OH and benzoyl) | Benzoyl gomisin O | C30H32O8 | 521.2175 | 18.63 | 521.2138 | 7.10 | 521 [M + H]+ 543 [M + Na]+ 399 [M + H − C6H5COOH]+ 369 [M + H − C6H5COOH − CH2O]+ 384 [M + H − C6H5COOH − CH3]+ | [10] |
| 12 | Type 3 biphenyl cyclooctene lignans (with OH and benzoyl) | Benzoyl iso- gomisin O | C30H32O8 | 521.2175 | 18.62 | 521.2128 | 9.01 | 521 [M + H]+ 399 [M + H − C6H5COOH]+ 384 [M + H − C6H5COOH − CH3]+ | [33] |
| 13 | Other lignans | Gomisin K1 | C23H31O6 | 404.2199 | 15..36 | 404.2158 | 10.14 | 403 [M]+ | [27] |
| 14 | Other lignans | Pregomisin | C22H31O6 | 387.1807 | 16.39 | 387.1801 | 1.55 | 391 [M + H]+ 361 [M + H − CH2O]+ 331 [M + H − 2CH2O]+ 373 [M + H − H2O]+ 358 [M + H − H2O − CH3]+ | [10] |
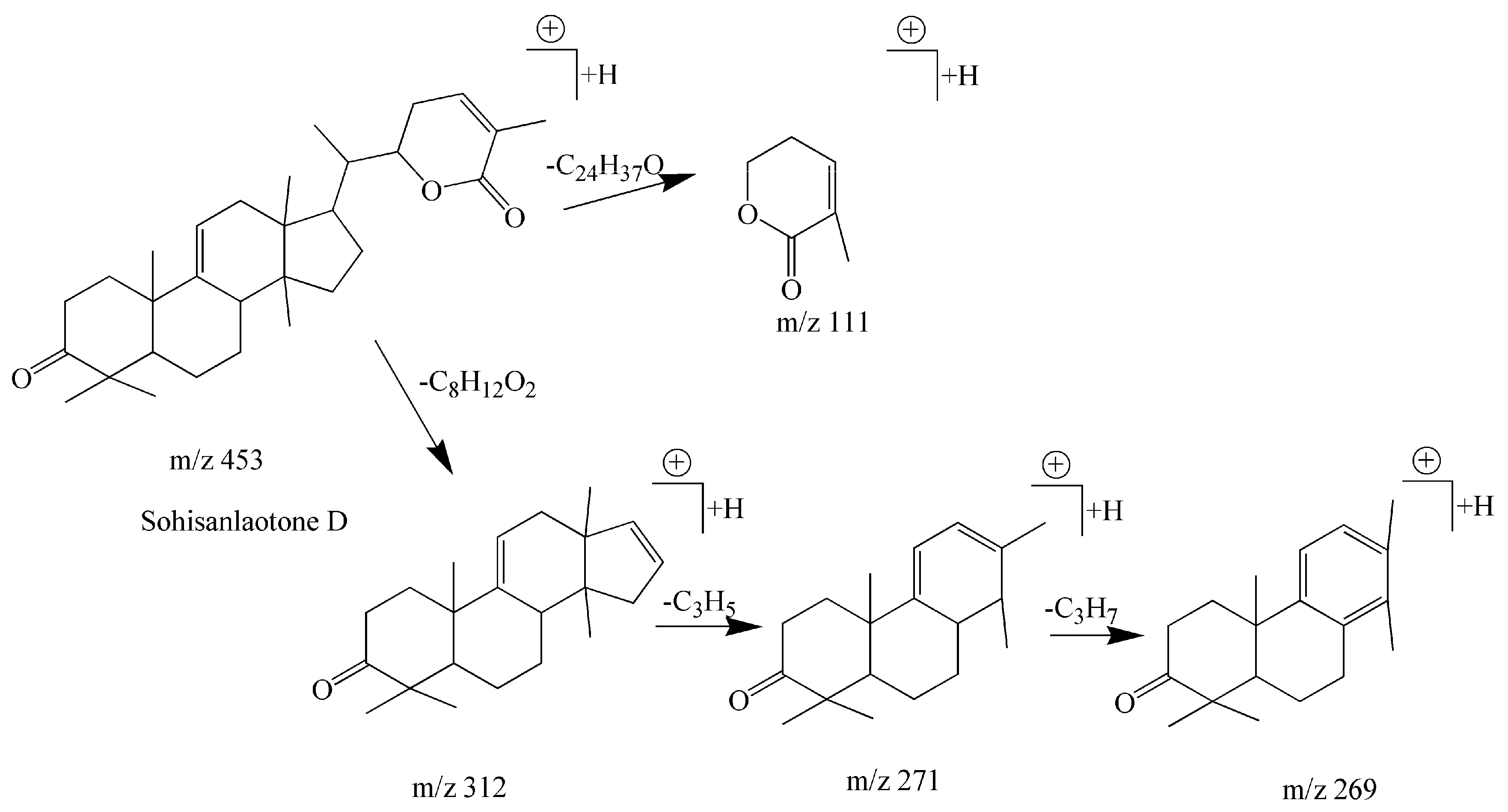
| 15 | Cycloartane-type triterpenoids | Sohisanlaotone D | C30H44O3 | 453.3368 | 20.48 | 453.3349 | 4.19 | 453 [M + H]+ 312 [M − C8H12O2]+ 271 [M − C8H12O2 − C3H5]+ 269 [M − C8H12O2 − C3H7]+ 111 [M − C24H37O]+ | [35] |
| 16 | Fatty Acids | Eicosapentaenoic Acid | C20H30O2 | 303.2324 | 20.33 | 303.2294 | 9.89 | 303 [M + H]+ 79 [C6H7]+ | [36] |
| 17 | Lanostane-type triterpenoids | Micranoic acid B | C22H32O3 | 343.2273 | 20.03 | 345.2415 | 4.06 | 345 [M + H]+ 299 [M + H − HCOOH]+ | [35] |
| No. | Classification | Name | Formula | m/z [M − H]− | Time (min) | Experimental m/z [M − H]− | ppm | Fragment Ions | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 18 | Open-loop lignans | 3′,4′-Dimethoxybenzoicacid-(3′′,4′′-dimethoxyphenyl)-2-methyl-3-oxobutyl ester | C22H26O7 | 401.1601 | 12.68 | 401.1614 | 3.24 | 401 [M − H]− 182 [C9H10O4]− | [32] |
