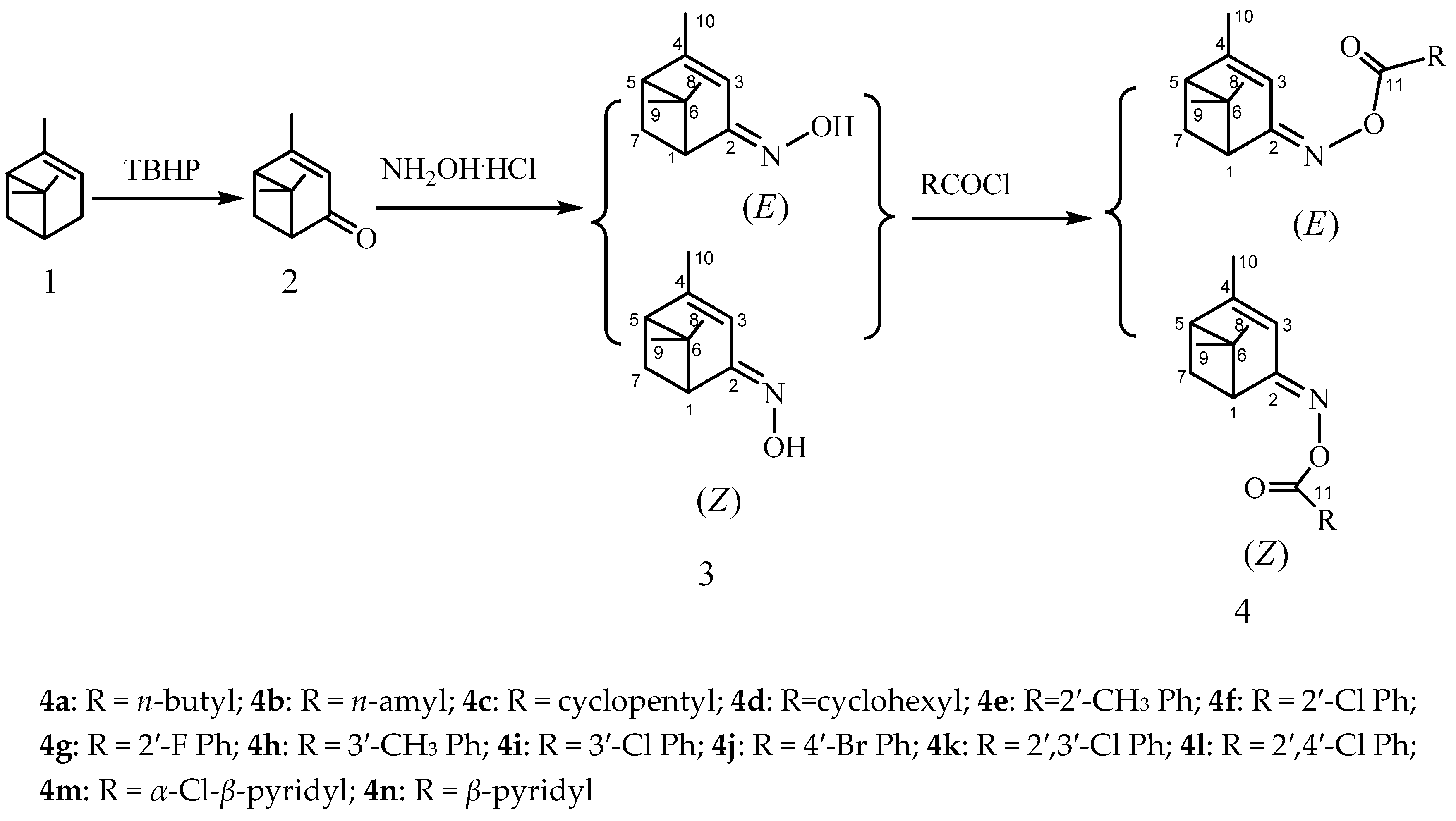
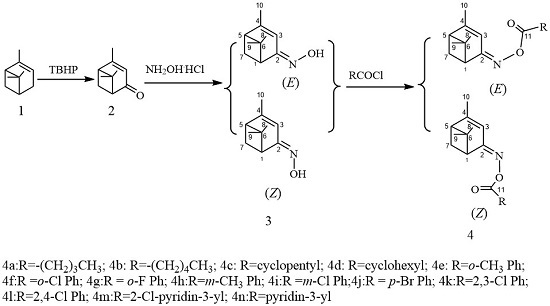
3.4. General Procedure for the Synthesis of (Z)- and (E)-verbenone Oxime Esters 4
Under an anhydrous atmosphere, acyl chloride (1.1 mmol) was added slowly to a stirred solution of (Z)- or (E)-verbenone oxime (3, 0.17 g, 1.00 mmol) in dichloromethane (5 mL) and ten drops of triethylamine in an ice-water bath. The reaction process was monitored by TLC. Upon completion, 5 mL deionized water was added to destroy the unreacted acyl chloride. Then, the organic layer was separated, washed with deionized water three times, and concentrated in vacuum. The crude product was further purified by silica gel chromatography to afford the target compounds (Z)- and (E)-4a–4n.
(Z)-verbenone O-n-pentanoyl oxime ((Z)-4a). Light yellow liquid. Yield: 77.0%, melting point: 69.3–70.3 °C. UV-Vis (EtOH) λmax(log ε): 255.4 (4.33) nm; IR (KBr, cm−1): 3084 (=C-H), 2958, 2930, 2872 (C-H), 1759 (C=O),1622, 1603 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 6.46–6.23 (m, 1H, H-3), 2.93 (t, J = 5.9 Hz, 1H, H-1, 2.74–2.70 (m, 1H, H-5), 2.43 (t, J = 7.6 Hz, 2H, H-12), 2.31 (t, J = 5.7 Hz, 1H, H-7a), 1.97 (dd, J = 1.5, 0.7 Hz, 3H, H-10), 1.80 (d, J = 9.2 Hz, 1H, H-7b), 1.73–1.69 (m, 2H, H-13), 1.45 (s, 3H, H-9), 1.42–1.39 (m, 2H, H-14), 0.95–0.92 (m, 6H, H-8,15); 13C-NMR (150 MHz, CDCl3): δ = 171.5 (C-11), 165.8 (C-2), 163.4 (C-4), 110.0 (C-3), 49.7 (C-1), 49.4 (C-6), 48.3 (C-5), 38.4 (C-12), 32.8 (C-7), 27.1 (C-13), 26.1 (C-8), 23.8 (C-14), 22.3 (C-10), 21.8 (C-9), 13.7 (C-15); ESI-MS m/z: 249.91 [M + H]+. Anal. calcd. For C15H23NO2: C, 72.25; H, 9.30; N, 5.62; Found: C, 72.22; H, 9.21; N, 5.59.
