Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy
Abstract
:1. Introduction
2. Principle and History of Metabolic Syndrome
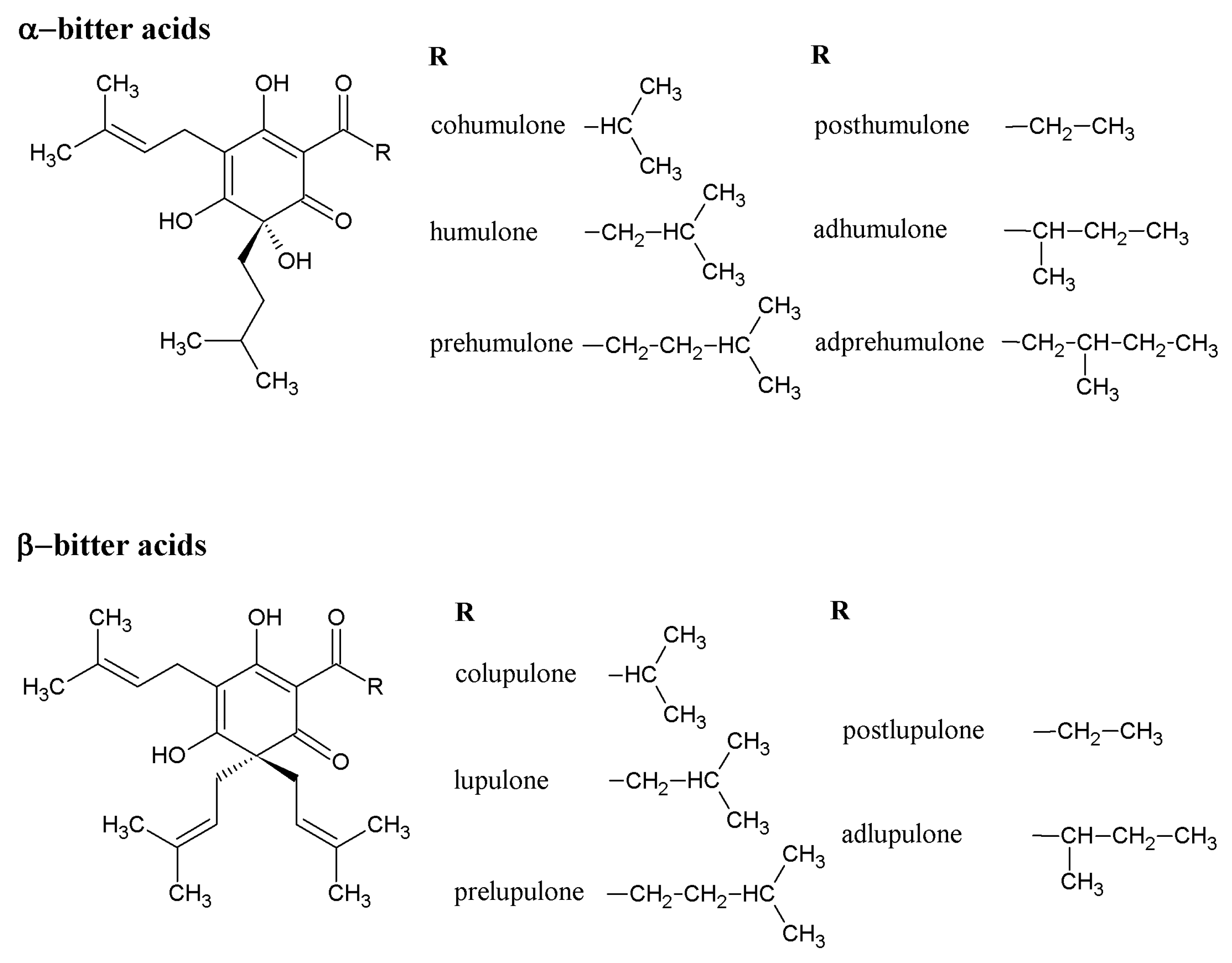
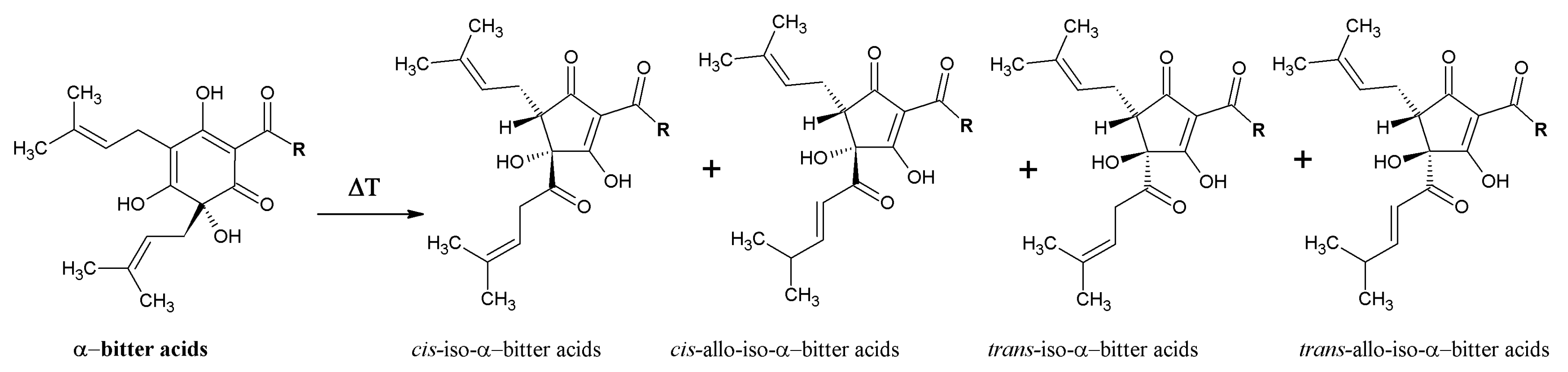
3. Beneficial Effects of Hop Phytochemicals on Metabolic Syndrome
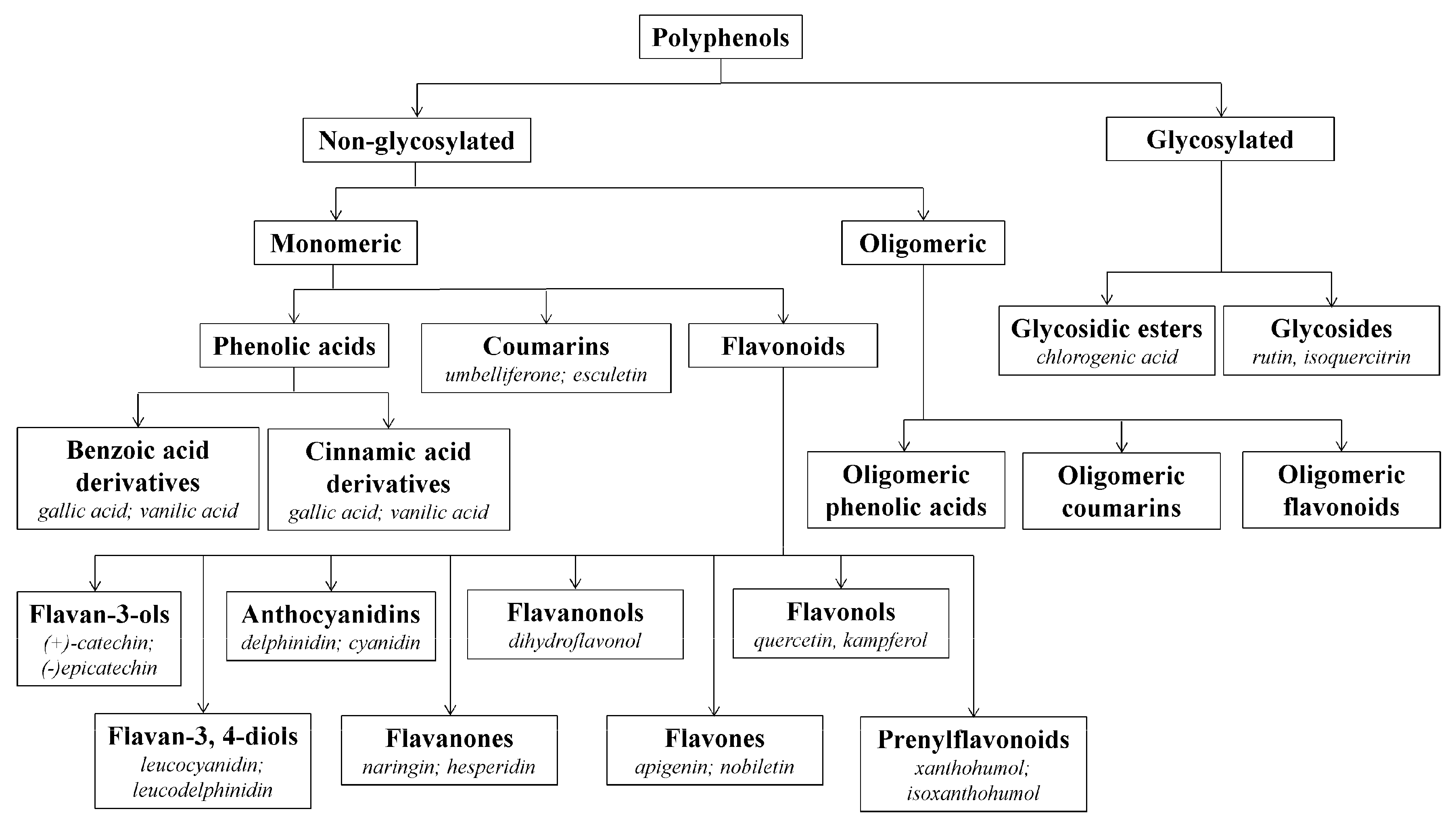
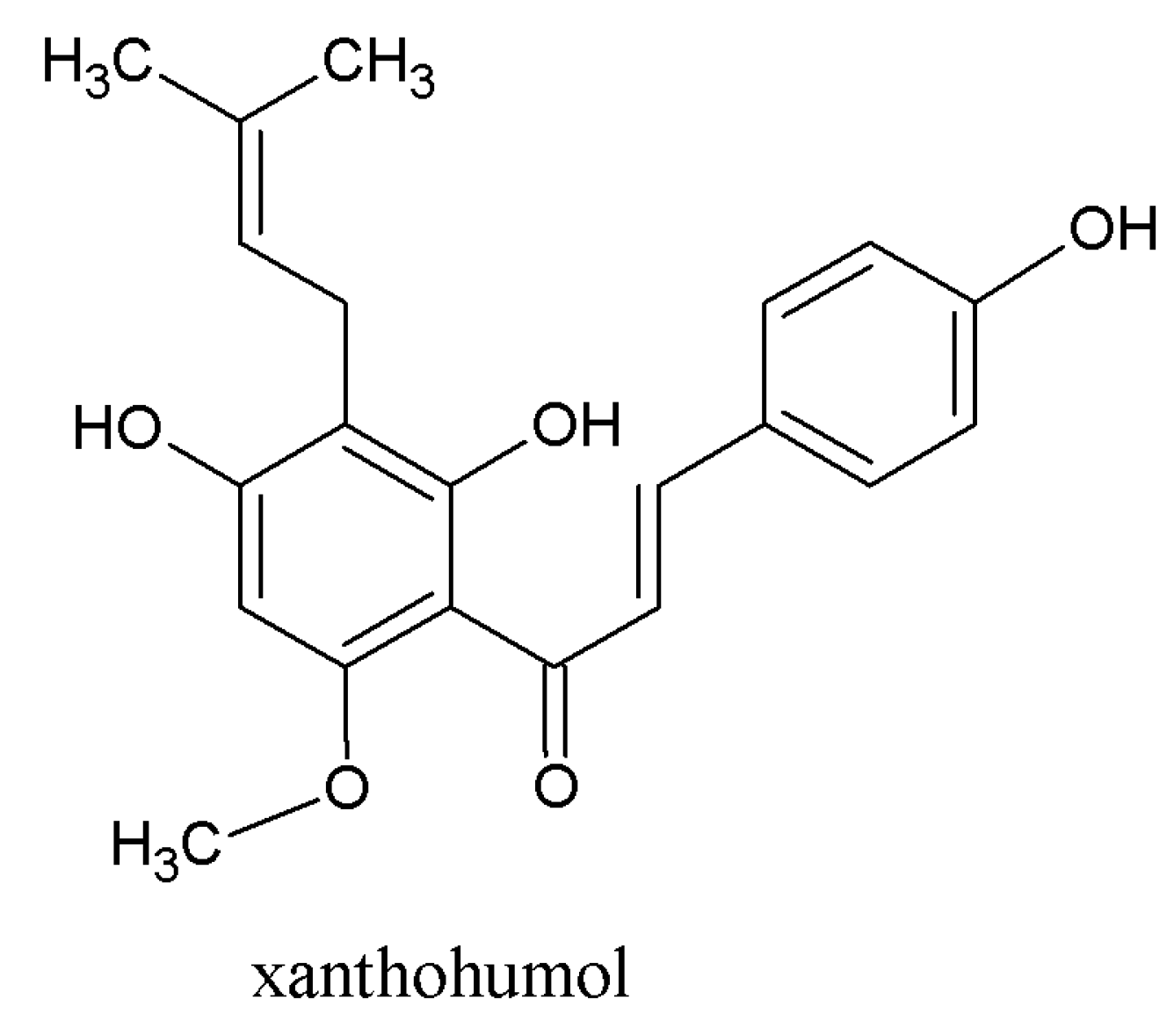
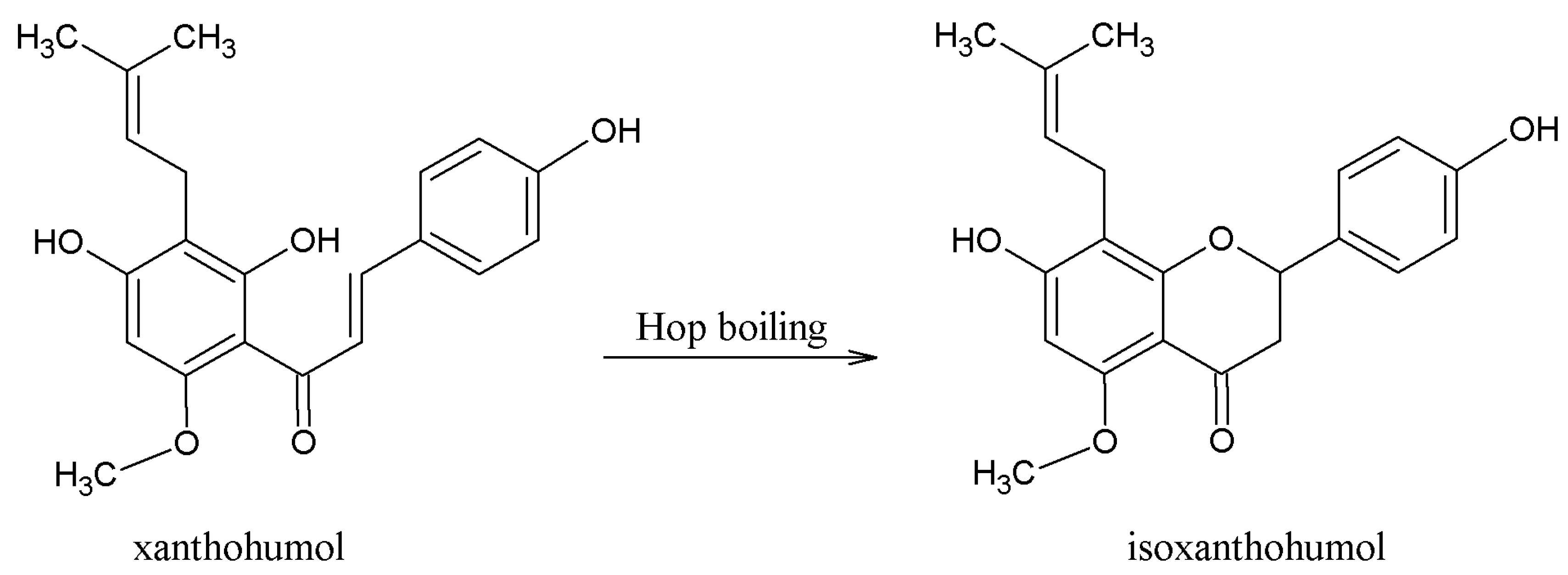
3.1. Beneficial Effects of Hop Polyphenols
3.2. Beneficial Effects of Hop IAA and MHBA
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High added-value compounds from Cannabis threshing residues. Ind. Crop Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R.; Wilson, R.J.H. Hops. In Handbook of Brewing, 2nd ed.; Priest, F.G., Stewart, G.G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2006; pp. 177–279. [Google Scholar]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Koetter, U.; Biendl, M. Hops (Humulus lupulus): A review of its historic and medicinal uses. HerbalGram 2010, 87, 44–57. [Google Scholar]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W.; Suter, A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract. Eur. J. Med. Res. 2008, 13, 200–204. [Google Scholar] [PubMed]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. MeTab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Qian, X.K.; Dong, J.J.; Qian, Z.H.; Miao, J.L. Xanthohumol, a prenylated chalcone from hops, inhibits the viability and stemness of doxorubicin-resistant MCF-7/ADR cells. Molecules 2017, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.R.; Frank, N.; Bartsch, H.; et al. Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol. Cancer Ther. 2002, 1, 959–969. [Google Scholar] [PubMed]
- Gerhäuser, C. Phenolic beer compounds to prevent cancer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 669–684. [Google Scholar]
