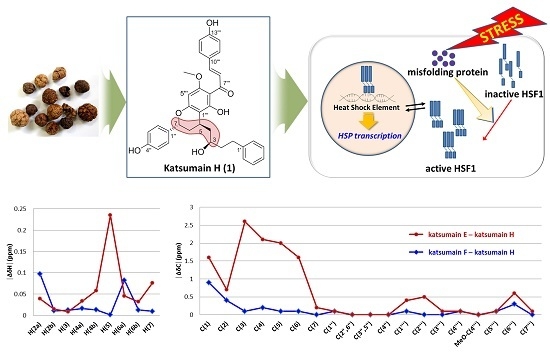
Heat Shock Protein-Inducing Property of Diarylheptanoid Containing Chalcone Moiety from Alpinia katsumadai
Abstract
:1. Introduction
2. Results and Discussion
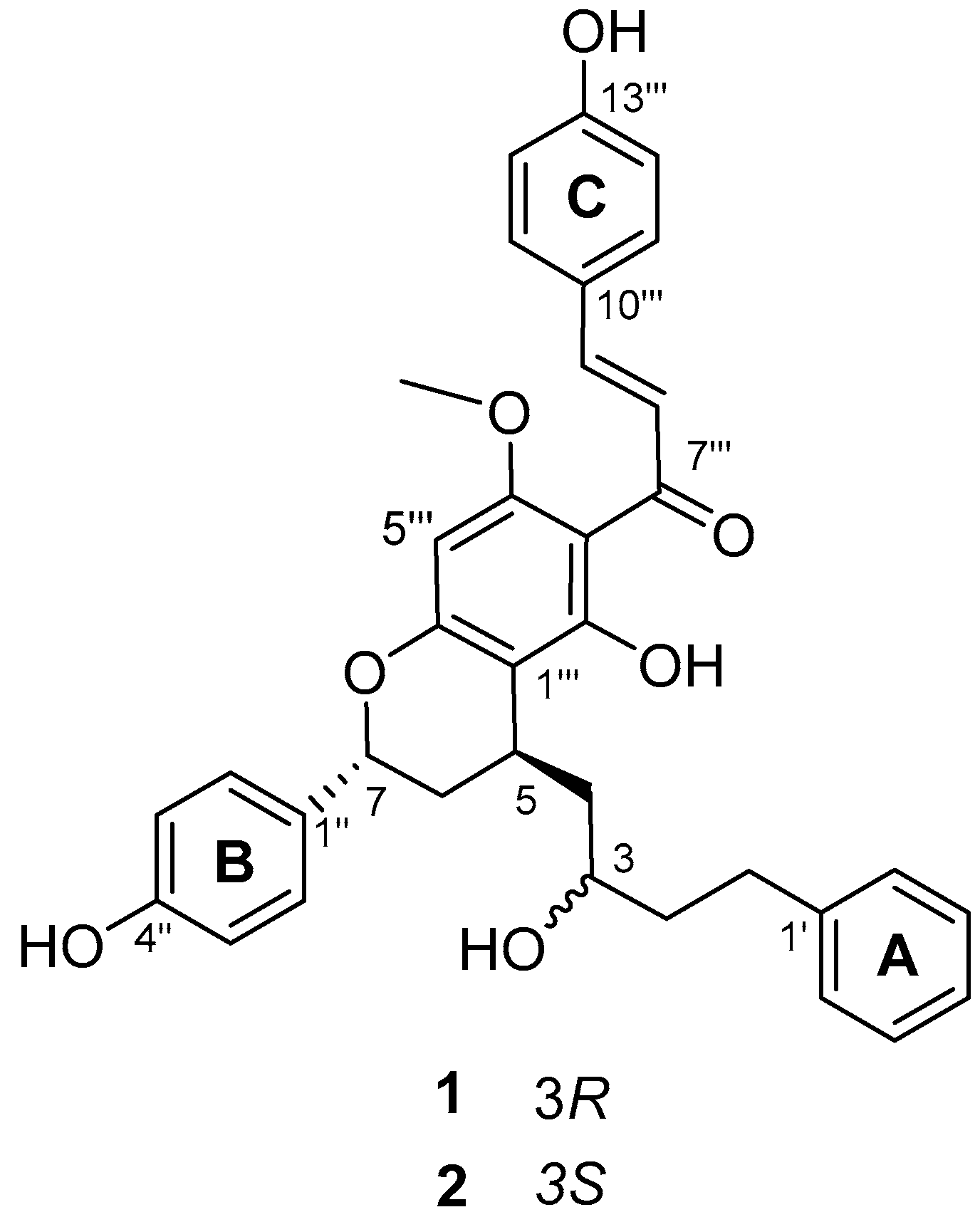
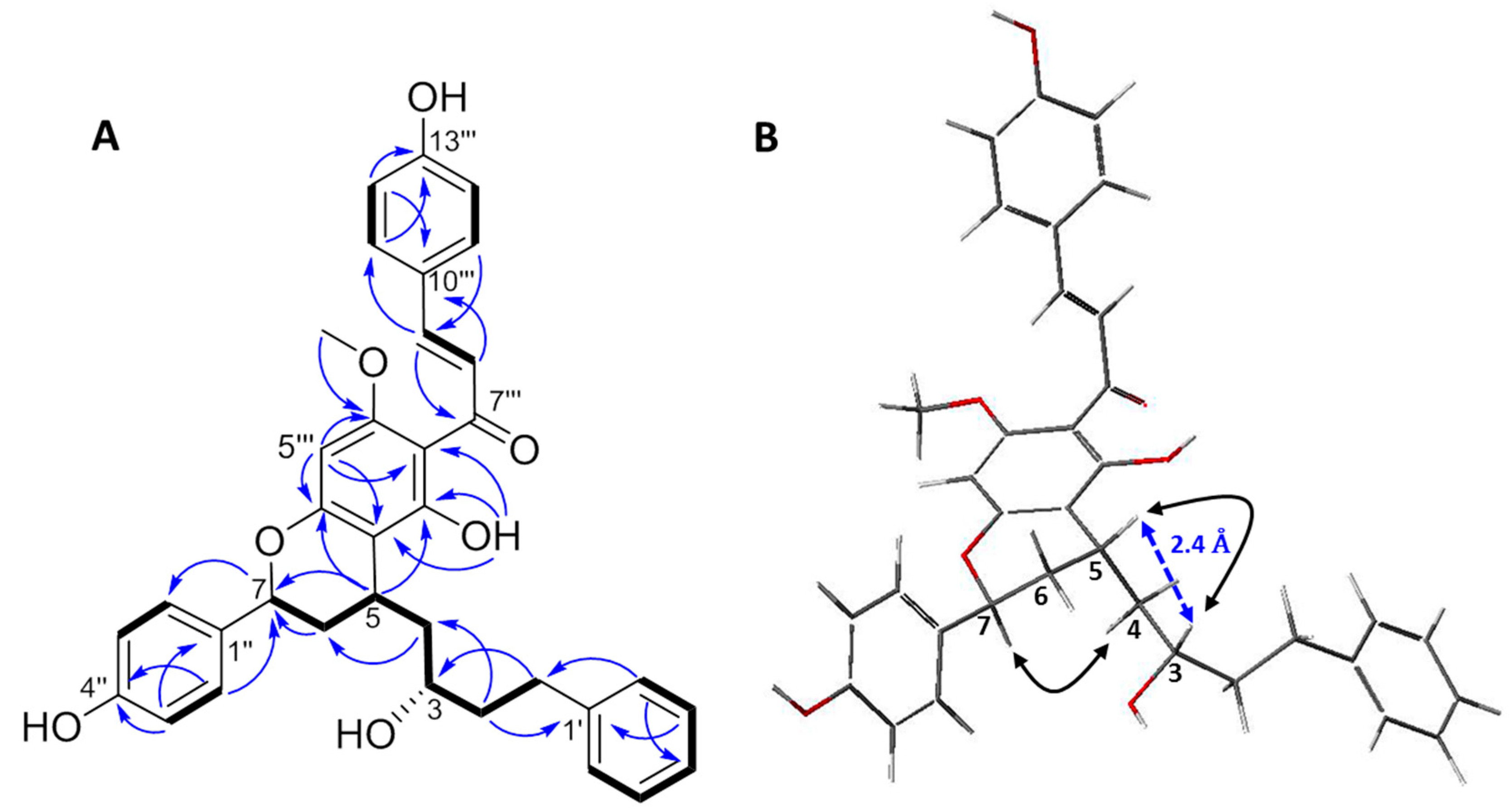
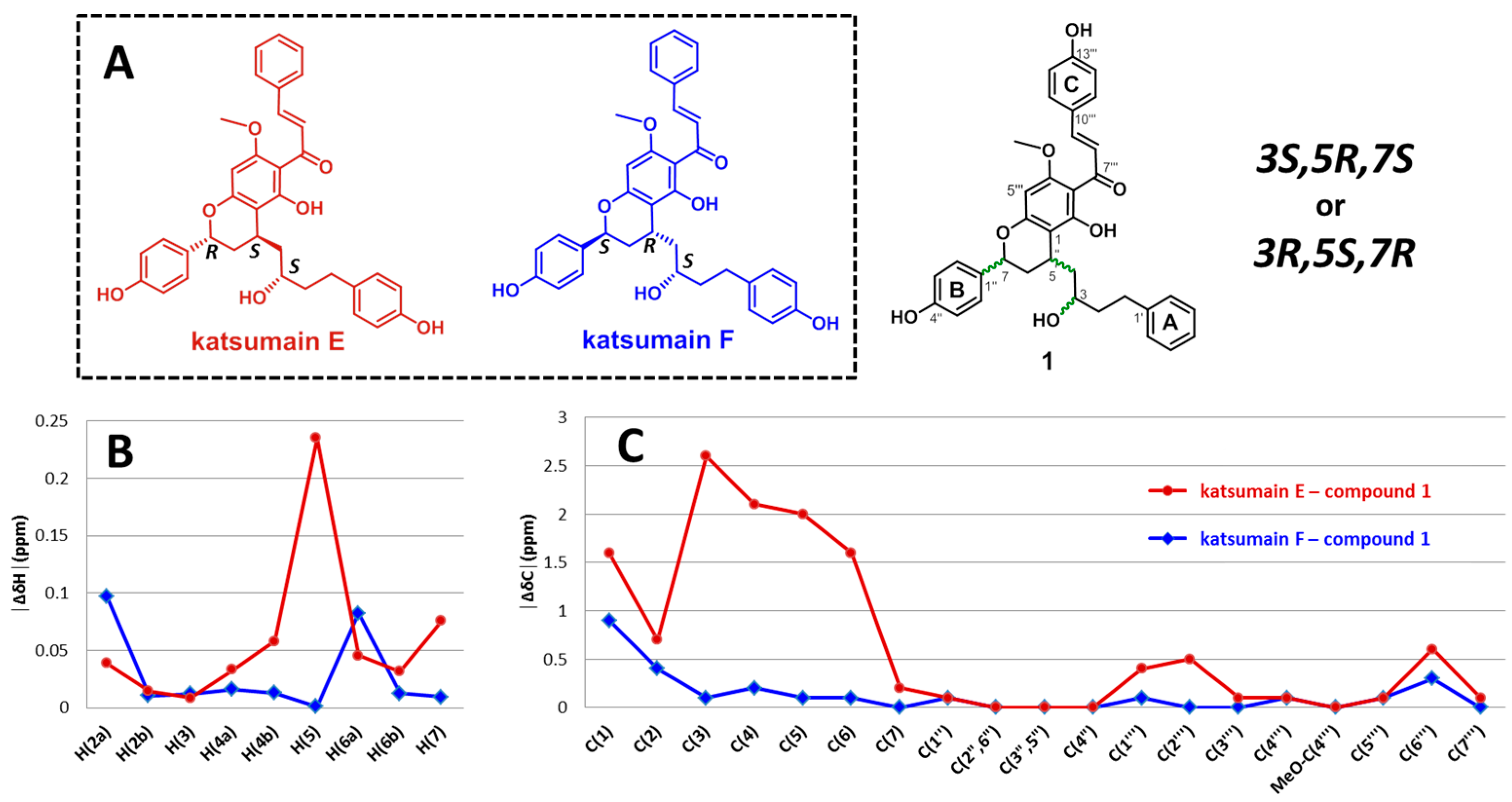
2.1. Structure Elucidation of Compound 1
2.2. Induction of HSF1 and HSPs by Compounds 1 and 2
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Western Blot Analysis
3.5. MTT Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Tai, T.; Nunoura, Y.; Watanabe, K. Anti-emetic principles of Alpinia katsumadai Hayata. Nat. Prod. Sci. 1999, 5, 20–24. [Google Scholar]
- Nam, J.-W.; Seo, E.-K. Structural characterization and biological effects of constituents of the seeds of Alpinia katsumadai (Alpina katsumadai seed). Nat. Prod. Commun. 2012, 7, 795–798. [Google Scholar] [PubMed]
- Nam, J.-W.; Kang, G.-Y.; Han, A.-R.; Lee, D.; Lee, Y.-S.; Seo, E.-K. Diarylheptanoids from the seeds of Alpinia katsumadai as heat shock factor 1 inducers. J. Nat. Prod. 2011, 74, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Chou, G.-X.; Wang, Z.-T. New diarylheptanoids and kavalactone from Alpinia katsumadai Hayata. Helv. Chim. Acta 2010, 93, 382–388. [Google Scholar] [CrossRef]