(E)-verbenone O-n-pentanoyl oxime ((E)-4a) as a yellow liquid, Yield 86.0% melting point: 78.8–81.3 °C. UV-Vis (EtOH) λmax(log ε): 252.1 (4.37) nm; IR (KBr, cm−1): 3053 (=C-H), 2958, 2933, 2872 (C-H), 1760 (C=O),1628, 1595 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 6.00–5.97 (m, 1H, H-3), 3.52 (td, J = 5.8, 1.6 Hz, 1H, H-1), 2.66–2.63 (m, 1H, H-5), 2.42–2.39 (m, 2H, H-12), 2.30–2.27 (m, 1H, H-7a), 1.93 (d, J = 1.5 Hz, 3H, H-10), 1.73 (d, J = 9.1 Hz, 1H, H-7b), 1.69–1.66 (m, 2H, H-13), 1.48 (s, 3H, H-9), 1.41–1.38 (m, 2H, H-14), 0.92 (t, J = 3.7 Hz, 6H, H-8,15); 13C-NMR (150 MHz, CDCl3): δ = 171.7 (C-11), 168.1 (C-2), 159.2 (C-4), 115.1 (C-3), 49.2 (C-1), 49.1 (C-6), 43.7 (C-5), 37.2 (C-12), 32.8 (C-7), 27.0 (C-13), 26.2 (C-8), 23.4 (C-14), 22.3 (C-10), 22.2 (C-9), 13.7 (C-15); ESI-MS m/z: 249.84 [M + H]+. Anal. calcd. For C15H23NO2: C, 72.25; H, 9.30; N, 5.62; Found: C, 72.20; H, 9.23; N, 5.60.
(Z)-verbenone O-n-hexanoyl oxime ((Z)-4b). Yellow liquid. Yield: 80.0%, melting point: 62.1–63.2 °C. UV-Vis (EtOH) λmax(log ε): 254.0 (4.38) nm; IR (KBr, cm−1): 3050 (=C-H), 2958, 2933, 2872 (C-H), 1760 (C=O),1628, 1600 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 6.37–6.33 (m, 1H, H-3), 2.93 (t, J = 5.9 Hz, 1H, H-1), 2.72 (dt, J = 10.9, 5.5 Hz, 1H, H-5), 2.42 (t, J = 7.6 Hz, 2H, H-12), 2.31 (t, J = 5.7 Hz, 1H, H-7a), 1.96 (d, J = 1.6 Hz, 3H, H-10), 1.80 (d, J = 9.2 Hz, 1H, H-7b), 1.74–1.69 (m, 2H, H-13), 1.45 (s, 3H, H-9), 1.37–1.34 (m, 4H, H-14,15), 0.94 (s, 3H, H-8), 0.91 (t, J = 6.2 Hz, 3H, H-16); 13C-NMR (150 MHz, CDCl3): δ = 171.5 (C-11), 165.8 (C-2), 163.4 (C-4), 110.0 (C-3), 49.7 (C-1), 49.4 (C-6), 48.3 (C-5), 38.4 (C-12), 33.1 (C-14), 31.4 (C-7), 26.1 (C-8), 24.7 (C-13), 23.8 (C-15), 22.3 (C-10), 21.8 (C-9), 13.9 (C-16); ESI-MS m/z: 263.80 [M + H]+. Anal. calcd. For C16H25NO2: C, 72.97; H, 9.57; N, 5.32; Found: C, 72.65; H, 9.47; N, 5.29.
(E)-verbenone O-n-hexanoyl oxime ((E)-4b). Light yellow liquid. Yield: 72.0%, melting point: 78.2–79.6 °C. UV-Vis (EtOH) λmax(log ε): 252.5 (4.33) nm; IR (KBr, cm−1): 3053 (=C-H), 2957, 2933, 2872 (C-H), 1760 (C=O), 1629, 1600 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 5.99 (d, J = 1.7 Hz, 1H, H-3), 3.52 (td, J = 5.8, 1.6 Hz, 1H, H-1), 2.65 (dt, J = 9.1, 5.5 Hz, 1H, H-5), 2.40 (t, J = 7.6 Hz, 2H, H-12), 2.30–2.27 (m, 1H, H-7a), 1.93 (d, J = 1.6 Hz, 3H, H-10), 1.73 (d, J = 9.1 Hz, 1H, H-7b), 1.71–1.66 (m, 2H, H-13), 1.48 (s, 3H, H-9), 1.34 (q, J = 3.6 Hz, 4H, H-14,15), 0.92 (s, 3H, H-8), 0.91–0.89 (m, 3H, H-16); 13C-NMR (150 MHz, CDCl3): δ = 171.7 (C-11), 168.1 (C-2), 159.2 (C-4), 115.1 (C-3), 49.2 (C-1), 49.1 (C-6), 43.7 (C-5), 37.2 (C-12), 33.1 (C-14), 31.3 (C-7), 26.2 (C-8), 24.7 (C-13), 23.4 (C-15), 22.3 (C-10), 22.2 (C-9), 13.9 (C-16); ESI-MS m/z: 263.88 [M + H]+. Anal. calcd. For C16H25NO2: C, 72.97; H, 9.57; N, 5.32; Found: C, 72.71; H, 9.49; N, 5.30.