- Verzele, M.; Stockx, J.; Fontijn, F.; Anteunis, M. Xanthohumol, a new natural chalkone. Bull. Soc. Chim. Belg. 1957, 66, 452–475. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.Z.; Qiu, L.; Dong, J.J.; Yin, H.; Qian, Z.H.; Yang, M.; Miao, J.L. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, L.; Karabin, M.; Kincl, T.; Hudcova, T.; Kotlikova, B.; Dostalek, P. Xanthohumol: Possible isolation and beer enrichment. Chem. Listy 2013, 107, 209–213. [Google Scholar]
- Magalhaes, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [PubMed]
- Karabin, M.; Jelinek, L.; Kincl, T.; Hudcova, T.; Kotlikova, B.; Dostalek, P. New approach to the production of xanthohumol-enriched beers. J. Inst. Brew. 2013, 119, 98–102. [Google Scholar] [CrossRef]
- Magalhaes, P.J.; Dostalek, P.; Cruz, J.M.; Guido, L.F.; Barros, A.A. The impact of a xanthohumol-enriched hop product on the behavior of xanthohumol and isoxanthohumol in pale and dark beers: A pilot scale approach. J. Inst. Brew. 2008, 114, 246–256. [Google Scholar] [CrossRef]
- Wunderlich, S.; Zurcher, A.; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005, 49, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Verzele, M.; De Keukeleire, D. Developments in Food Science—Chemistry and Analysis of Hop and Beer Bitter Acids; Elsevier: Amsterdam, The Netherlands, 1991; Volume 27, pp. 1–417. [Google Scholar]
- Hofta, P.; Dostalek, P.; Sykora, D. Liquid chromatography-diode array and electrospray high-accuracy mass spectrometry of iso-a-acids in DCHA-iso standard and beer. J. Inst. Brew. 2007, 113, 48–54. [Google Scholar] [CrossRef]
- Karabin, M.; Ryparova, A.; Jelinek, L.; Kunz, T.; Wietstock, P.; Methner, F.J.; Dostalek, P. Relationship of iso-a-acid content and endogenous antioxidative potential during storage of lager beer. J. Inst. Brew. 2014, 120, 212–219. [Google Scholar] [CrossRef]
- De Keukeleire, D.; Verzele, M. The absolute configuration of the isohumulones and the humulinic acids. Tetrahedron 1971, 27, 4939–4945. [Google Scholar] [CrossRef]