- Ngo, K.-S.; Brown, G.D. Stilbenes, monoterpenes, diarylheptanoids, labdanes and chalcones from Alpinia katsumadai. Phytochemistry 1998, 47, 1117–1123. [Google Scholar] [CrossRef]
- Nguyen Xuan, D.; Dao Lan, P.; Leclercq, P.A. Constituents of the stem, leaf and seed oils of Alpinia katsumadai Hayata L. from Vietnam. J. Essent. Oil Res. 1990, 2, 259–261. [Google Scholar]
- Wang, X.-B.; Yang, C.-S.; Luo, J.-G.; Zhang, C.; Luo, J.; Yang, M.-H.; Kong, L.-Y. Experimental and theoretical calculation studies on the structure elucidation and absolute configuration of calyxins from Alpinia katsumadai. Fitoterapia 2017, 119, 121–129. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Fu, Y.; Yang, Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci. Rep. 2016, 6, 28370. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.-R.; Ren, S.-J.; Li, J. A new flavonone from seeds of Alpinia katsumadai and its neuroprotective effect on PC12 cells. Zhongguo Zhongyao Zazhi 2014, 39, 2674–2678. [Google Scholar] [PubMed]
- Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Tai, T.; Nunoura, Y.; Watanabe, K. Two novel anti-emetic principles of Alpinia katsumadai. J. Nat. Prod. 1999, 62, 1672–1674. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Nykanen, P.; Sistonen, L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-W.; Kim, S.-Y.; Yoon, T.; Lee, Y.J.; Kil, Y.