(Z)-verbenone O-cyclopentylcarbonyl oxime ((Z)-4c). Slight brown liquid. Yield: 90.0%, melting point: 90.2–91.3 °C. UV-Vis (EtOH) λmax(log ε): 256.9 (4.28) nm; IR (KBr, cm−1): 3082, 3061 (Ar-H, =C-H), 2961, 2899, 2876 (C-H), 1755 (C=O), 1621, 1599, 1577 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 6.35 (d, J = 1.5 Hz, 1H, H-3), 2.94 (td, J = 6.0, 1.6 Hz, 1H, H-12), 2.85 (p, J = 8.1 Hz, 1H, H-1), 2.72 (dt, J = 9.2, 5.5 Hz, 1H, H-5), 2.33–2.29 (m, 1H, H-7a), 1.97 (d, J = 1.6 Hz, 3H, H-10), 1.94 (d, J = 5.5 Hz, 2H, H-16), 1.93–1.85 (m, 2H, H-13), 1.80 (d, J = 9.2 Hz, 1H, H-7b), 1.78–1.72 (m, 2H, H-15), 1.61 (dt, J = 9.0, 4.2 Hz, 2H, H-9), 1.45 (s, 3H, H-14), 0.94 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 174.3 (C-11), 165.8 (C-2), 163.3 (C-4), 110.0 (C-3), 49.7 (C-1), 49.4 (C-6), 48.3 (C-5), 42.8 (C-12), 38.4 (C-16), 30.2 (C-13), 30.1 (C-7), 26.1 (C-15), 25.9 (C-14), 25.9 (C-8), 23.9 (C-10), 21.8 (C-9); ESI-MS m/z: 261.85 [M + H]+. Anal. calcd. For C16H23NO2: C, 73.53; H, 8.87; N, 5.36; Found: C, 73.25; H, 8.78; N, 5.33.
(E)-verbenone O-cyclopentylcarbonyl oxime ((E)-4c). Brown liquid. Yield: 90.0%, melting point: 102.11–104.35 °C. UV-Vis (EtOH) λmax(log ε): 252.6 (4.25) nm; IR (KBr, cm−1): 3066 (= C-H), 2959, 2933, 2873 (C-H), 1741 (C=O), 1619, 1436 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 5.99 (q, J = 1.6 Hz, 1H, H-3), 3.51 (td, J = 5.8, 1.7 Hz, 1H, H-12), 2.82 (p, J = 8.1 Hz, 1H, H-1), 2.64 (dt, J = 9.1, 5.5 Hz, 1H, H-5), 2.28 (td, J = 5.9, 1.4 Hz, 1H, H-7a), 1.92 (d, J = 1.6 Hz, 3H, H-10), 1.90–1.85 (m, 2H, H-16), 1.78–1.74 (m, 1H, H-7b), 1.74–1.72 (m, 2H, H-13), 1.60 (dd, J = 7.2, 4.0 Hz, 2H, H-15), 1.48 (s, 3H, H-9), 1.46–1.24 (m, 2H, H-14), 0.92 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 174.5 (C-11), 168.1 (C-2), 159.1 (C-4), 115.2 (C-3), 49.2 (C-1), 49.1 (C-6), 43.7 (C-5), 42.7 (C-12), 37.2 (C-16), 30.2 (C-13), 30.1 (C-7), 26.2 (C-15), 25.8 (C-14), 25.8 (C-8), 23.4 (C-10), 22.3 (C-9); ESI-MS m/z: 261.86 [M + H]+. Anal. calcd. For C16H23NO2: C, 73.53; H, 8.87; N, 5.36; Found: C, 73.29; H, 8.80; N, 5.34.
(Z)-verbenone O-cyclohexylcarbonyl oxime ((Z)-4d). Yellow liquid. Yield: 93.0%, melting point: 95.7–98.2 °C. UV-Vis (EtOH) λmax(log ε): 256.5 (4.57) nm; IR (KBr, cm−1): 3071 (=C-H), 2980, 2933, 2856 (C-H), 1756 (C=O), 1621, 1600 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 6.34 (q, J = 1.6 Hz, 1H, H-3), 2.94 (td, J = 5.9, 1.7 Hz, 1H, H-12), 2.71 (dt, J = 9.2, 5.5 Hz, 1H, H-1), 2.45 (ddt, J = 11.5, 7.8, 3.6 Hz, 1H, H-5), 2.32–2.29 (m, 1H, H-7a), 2.01–1.98 (m, 2H, H-17), 1.97 (d, J = 1.6 Hz, 3H, H-10), 1.80 (d, J = 9.2 Hz, 2H, H-13), 1.67 (d, J = 10.9 Hz, 1H, H-7b), 1.60–1.54 (m, 2H, H-15), 1.45 (s, 3H, H-9), 1.34–1.25 (m, 4H, H-14,16), 0.94 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 173.4 (C-11), 165.9 (C-2), 163.3 (C-4), 110.0 (C-3), 49.7 (C-1), 49.4 (C-6), 48.3 (C-5), 42.5 (C-12), 38.4 (C-17), 29.2 (C-13), 29.1 (C-7), 26.1 (C-15), 25.7 (C-16), 25.5 (C-14), 25.5 (C-8), 23.8 (C-10), 21.8 (C-9); ESI-MS m/z: 275.86 [M + H]+. Anal. calcd. For C17H25NO2: C, 74.14; H, 9.15; N, 5.09; Found: C, 73.85; H, 9.06; N, 5.06.
(E)-verbenone O-cyclohexylcarbonyl oxime ((E)-4d). Yellow liquid. Yield: 95.0%, melting point: 104.3–105.4 °C. UV-Vis (EtOH) λmax(log ε): 251.9 (4.33) nm; IR (KBr, cm−1): 3048 (=C-H), 2985, 2933, 2856 (C-H), 1759 (C=O), 1630, 1598 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 5.99 (q, J = 1.6 Hz, 1H, H-3), 3.51 (td, J = 5.8, 1.7 Hz, 1H, H-12), 2.64 (dt, J = 9.1, 5.5 Hz, 1H, H-1), 2.41 (tt, J = 11.4, 3.6 Hz, 1H, H-5), 2.28 (td, J = 5.7, 1.5 Hz, 1H, H-7a), 1.97 (d, J = 1.6 Hz, 2H, H-17), 1.92 (d, J = 1.6 Hz, 3H, H-10), 1.79–1.76 (m, 2H, H-13), 1.67–1.64 (m, 1H, H-7b), 1.56–1.50 (m, 2H, H-15), 1.48 (s, 3H, H-9), 1.32–1.23 (m, 4H, H-14,16), 0.92 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 173.6 (C-11), 168.2 (C-2), 159.1 (C-4), 115.2 (C-3), 49.2 (C-1), 49.1 (C-6), 43.7 (C-5), 42.4 (C-12), 37.2 (C-17), 29.1 (C-13), 29.0 (C-7), 26.3 (C-15), 25.7 (C-16), 25.5 (C-14), 25.5 (C-8), 23.4 (C-10), 22.3 (C-9); ESI-MS m/z: 275.94 [M + H]+. Anal. calcd. For C17H25NO2: C, 74.14; H, 9.15; N, 5.09; Found: C, 73.89; H, 9.08; N, 5.07.