- Urban, J.; Dahlberg, C.J.; Carroll, B.J.; Kaminsky, W. Absolute configuration of beer’s bitter compounds. Angew. Chem. Int. Ed. 2013, 52, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Cermak, P.; Paleckova, V.; Houska, M.; Strohalm, J.; Novotna, P.; Mikyska, A.; Jurkova, M.; Sikorova, M. Inhibitory effects of fresh hops on Helicobacter pylori strains. Czech J. Food Sci. 2015, 33, 302–307. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Matsukura, Y.; Ozaki, H.; Nishimura, K.; Shindo, K. Identification and quantification of the oxidation products derived from α-acids and β-acids during storage of hops (Humulus lupulus L.). J. Agric. Food. Chem. 2013, 61, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Taniguchi, H.; Matsukura, Y.; Kawachi, Y.; Shindo, K. Structural elucidation of humulone autoxidation products and analysis of their occurrence in stored hops. J. Nat. Prod. 2014, 77, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Matsukura, Y.; Taniguchi, H.; Koizumi, H.; Katayama, M. Development of preparative and analytical methods of the hop bitter acid oxide fraction and chemical properties of its components. Biosci. Biotechnol. Biochem. 2015, 79, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Schonberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus: A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Clawson, J.E.; Deinzer, M.L. Fate of xanthohumol and related prenylflavonoids from hops to beer. J. Agric. Food Chem. 1999, 47, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Kylin, E. Studien ueber das hypertonie-hyperglykämie-hyperurikämiesyndrom. Zent. Inn. Med. 1923, 44, 105–127. [Google Scholar]
- Vague, J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am. J. Clin. Nutr. 1956, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Himsworth, H.P. Diabetes mellitus: Its differentiation into insulin-sensitive and insulin-insensitive types. Lancet 1936, 227, 127–130. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human-disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M. The deadly quartet: Upper-body obesity, glucose-intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Mohan, V. Changing definitions of metabolic syndrome. Indian J. Endocrinol. MeTab. 