-S.; Lee, Y.-S.; Seo, E.-K. Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents. Chem. Biodivers. 2013, 10, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-W.; Seo, E.-K. Identification of six new minor diarylheptanoids from the seeds of Alpinia katsumadai. Helv. Chim. Acta 2013, 96, 1670–1680. [Google Scholar] [CrossRef]
- Xu, J.-J.; Tan, N.-H.; Chen, Y.-S.; Pan, X.-L.; Zeng, G.-Z.; Han, H.-J.; Ji, C.-J.; Zhu, M.-J. Three unusual new sesquiterpenes from Alpinia oxyphylla. Helv. Chim. Acta 2009, 92, 1621–1625. [Google Scholar] [CrossRef]
- Huang, S.-X.; Yang, J.; Xiao, W.-L.; Zhu, Y.-L.; Li, R.-T.; Li, L.-M.; Pu, J.-X.; Li, X.; Li, S.-H.; Sun, H.-D. Three novel terpenoids from Schisandra pubescens var. pubinervis. Helv. Chim. Acta 2006, 89, 1169–1175. [Google Scholar] [CrossRef]
- Higashibayashi, S.; Czechtizky, W.; Kobayashi, Y.; Kishi, Y. Universal NMR databases for contiguous polyols. J. Am. Chem. Soc. 2003, 125, 14379–14393. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tan, C.-H.; Kishi, Y. Toward creation of a universal NMR database for stereochemical assignment: Complete structure of the desertomycin/oasomycin class of natural products. J. Am. Chem. Soc. 2001, 123, 2076–2078. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Lee, J.; Tezuka, K.; Kishi, Y. Toward creation of a universal NMR database for the stereochemical assignment of acyclic compounds: The case of two contiguous propionate units. Org. Lett. 1999, 1, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jaber James, J.; Rychnovsky Scott, D. Synthesis and structure revision of calyxin natural products. J. Org. Chem. 2006, 71, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kim, E.-H.; Lee, J.S.; Jeoung, D.; Bae, S.; Kwon, S.H.; Lee, Y.-S. HSF1 as a mitotic regulator: Phosphorylation of HSF1 by Plk1 Is essential for mitotic progression. Cancer Res. 2008, 68, 7550–7560. [Google Scholar] [CrossRef] [PubMed]
- Bava, S.V.; Puliappadamba, V.T.; Deepti, A.; Nair, A.; Karunagaran, D.; Anto, R.J. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J. Biol. Chem. 2005, 280, 6301–6308. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Compound | Fold Increase a | IC50 (μM) b | ||
|---|---|---|---|---|
| HSF1 | HSP27 | HSP70 | ||
| 1 | 1.056 ± 0.023 | 1.312 ± 0.013 | 1.234 ± 0.016 | 44.1 |
| 2 | 1.200 ± 0.030 | 1.242 ± 0.016 | 1.271 ± 0.026 | 42.1 |
| Celastrol c | 1.066 ± 0.009 | 1.216 ± 0.022 | 1.371 ± 0.037 | 12.3 |
| Taxol d | ND e | ND e | ND e | 8.0 |
| Position | 1 | |
|---|---|---|
| δH | δC | |
| 1 | 2.71 m 2.88 m | 32.7 |
| 2 | 1.83 m | 40.7 |
| 3 | 3.84 m | 70.6 |
| 4 | 1.74 m | 43.7 |
| 2.01 m | ||
| 5 | 3.20 m | 28.3 |