(Z)-verbenone O-(2′-methylbenzoyl) oxime ((Z)-4e). White solid. Yield: 95.0%, melting point: 86.3–87.4 °C. UV-Vis (EtOH) λmax(log ε): 262.9 (4.42), 237.8 (4.28) nm; IR (KBr, cm−1): 3027 (Ar-H), 2962, 2943, 2897 (C-H), 1740 (C=O), 1623, 1600, 1575 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.90 (dd, J = 8.1, 1.2 Hz, 1H, H-17), 7.42 (td, J = 7.5, 1.3 Hz, 1H, H-15), 7.29–7.26 (m, 2H, H-16,14), 6.43 (d, J = 1.5 Hz, 1H, H-3), 3.03 (td, J = 6.0, 1.5 Hz, 1H, H-5), 2.76 (dt, J = 9.3, 5.5 Hz, 1H, H-1), 2.64 (s, 3H, H-18), 2.35–2.32 (m, 1H, H-7a), 1.98 (d, J = 1.5 Hz, 3H, H-10), 1.85 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 166.3 (C-11), 165.5 (C-2), 163.7 (C-4), 134.0 (C-13), 131.8 (C-15), 131.6 (C-12), 130.1 (C-14), 129.3 (C-17), 125.7 (C-16), 110.3 (C-3), 49.8 (C-1), 49.5 (C-6), 48.4 (C-5), 38.5 (C-7), 26.2 (C-8), 23.9 (C-10), 21.9 (C-9), 21.4 (C-18); ESI-MS m/z: 283.84 [M + H]+. Anal. calcd. For C18H21NO2: C, 76.30; H, 7.47; N, 4.94; Found: C, 75.98; H, 7.40; N, 4.92.
(E)-verbenone O-(2′-methylbenzoyl) oxime ((E)-4e). White solid. Yield: 90.0%, melting point: 87.8–90.4 °C. UV-Vis (EtOH) λmax(log ε): 262.7 (4.33), 238.0 (4.19) nm; IR (KBr, cm−1): 3027 (Ar-H), 2962, 2943, 2868 (C-H), 1740 (C=O), 1623, 1560, 1574 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.90 (dd, J = 8.0, 1.5 Hz, 1H, H-17), 7.42 (td, J = 7.5, 1.5 Hz, 1H, H-15), 7.31–7.26 (m, 2H, H-16,14), 6.43 (d, J = 1.5 Hz, 1H, H-3), 3.03 (td, J = 5.9, 1.7 Hz, 1H, H-5), 2.78–2.74 (m, 1H, H-1), 2.64 (s, 3H, H-18), 2.33 (ddd, J = 6.5, 5.3, 1.4 Hz, 1H, H-7a), 1.98 (d, J = 1.7 Hz, 3H, H-10), 1.85 (d, J = 9.3 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 166.3 (C-11), 165.5 (C-2), 163.7 (C-4), 134.0 (C-13), 131.8 (C-15), 131.6 (C-12), 130.1 (C-14), 129.3 (C-17), 125.7 (C-16), 110.3 (C-3), 49.8 (C-1), 49.5 (C-6), 48.4 (C-5), 38.5 (C-7), 26.2 (C-8), 23.9 (C-10), 21.9 (C-9), 21.4 (C-18); ESI-MS m/z: 283.82 [M + H]+. Anal. calcd. For C18H21NO2: C, 76.30; H, 7.47; N, 4.94; Found: C, 76.05; H, 7.41; N, 4.93.
(Z)-verbenone-based O-(2′-chlorobenzoyl) oxime ((Z)-4f). Yellow solid. Yield: 91.2%, melting point: 101.1–104.0 °C. UV-Vis (EtOH) λmax(log ε): 263.1 (3.92), 202.5 (4.36) nm; IR (KBr, cm−1): 3083, 3060 (Ar-H, =C-H), 2975, 2955, 2867 (C-H), 1736 (C=O), 1620, 1591, 1469 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.85 (dd, J = 7.7, 1.6 Hz, 1H, H-17), 7.48 (dd, J = 8.0, 1.1 Hz, 1H, H-14), 7.44 (td, J = 7.7, 1.7 Hz, 1H, H-15), 7.35 (td, J = 7.6, 1.3 Hz, 1H, H-16), 6.48 (d, J = 1.7 Hz, 1H, H-3), 3.02 (td, J = 6.0, 1.6 Hz, 1H, H-1), 2.76 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.36 – 2.31 (m, 1H, H-7a), 1.97 (d, J = 1.6 Hz, 3H, H-10), 1.86 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.0 (C-11), 164.2 (C-2), 164.0 (C-4), 133.3 (C-15), 132.5 (C-13), 131.5 (C-12), 130.9 (C-17), 130.2 (C-14), 126.7 (C-16), 110.5 (C-3), 50.1 (C-1), 49.5 (C-6), 48.3 (C-5), 38.6 (C-7), 26.2 (C-8), 23.9 (C-10), 21.8 (C-9); ESI-MS m/z: 303.72 [M + H]+. Anal. calcd. For C17H18ClNO2: C, 66.95; H, 5.97; N, 4.61; Found: C, 67.21; H, 5.91; N, 4.59.
(E)-verbenone O-(2′-chlorobenzoyl) oxime ((E)-4f). Yellow solid. Yield: 91.0%, melting point: 104.4–107.2 °C. UV-Vis (EtOH) λmax(log ε): 262.3 (4.01), 203.2 (4.35) nm; IR (KBr, cm−1): 3081, 3060 (Ar-H, =C-H), 2979, 2952, 2865 (C-H), 1747 (C=O),1621, 1591, 1466 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.80 (dd, J = 7.7, 1.6 Hz, 1H, H-17), 7.47 (d, J = 1.5 Hz, 1H, H-14), 7.45–7.41 (m, 1H, H-15), 7.35–7.33 (m, 1H, H-16), 6.07 (d, J = 1.6 Hz, 1H, H-3), 3.67 (td, J = 5.8, 1.8 Hz, 1H, H-1), 2.68–2.64 (m, 1H, H-5), 2.31 (td, J = 5.7, 1.5 Hz, 1H, H-7a), 1.96 (d, J = 1.7 Hz, 3H, H-10), 1.78 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.96 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.3 (C-11), 164.1 (C-2), 160.1 (C-4), 133.2 (C-15), 132.5 (C-13), 131.5 (C-12), 130.9 (C-17), 130.1 (C-14), 126.7 (C-16), 114.8 (C-3), 49.7 (C-1), 49.1 (C-6), 44.4 (C-5), 37.4 (C-7), 26.2 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 303.72 [M + H]+. Anal. calcd. For C17H18ClNO2: C, 67.21; H, 5.97; N, 4.61; Found: C, 66.97; H, 5.93; N, 4.60.