2012, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; von Eckardstein, A.; Widmann, C. HDLs, diabetes, and metabolic syndrome. In High Density Lipoproteins: From Biological Understanding to Clinical Exploitation; von Eckardstein, A., Kardassis, D., Eds.; Springer: Cham, Switzerland, 2015; pp. 405–421. [Google Scholar]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Luna, A.Y.M.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Bland, J.S. Metabolic syndrome: The complex relationship of diet to conditions of disturbed. Funct. Foods Health Dis. 2011, 1, 1–12. [Google Scholar]
- Jones, J.L.; Comperatore, M.; Barona, J.; Calle, M.C.; Andersen, C.; McIntosh, M.; Najm, W.; Lerman, R.H.; Fernandez, M.L. A mediterranean-style, low-glycemic-load diet decreases atherogenic lipoproteins and reduces lipoprotein (a) and oxidized low-density lipoprotein in women with metabolic syndrome. Metab. Clin. Exp. 2012, 61, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanan, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Mok, S.Y.; Lee, S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase. Food Chem. 2013, 136, 969–974. [Google Scholar] [PubMed]
- Kantsadi, A.L.; Apostolou, A.; Theofanous, S.; Stravodimos, G.A.; Kyriakis, E.; Gorgogietas, V.A.; Chatzileontiadou, D.S.M.; Pegiou, K.; Skamnaki, V.T.; Stagos, D.; et al. Biochemical and biological assessment of the inhibitory potency of extracts from vinification byproducts of Vitis vinifera extracts against glycogen phosphorylase. Food Chem. Toxicol. 2014, 67, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.; Harris, M. Key elements of plant-based diets associated with reduced risk of metabolic syndrome. Curr. Diab. Rep. 2015, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monforte, M.; Sanchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, Y.J.; Yang, Y.K.; Kim, J.Y.; Kwon, O. Dietary flavan-3-ols intake and metabolic syndrome risk in Korean adults. Nutr. Res. Pract. 2012, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N. Flavan 3-ols improve metabolic syndrome risk factors: Evidence and mechanisms. J. Clin. Biochem. Nutr. 2013, 52, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Milenkovic, D.; Morand, C. Vascular protective effects of fruit polyphenols. In Polyphenols in Human Health and Disease; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 875–893. [Google Scholar]