| 6 | 1.97 dd (13.8, 5.2) | 35.0 |
| 2.30 d (13.8) | ||
| 7 | 5.25 dd (12.4, 1.6) | 75.5 |
| 1′ | 143.8 | |
| 2′,6′ | 7.23 m | 129.1 |
| 3′,5′ | 7.23 m | 129.3 |
| 4′ | 7.13 m | 126.4 |
| 1′′ | 133.1 | |
| 2′′,6′′ | 7.33 d (8.4) | 128.7 |
| 3′′,5′′ | 6.89 d (8.4) | 116.0 |
| 4′′ | 158.2 | |
| 1′′′ | 108.0 | |
| 2′′′ | 166.5 | |
| 3′′′ | 106.3 | |
| 4′′′ | 161.9 | |
| 5′′′ | 6.05 s | 92.6 |
| 6′′′ | 162.6 | |
| 7′′′ | 193.4 | |
| 8′′′ | 7.94 d (15.6) | 125.2 |
| 9′′′ | 7.80 d (15.6) | 143.7 |
| 10′′′ | 128.1 | |
| 11′′′,15′′′ | 7.63 d (8.8) | 131.4 |
| 12′′′,14′′′ | 6.93d (8.8) | 116.9 |
| 13′′′ | 160.8 | |
| OCH3-4′′′ | 3.98 s | 56.4 |
| OH-2′′′ | 15.32 s | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.-W.; Lee, Y.-S. Heat Shock Protein-Inducing Property of Diarylheptanoid Containing Chalcone Moiety from Alpinia katsumadai. Molecules 2017, 22, 1750. https://doi.org/10.3390/molecules22101750
Nam J-W, Lee Y-S. Heat Shock Protein-Inducing Property of Diarylheptanoid Containing Chalcone Moiety from Alpinia katsumadai. Molecules. 2017; 22(10):1750. https://doi.org/10.3390/molecules22101750
Chicago/Turabian StyleNam, Joo-Won, and Yun-Sil Lee. 2017. "Heat Shock Protein-Inducing Property of Diarylheptanoid Containing Chalcone Moiety from Alpinia katsumadai" Molecules 22, no. 10: 1750. https://doi.org/10.3390/molecules22101750