(Z)-verbenone O-(2′-fluorobenzoyl) oxime ((Z)-4g). Yellow solid. Yield: 89.8%, melting point: 68.9–71.9 °C. UV-Vis (EtOH) λmax(log ε): 265.1 (4.28), 226.6 (4.26) nm; IR (KBr, cm−1): 3064, 3041 (Ar-H, =C-H), 3000, 2964, 2865 (C-H), 1731 (C=O), 1613, 1488, 1456 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.05 (td, J = 7.5, 1.8 Hz, 1H, H-17), 7.56–7.53 (m, 1H, H-15), 7.26–7.24 (m, 1H, H-14), 7.17 (dd, J = 10.3, 8.7 Hz, 1H, H-16), 6.55 – 6.50 (m, 1H, H-3), 3.03 (td, J = 6.0, 1.5 Hz, 1H, H-1), 2.76 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.35–2.32 (m, 1H, H-7a), 1.99 (d, J = 1.5 Hz, 3H, H-10), 1.86 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.0 (C-11), 164.1 (C-2), 134.6 (C-4), 134.5, 132.6 (C-13), 124.2 (C-15), 124.2 (C-17), 117.0 (C-16), 116.8 (C-12), 110.5 (C-14), 110.5 (C-3), 50.0 (C-1), 49.5 (C-6), 48.2 (C-5), 38.6 (C-7), 26.2 (C-8), 23.9 (C-10), 21.8 (C-9); ESI-MS m/z: 287.80 [M + H]+. Anal. calcd. For C17H18FNO2: C, 71.06; H, 6.31; N, 4.87; Found: C, 70.71; H, 6.25; N, 4.85.
(E)-verbenone-based O-(2′-fluorobenzoyl) oxime ((E)-4g). Faint yellow solid. Yield: 90.5%, melting point: 101.3–103.7 °C. UV-Vis (EtOH) λmax(log ε): 264.7 (4.34), 227.3 (4.23) nm; IR (KBr, cm−1): 3067, 3043 (Ar-H, =C-H), 2979, 2958, 2870 (C-H), 1741 (C=O), 1626, 1610, 1597 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.01 (td, J = 7.5, 1.9 Hz, 1H, H-17), 7.54 (ddd, J = 15.4, 4.9, 1.8 Hz, 1H, H-15), 7.24 (td, J = 7.6, 1.1 Hz, 1H, H-14), 7.17–7.13 (m, 1H, H-16), 6.08 (d, J = 1.7 Hz, 1H, H-3), 3.71 (td, J = 5.8, 1.7 Hz, 1H, H-1), 2.69 (dt, J = 9.1, 5.5 Hz, 1H, H-5), 2.36–2.27 (m, 1H, H-7a), 1.96 (d, J = 1.6 Hz, 3H, H-10), 1.79 (d, J = 9.2 Hz, 1H, H-7b), 1.50 (s, 3H, H-9), 0.96 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.2 (C-11), 160.0 (C-2), 134.6 (C-4), 134.6, 132.5 (C-13), 132.5 (C-15), 124.2 (C-17), 124.2 (C-16), 117.0 (C-12), 116.8 (C-14), 114.9 (C-3), 49.6 (C-1), 49.2 (C-6), 44.4 (C-5), 37.4 (C-7), 26.3 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 287.80 [M + H]+. Anal. calcd. For C17H18FNO2: C, 71.06; H, 6.31; N, 4.87; Found: C, 70.82; H, 6.26; N, 4.86.
(Z)-verbenone O-(3′-methylbenzoyl) oxime ((Z)-4h). brown liquid. Yield: 90.5%, melting point: 85.8–89.4 °C. UV-Vis (EtOH) λmax(log ε): 262.8 (4.28), 236.0 (4.29) nm; IR (KBr, cm−1): 3066 (Ar-H), 2953, 2933, 2865 (C-H), 1740 (C=O), 1619, 1590, 1490 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.92 (s, 1H, H-17), 7.90 (d, J = 7.7 Hz, 1H, H-13), 7.39 (d, J = 7.6 Hz, 1H, H-16), 7.36 (d, J = 7.5 Hz, 1H, H-15), 6.49 (q, J = 1.6 Hz, 1H, H-3), 3.04 (td, J = 5.9, 1.7 Hz, 1H, H-1), 2.76 (dt, J = 9.2, 5.5 Hz, 1H, H-5), 2.43 (s, 3H, H-17), 2.36–2.32 (m, 1H, H-7a), 2.01 (d, J = 1.7 Hz, 3H, H-10), 1.86 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 166.6 (C-11), 164.5 (C-2), 163.8 (C-4), 138.3 (C-14), 133.8 (C-15), 130.1 (C-13), 129.5 (C-12), 128.3 (C-17), 126.6 (C-16), 110.1 (C-3), 49.9 (C-1), 49.5 (C-6), 48.3 (C-5), 38.5 (C-7), 26.1 (C-8), 23.9 (C-10), 21.9 (C-9), 21.3 (C-18); ESI-MS m/z: 283.83 [M + H]+. Anal. calcd. For C18H21NO2: C, 76.30; H, 7.47; N, 4.94; Found: C, 75.95; H, 7.41; N, 4.92.
(E)-verbenone O-(3′-methylbenzoyl) oxime ((E)-4h). Faint brown liquid. Yield: 90.5%, melting point: 96.8–97.5 °C. UV-Vis (EtOH) λmax(log ε): 263.2 (4.28), 237.1 (4.25) nm; IR (KBr, cm−1): 3071 (Ar-H), 2957, 2928, 2870 (C-H), 1741 (C=O), 1619, 1592, 1508 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.92 (s, 1H, H-17), 7.90 (d, J = 7.6 Hz, 1H, H-13), 7.39 (d, J = 7.6 Hz, 1H, H-16), 7.36 (t, J = 7.5 Hz, 1H, H-15), 6.49 (q, J = 1.6 Hz, 1H, H-3), 3.04 (td, J = 5.9, 1.7 Hz, 1H, H-1), 2.76 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.43 (s, 3H, H-17), 2.36–2.33 (m, 1H, H-7a), 2.01 (d, J = 1.7 Hz, 3H, H-10), 1.86 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 166.6 (C-11), 164.5 (C-2), 163.8 (C-4), 138.3 (C-14), 133.8 (C-15), 130.2 (C-13), 129.5 (C-12), 128.3 (C-17), 126.6 (C-16), 110.1 (C-3), 49.9 (C-1), 49.5 (C-6), 48.3 (C-5), 38.5 (C-7), 26.1 (C-8), 23.9 (C-10), 21.9 (C-9), 21.3 (C-18); ESI-MS m/z: 283.83 [M + H]+. Anal. calcd. For C18H21NO2: C, 76.30; H, 7.47; N, 4.94; Found: C, 76.05; H, 7.42; N, 4.93.