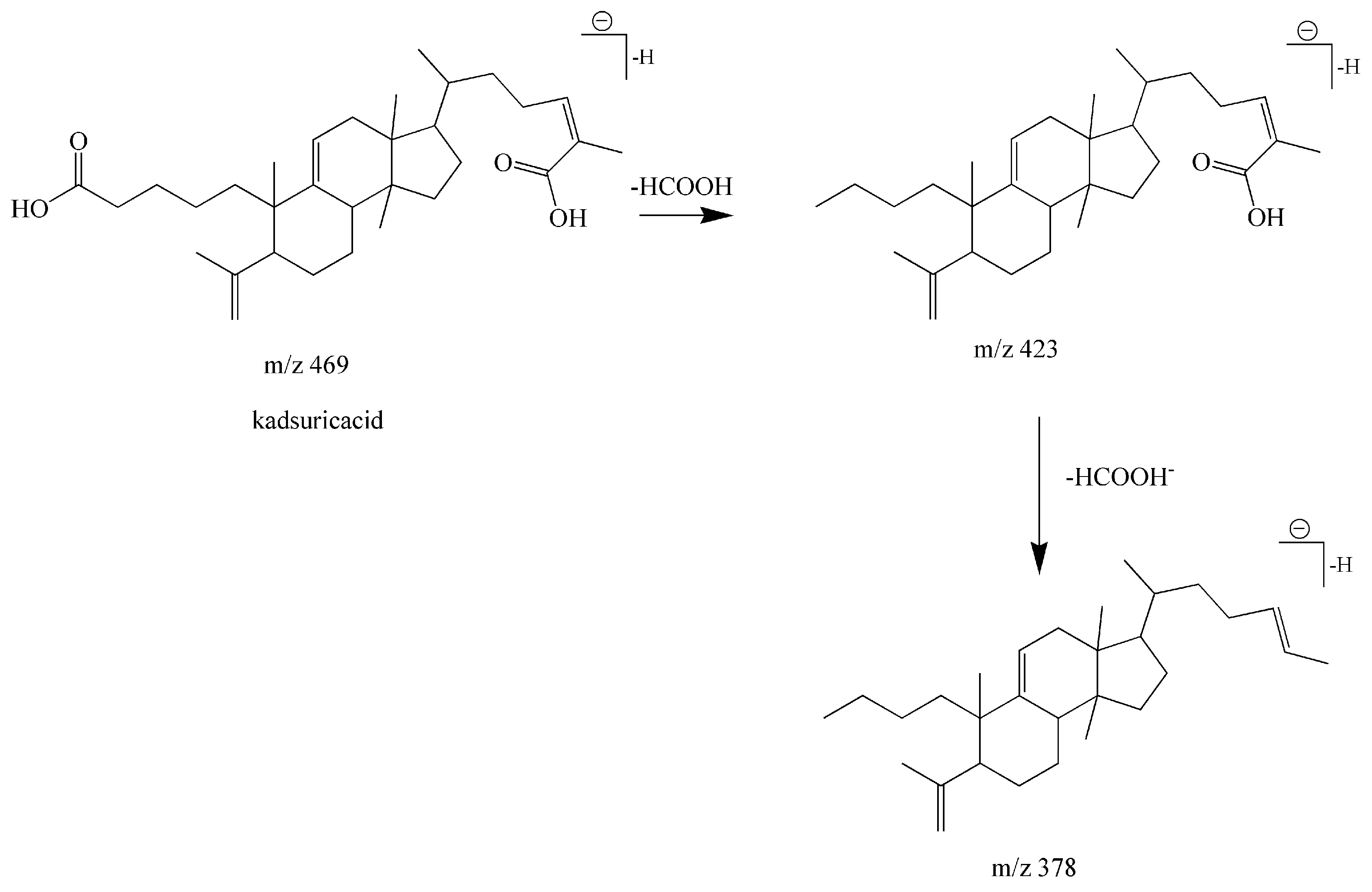
| 19 | Lanostane-type triterpenoids | Kadsuricacid | C30H46O4 | 469.3318 | 19.56 | 469.3318 | 0.00 | 469 [M − H]− 423 [M − H − HCOOH]− 378 [M − H − HCOOH − HCOO]− | [35] |
| 20 | Lanostane-type triterpenoids | Kadsuricacid 3-methyl dster | C31H48O4 | 483.3475 | 23.02 | 483.3483 | 1.66 | 483 [M − H]− 452 [M − H − OCH3]− 407 [M − H − OCH3 − HCOO]− | [34] |
| 21 | Cycloartane-type triterpenoids | Ganwuweizic acid | C30H46O3 | 453.3369 | 21.77 | 453.3368 | 0.22 | 453[M − H]− 435 [M − H − H2O]− | [35] |
| 22 | Schisanra-type triterpenoids | Henridilactone A | C29H35O10 | 542.2152 | 8.91 | 542.2133 | 3.50 | 542 [M − H]−524 [M – H − H2O]−464 [M − H − H2O − CH2C(OH)2]−390 [M – H − H2O − CH2C(OH)2 − CH3CH=C(OH)]− | [33] |
| 23 | Schisanra-type triterpenoids | Lancifodilactone D | C29H35O9 | 526.2203 | 9.50 | 526.2167 | 6.84 | 526 [M − H]− 466 [M – H − CH2C(OH)2]− 448 [M − H − CH2C(OH)2 − H2O]− | [33] |
| 24 | Schisanra-type triterpenoids | Schindilactone A | C29H35O10 | 542.2152 | 8.79 | 542.2139 | 2.40 | 542 [M − H]− 524 [M – H − H2O]− 482 [M – H − CH2C(OH)2]− 408 [M − H − CH2C(OH)2 − CH3CH=C(OH]− 390 [M − H− H2O − CH2C(OH)2 − CH3CH=C(OH]− | [33] |
| 25 | Other terpenoids | Lancifodilactone c | C29H36O10 | 543.223 | 5.07 | 543.2225 | 0.92 | 543 [M − H]− 525 [M − H − H2O]− 445[M − H − C4H7O2]− 499 [M − H − COO]− 481 [M − H − H2O − COO]− | [34] |