- Liu, M.; Yin, H.; Liu, G.; Dong, J.J.; Qian, Z.H.; Miao, J.L. Xanthohumol, a prenylated chalcone from beer hops, acts as an a-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014, 62, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, K.; Watson, R. Polyphenols and public health. In Polyphenols in Human Health and Disease; Watson, R., Preedy, V., Zibadi, S., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 9–15. [Google Scholar]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, M. Anthocyanins and diabetes regulation. In Polyphenols in Human Health and Disease; Watson, R., Preedy, V., Zibadi, S., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 83–93. [Google Scholar]
- Tan, B.; Ong, K. Influence of dietary polyphenols on carbohydrates metabolism. In Polyphenols in Human Health and Disease; Watson, R., Preedy, V., Zibadi, S., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 95–111. [Google Scholar]
- Unnikrishnan, M.; Veerapur, V.; Nayak, Y.; Mudgal, P.; Mathew, G. Antidiabetic, antihyperlipidemic and antioxidant effect of the flavonoids. In Polyphenols in human health and disease; Watson, R., Preedy, V., Zibadi, S., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 143–161. [Google Scholar]
- Prieto, M.A.V.; Bettaieb, A.; Lanzi, C.R.; Soto, V.C.; Perdicaro, D.J.; Galmarini, C.R.; Haj, F.G.; Miatello, R.M.; Oteiza, P.I. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2015, 59, 622–633. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.; Badawy, D.; Neamatallah, T.; Fahmy, A. Ferulic acid, a natural polyphenol, alleviates insulin resistance and hypertension in fructose fed rats: Effect on endothelial-dependent relaxation. Chem. Biol. Interact. 2016, 254, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Della-Fera, M.A.; Rayalam, S.; Baile, C.A. Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis 2007, 12, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Monteiro, R.; Pestana, D.; Teixeira, D.; Calhau, C.; Azevedo, I. Xanthohumol influences preadipocyte differentiation: Implication of anti proliferative and apoptotic effects. J. Agric. Food. Chem. 2008, 56, 11631–11637. [Google Scholar] [CrossRef] [PubMed]