(Z)-verbenone O-(3′-chlorobenzoyl) oxime ((Z)-4i). Pink solid. Yield: 90.5%, melting point: 84.2–87.5 °C. UV-Vis (EtOH) λmax(log ε): 265.9 (4.17), 231.4 (4.10) nm; IR (KBr, cm−1): 3075 (Ar-H), 2999, 2937, 2869 (C-H), 1734 (C=O), 1619, 1571, 1469 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.07 (t, J = 1.8 Hz, 1H, H-13), 8.00 (dt, J = 7.8, 1.3 Hz, 1H, H-17), 7.56 (ddd, J = 8.0, 2.1, 1.1 Hz, 1H, H-15), 7.43 (t, J = 7.9 Hz, 1H, H-16), 6.46 (q, J = 1.5 Hz, 1H, H-3), 3.03 (td, J = 6.0, 1.6 Hz, 1H, H-1), 2.77 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.37–2.34 (m, 1H, H-7a), 2.02 (d, J = 1.6 Hz, 3H, H-10), 1.87 (d, J = 9.3 Hz, 1H, H-7b), 1.49 (s, 3H, H-9), 0.99 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.1 (C-11), 164.5 (C-2), 163.2 (C-4), 134.6 (C-14), 133.1 (C-15), 131.4 (C-12), 129.8 (C-13), 129.6 (C-16), 127.7 (C-17), 109.9 (C-3), 50.1 (C-1), 49.6 (C-6), 48.3 (C-5), 38.6 (C-7), 26.1 (C-8), 24.0 (C-10), 21.9 (C-9); ESI-MS m/z: 303.76 [M + H]+. Anal. calcd. For C17H18ClNO2: C, 67.21; H, 5.97; N, 4.61; Found: C, 66.92; H, 5.91; N, 4.59.
(E)-verbenone O-(3′-chlorobenzoyl) oxime ((E)-4i). Faint pink solid. Yield: 90.5%, melting point: 89.4–94.8 °C. UV-Vis (EtOH) λmax(log ε): 265.1 (4.08), 232.5 (3.99) nm; IR (KBr, cm−1): 3077, 3048 (Ar-H, =C-H), 2983, 2959, 2871 (C-H), 1743 (C=O), 1632, 1601, 1569 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.01 (s, 1H, H-13), 7.93 (d, J = 7.8 Hz, 1H, H-17), 7.57 – 7.53 (m, 1H, H-15), 7.41 (t, J = 7.9 Hz, 1H, H-16), 6.14–6.03 (m, 1H, H-3), 3.64 (td, J = 5.8, 1.5 Hz, 1H, H-1), 2.72 (dt, J = 9.2, 5.5 Hz, 1H, H-5), 2.36–2.31 (m, 1H, H-7a), 1.97 (d, J = 1.3 Hz, 3H, H-10), 1.82 (d, J = 9.2 Hz, 1H, H-7b), 1.53 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.3 (C-11), 163.2 (C-2), 156.0 (C-4), 134.6 (C-14), 133.1 (C-15), 131.3 (C-12), 129.8 (C-13), 129.6 (C-16), 127.7 (C-17), 115.0 (C-3), 49.6 (C-1), 49.1 (C-6), 44.1 (C-5), 37.5 (C-7), 26.3 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 303.73 [M + H]+. Anal. calcd. For C17H18ClNO2: C, 67.21; H, 5.97; N, 4.61; Found: C, 67.10; H, 5.94; N, 4.60.
(Z)-verbenone O-(4′-bromobenzoyl) oxime ((Z)-4j). White solid. Yield: 90.5%, melting point: 124.6–128.0°C. UV-Vis (EtOH) λmax(log ε): 262.6 (4.29), 249.2 (4.30) nm; IR (KBr, cm−1): 3093, 3074 (Ar-H, =C-H), 2963, 2943, 2869 (C-H), 1737 (C=O), 1619, 1586, 1482 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.00–7.94 (m, 2H, H-13,17), 7.67–7.59 (m, 2H, H-14,16), 6.45 (q, J = 1.4 Hz, 1H, H-3), 3.03 (td, J = 5.8, 1.7 Hz, 1H, H-1), 2.77 (dt, J = 9.3, 5.4 Hz, 1H, H-5), 2.40–2.30 (m, 1H, H-7a), 2.01 (d, J = 1.6 Hz, 3H, H-10), 1.86 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 166.9 (C-11), 164.3 (C-2), 163.6 (C-4), 131.8 (C-13,17), 131.1 (C-14,16), 128.5 (C-12), 128.1 (C-15), 109.9 (C-3), 50.0 (C-1), 49.5 (C-6), 48.3 (C-5), 38.6 (C-7), 26.1 (C-8), 24.0 (C-10), 21.8 (C-9); ESI-MS m/z: 347.65 [M + H]+. Anal. calcd. For C17H18BrNO2: C, 58.63; H, 5.21; N, 4.02; Found: C, 58.39; H, 5.17; N, 4.00.