| 26 | Fatty Acids | 9,12-Linoleic acid | C18H32O2 | 279.2324 | 20.33 | 279.2329 | 1.79 | 66 [C5H6]− 261 [M − H − H2O]− 279 [M − H]− | [36] |
| 27 | Fatty Acids | α-Linolenic acid | C18H30O2 | 277.2168 | 19.31 | 277.2173 | 1.80 | 277 [M − H]− 78 [C6H6]− | [36] |
| 28 | Organic acids | Citric acid | C6H8O7 | 191.0192 | 0.78 | 191.0199 | 3.66 | 191 [M − H]− 146 [M − HCOOH]− 147 [M − H − CO2]− 129 [M − H − CO2 − H2O]− 85 [M − H − CO2 − CO2 − H2O]− | [37,38] |
| 29 | Organic acids | 6-methyl citrate | C7H10O7 | 205.0349 | 0.988 | 205.0353 | 1.95 | 205 [M − H]− 161 [M − H − CO2]− 174 [M − H − OCH3]− | [37] |
| 30 | Organic acids | Dimethyl citrate | C8H12O7 | 219.0505 | 1.08 | 219.0508 | 1.37 | 219 [M − H]− 175 [M − H − CO2]− 188 [M − H − OCH3]− | [37] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid Classification and Identification of Chemical Components of Schisandra Chinensis by UPLC-Q-TOF/MS Combined with Data Post-Processing. Molecules 2017, 22, 1778. https://doi.org/10.3390/molecules22101778
Yang S, Shan L, Luo H, Sheng X, Du J, Li Y. Rapid Classification and Identification of Chemical Components of Schisandra Chinensis by UPLC-Q-TOF/MS Combined with Data Post-Processing. Molecules. 2017; 22(10):1778. https://doi.org/10.3390/molecules22101778
Chicago/Turabian StyleYang, Shenshen, Lanlan Shan, Houmin Luo, Xue Sheng, Jun Du, and Yubo Li. 2017. "Rapid Classification and Identification of Chemical Components of Schisandra Chinensis by UPLC-Q-TOF/MS Combined with Data Post-Processing" Molecules 22, no. 10: 1778. https://doi.org/10.3390/molecules22101778