- Bartmanska, A.; Tronina, T.; Poplonski, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug MeTab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; et al. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.J.; Holick, M.F.; Lerman, R.H.; Konda, V.R.; Minich, D.M.; Desai, A.; Chen, T.C.; Austin, M.; Komberg, J.; Chang, J.L.; et al. Nutritional supplementation of hop rho iso-a-acids, berberine, vitamin D3, and vitamin K1 produces a favorable bone biomarker profile supporting healthy bone metabolism in postmenopausal women with metabolic syndrome. Nutr. Res. 2011, 31, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.S.; Minich, D.; Lerman, R.; Darland, G.; Lamb, J.; Tripp, M.; Grayson, N. Isohumulones from hops (Humulus lupulus) and their potential role in medical nutrition therapy. PharmaNutrition 2015, 3, 46–52. [Google Scholar] [CrossRef]
- Kern, P.A.; Finlin, B.S.; Ross, D.; Boyechko, T.; Zhu, B.; Grayson, N.; Sims, R.; Bland, J.S. Effects of KDT501 on metabolic parameters in insulin-resistant prediabetic humans. J. Endocr. Soc. 2017, 1, 650–659. [Google Scholar] [CrossRef]
- Yajima, H.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Fujiwara, D.; Odai, H.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor a and g and reduce insulin resistance. J. Biol. Chem. 2004, 279, 33456–33462. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Noguchi, T.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int. J. Obes. 2005, 29, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Lerman, R.H.; Minich, D.M.; Darland, G.; Lamb, J.J.; Chang, J.L.; Hsi, A.; Bland, J.S.; Tripp, M.L. Subjects with elevated LDL cholesterol and metabolic syndrome benefit from supplementation with soy protein, phytosterols, hops rho iso-a acids, and Acacia nilotica proanthocyanidins. J. Clin. Lipidol. 