(Z)-verbenone O-(2′,3′-dichlorobenzoyl) oxime ((Z)-4k). Yellow solid. Yield: 90.5%, melting point: 50.2–52.3 °C. UV-Vis (EtOH) λmax(log ε): 262.4 (4.26), 204.9 (4.61) nm; IR (KBr, cm−1): 3074 (Ar-H), 2953, 2933, 2868 (C-H), 1754 (C=O), 1650, 1622, 1593 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.71–7.68 (m, 1H, H-15), 7.64–7.61 (m, 1H, H-17), 7.31 (s, 1H, H-16), 6.44 (d, J = 1.4 Hz, 1H, H-3), 3.02 (td, J = 6.0, 1.5 Hz, 1H, H-1), 2.78 (dt, J = 9.4, 5.5 Hz, 1H, H-5), 2.35 (t, J = 5.3 Hz, 1H, H-7a), 1.99 (d, J = 1.3 Hz, 3H, H-10), 1.88 (d, J = 9.2 Hz, 1H, H-7b), 1.50 (s, 3H, H-9), 0.99 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.2 (C-11), 164.6 (C-2), 163.7 (C-4), 134.4 (C-14), 133.0 (C-15), 132.8 (C-12), 131.3 (C-13), 129.1 (C-17), 127.3 (C-16), 110.2 (C-3), 50.2 (C-1), 49.5 (C-6), 48.0 (C-5), 38.6 (C-7), 26.1 (C-8), 23.9 (C-10), 21.8 (C-9); ESI-MS m/z: 337.66 [M + H]+. Anal. calcd. For C17H17Cl2NO2: C, 60.37; H, 5.07; N, 4.14; Found: C, 60.07; H, 5.02; N, 4.12.
(E)-verbenone O-(2′,3′-dichlorobenzoyl) oxime ((E)-4k). Faint yellow solid. Yield: 90.5%, melting point: 62.5–63.8 °C. UV-Vis (EtOH) λmax(log ε): 261.4 (4.22), 204.8 (4.58) nm; IR (KBr, cm−1): 3078, 3045 (Ar-H, =C-H), 2954, 2932, 2868 (C-H), 1754 (C=O), 1625, 1595, 1558 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.64 (d, J = 7.7 Hz, 1H, H-15), 7.60 (d, J = 8.0 Hz, 1H, H-17), 7.29 (d, J = 7.9 Hz, 1H, H-16), 6.05 (s, 1H, H-3), 3.62 (td, J = 5.8, 1.7 Hz, 1H, H-1), 2.66 (dt, J = 9.0, 5.5 Hz, 1H, H-5), 2.32 (t, J = 5.6 Hz, 1H, H-7a), 1.96 (s, 3H, H-10), 1.79 (s, 1H, H-7b), 1.48 (s, 3H, H-9), 0.96 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.6 (C-11), 163.8 (C-2), 160.5 (C-4), 134.4 (C-14), 133.1 (C-15), 132.7 (C-12), 131.2 (C-13), 129.1 (C-17), 127.4 (C-16), 114.6 (C-3), 49.8 (C-1), 49.2 (C-6), 44.3 (C-5), 37.4 (C-7), 26.2 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 337.65 [M + H]+. Anal. calcd. For C17H17Cl2NO2: C, 60.37; H, 5.07; N, 4.14; Found: C, 60.12; H, 5.04; N, 4.13.
(Z)-verbenone O-(2′,4′-dichlorobenzoyl) oxime ((Z)-4l). White solid. Yield: 90.5%, melting point: 78.9–80.3 °C. UV-Vis (EtOH) λmax(log ε): 262.8 (4.16), 206.7 (4.45) nm; IR (KBr, cm−1): 3060, 3022 (Ar-H, =C-H), 2934, 2902, 2870 (C-H), 1755 (C=O), 1622, 1581, 1553 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.83 (d, J = 8.4 Hz, 1H, H-17), 7.50 (d, J = 2.0 Hz, 1H, H-14), 7.34 (dd, J = 8.4, 2.0 Hz, 1H, H-16), 6.50–6.39 (m, 1H, H-3), 3.01 (td, J = 6.0, 1.4 Hz, 1H, H-1), 2.76 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.34 (t, J = 5.7 Hz, 1H, H-7a), 1.98 (d, J = 1.5 Hz, 3H, H-10), 1.86 (d, J = 9.3 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.97 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.1 (C-11), 164.5 (C-2), 163.2 (C-4), 138.2 (C-15), 134.4 (C-13), 132.6 (C-17), 130.8 (C-12), 128.4 (C-14), 127.1 (C-16), 110.4 (C-3), 50.1 (C-1), 49.5 (C-6), 48.2 (C-5), 38.6 (C-7), 26.1 (C-8), 24.0 (C-10), 21.8 (C-9); ESI-MS m/z: 337.74 [M + H]+. Anal. calcd. For C17H17Cl2NO2: C, 60.37; H, 5.07; N, 4.14; Found: C, 60.09; H, 5.03; N, 4.12.
(E)-verbenone O-(2′,4′-dichlorobenzoyl) oxime ((E)-4l). Faint yellow liquid. Yield: 90.5%, melting point: 112.7–121.9 °C. UV-Vis (EtOH) λmax(log ε): 262.1 (4.26), 207.1 (4.57) nm; IR (KBr, cm−1): 3084 (Ar-H), 2956, 2927, 2870 (C-H), 1752 (C=O), 1629, 1586, 1557 (C=N, Ar-C=C, C=C); 1H-NMR (600 MHz, CDCl3): δ = 7.78 (d, J = 8.4 Hz, 1H, H-17), 7.48 (d, J = 2.0 Hz, 1H, H-14), 7.33 (dd, J = 8.4, 2.0 Hz, 1H, H-16), 6.06 (q, J = 1.6 Hz, 1H, H-3), 3.64 (td, J = 5.8, 1.7 Hz, 1H, H-1), 2.67 (dt, J = 9.2, 5.5 Hz, 1H, H-5), 2.32 (td, J = 5.9, 1.4 Hz, 1H, H-7a), 1.96 (d, J = 1.6 Hz, 3H, H-10), 1.78 (d, J = 9.2 Hz, 1H, H-7b), 1.48 (s, 3H, H-9), 0.96 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.5 (C-11), 163.3 (C-2), 160.3 (C-4), 138.3 (C-15), 134.3 (C-13), 132.6 (C-17), 130.8 (C-12), 128.4 (C-14), 127.2 (C-16), 114.7 (C-3), 49.7 (C-1), 49.1 (C-6), 44.5 (C-5), 37.4 (C-7), 26.3 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 337.67 [M + H]+. Anal. calcd. For C17H17Cl2NO2: C, 60.37; H, 5.07; N, 4.14; Found: C, 60.12; H, 5.04; N, 4.13.