2010, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Hosono, M.; Oyamada, C.; Odai, H.; Oikawa, S.; Kondo, K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARa activations in C57BL/6 mice. Br. J. Nutr. 2005, 93, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Hasumi, A.; Minato, T.; Hosono, M.; Miura, Y.; Mizutani, S.; Kondo, K.; Oikawa, S.; Yoshida, A. Isohumulones modulate blood lipid status through the activation of PPARa. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1736, 51–60. [Google Scholar]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Mizutani, M.; Hitomi, Y.; Yajima, H.; Kondo, K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 2009, 28, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Morimoto-Kobayashi, Y.; Ohara, K.; Takahashi, C.; Kitao, S.; Wang, G.Y.; Taniguchi, Y.; Katayama, M.; Nagai, K. Matured hop bittering components induce thermogenesis in brown adipose tissue via sympathetic nerve activity. PLoS ONE 2015, 10, e0131042. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.; Seale, P. Beige can be slimming. Science 2010, 328, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Morimoto-Kobayashi, Y.; Ohara, K.; Ashigai, H.; Kanaya, T.; Koizumi, K.; Manabe, F.; Kaneko, Y.; Taniguchi, Y.; Katayama, M.; Kowatari, Y.; et al. Matured hop extract reduces body fat in healthy overweight humans: A randomized, double-blind, placebo-controlled parallel group study. Nutr. J. 2016, 15, 25. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dostálek, P.; Karabín, M.; Jelínek, L. Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy. Molecules 2017, 22, 1761. https://doi.org/10.3390/molecules22101761
Dostálek P, Karabín M, Jelínek L. Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy. Molecules. 2017; 22(10):1761. https://doi.org/10.3390/molecules22101761
Chicago/Turabian StyleDostálek, Pavel, Marcel Karabín, and Lukáš Jelínek. 2017. "Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy" Molecules 22, no. 10: 1761. https://doi.org/10.3390/molecules22101761