(Z)-verbenone O-2-chloropyridylcarbonyl oxime ((Z)-4m). White solid. Yield: 90.5%, melting point: 84.1–87.1 °C. UV-Vis (EtOH) λmax(log ε): 261.5 (4.39), 219.7 (4.34) nm; IR (KBr, cm−1): 3077 (=C-H), 2955, 2871 (C-H), 1758 (C=O), 1621, 1600 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.55 (dd, J = 4.8, 2.0 Hz, 1H, H-14), 8.20 (dd, J = 7.6, 2.0 Hz, 1H, H-16), 7.38 (dd, J = 7.7, 4.8 Hz, 1H, H-15), 6.48 (q, J = 1.5 Hz, 1H, H-3), 3.01 (td, J = 6.0, 1.6 Hz, 1H, H-1), 2.78 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.37–2.33 (m, 1H, H-7a), 1.99 (d, J = 1.6 Hz, 3H, H-10), 1.87 (d, J = 9.3 Hz, 1H, H-7b), 1.49 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.4 (C-11), 164.9 (C-2), 163.0 (C-4), 151.8 (C-14), 149.5 (C-13), 140.5 (C-15), 127.1 (C-12), 122.2 (C-16), 110.3 (C-3), 50.3 (C-1), 49.5 (C-6), 48.2 (C-5), 38.7 (C-7), 26.1 (C-8), 24.0 (C-10), 21.8 (C-9); ESI-MS m/z: 313.24 [M + H]+. Anal. calcd. For C16H17ClN2O2: C, 63.06; H, 5.62; N, 9.19; Found: C, 62.81; H, 5.57; N, 9.14.
(E)-verbenone O-2-chloropyridylcarbonyl oxime ((E)-4m). Faint yellow solid. Yield: 90.5%, melting point: 123.7–126.5 °C. UV-Vis (EtOH) λmax(log ε): 266.1(4.30), 218.8 (4.13) nm; IR (KBr, cm−1): 3074 (=C-H), 2964, 2943, 2866 (C-H), 1756 (C=O), 1622, 1578 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 8.55 (dd, J = 4.8, 2.0 Hz, 1H, H-14), 8.20 (dd, J = 7.6, 2.0 Hz, 1H, H-16), 7.38 (dd, J = 7.7, 4.8 Hz, 1H, H-15), 6.48 (q, J = 1.5 Hz, 1H, H-3), 3.01 (td, J = 5.9, 1.7 Hz, 1H, H-1), 2.78 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.38–2.33 (m, 1H, H-7a), 1.99 (d, J = 1.6 Hz, 3H, H-10), 1.87 (d, J = 9.3 Hz, 1H, H-7b), 1.49 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.4 (C-11), 164.9 (C-2), 163.0 (C-4), 151.8 (C-14), 149.5 (C-13), 140.5 (C-15), 127.1 (C-12), 122.2 (C-16), 110.3 (C-3), 50.3 (C-1), 49.5 (C-6), 48.2 (C-5), 38.7 (C-7), 26.1 (C-8), 24.0 (C-10), 21.8 (C-9); ESI-MS m/z: 312.88 [M + H]+. Anal. calcd. For C16H17ClN2O2: C, 63.06; H, 5.62; N, 9.19; Found: C, 62.85; H, 5.58; N, 9.16.
(Z)-verbenone O-β-pyridylcarbonyl oxime ((Z)-4n). Brown solid. Yield: 90.5%, melting point: 75.2–76.9 °C. UV-Vis (EtOH) λmax(log ε): 266.1 (4.45), 220.2 (4.34) nm; IR (KBr, cm−1): 3053 (=C-H), 2980, 2952, 2876 (C-H), 1745 (C=O), 1619, 1589, 1482 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 9.31 (d, J = 1.7 Hz, 1H, H-13), 8.83 (dd, J = 4.9, 1.6 Hz, 1H, H-14), 8.44 (dt, J = 7.9, 1.9 Hz, 1H, H-16), 7.50 (dd, J = 7.9, 4.9 Hz, 1H, H-14), 6.61–6.36 (m, 1H, H-3), 3.03 (td, J = 6.0, 1.5 Hz, 1H, H-1), 2.78 (dt, J = 9.3, 5.5 Hz, 1H, H-5), 2.38–2.36 (m, 1H, H-7a), 2.03 (d, J = 1.5 Hz, 3H, H-10), 1.88 (d, J = 9.3 Hz, 1H, H-7b), 1.50 (s, 3H, H-9), 0.99 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 167.3 (C-11), 164.9 (C-2), 162.7 (C-4), 152.8 (C-14), 150.0 (C-13), 137.8 (C-16), 126.0 (C-12), 123.8 (C-15), 109.8 (C-3), 50.2 (C-1), 49.6 (C-6), 48.3 (C-5), 38.7 (C-7), 26.1 (C-8), 24.0 (C-10), 21.8 (C-9); ESI-MS m/z: 270.83 [M + H]+. Anal. calcd. For C16H18N2O2: C, 71.09; H, 6.71; N, 10.36; Found: C, 70.80; H, 6.64; N, 10.31.
(E)-verbenone O-β-pyridylcarbonyl oxime ((E)-4n). Faint yellow solid. Yield: 90.5%, melting point: 77.5–82.4 °C. UV-Vis (EtOH) λmax(log ε): 261.5(4.39), 219.7 (4.34) nm; IR (KBr, cm−1): 3060 (=C-H), 2991, 2965, 2871 (C-H), 1748 (C=O), 1621, 1586 (C=N, C=C); 1H-NMR (600 MHz, CDCl3): δ = 9.23 (d, J = 1.5 Hz, 1H, H-13), 8.80 (dd, J = 4.8, 1.6 Hz, 1H, H-14), 8.34 (dt, J = 7.9, 1.9 Hz, 1H, H-16), 7.44 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, H-14), 6.13–6.04 (m, 1H, H-3), 3.66 (td, J = 5.8, 1.6 Hz, 1H, H-1), 2.72 (dt, J = 9.2, 5.5 Hz, 1H, H-5), 2.34 (td, J = 5.7, 1.6 Hz, 1H, H-7a), 1.97 (d, J = 1.5 Hz, 3H, H-10), 1.82 (d, J = 9.2 Hz, 1H, H-7b), 1.52 (s, 3H, H-9), 0.98 (s, 3H, H-8); 13C-NMR (150 MHz, CDCl3): δ = 169.5 (C-11), 163.0 (C-2), 160.3 (C-4), 153.5 (C-14), 150.6 (C-13), 137.2 (C-16), 125.6 (C-12), 123.5 (C-15), 114.8 (C-3), 49.7 (C-1), 49.1 (C-6), 44.1 (C-5), 37.6 (C-7), 26.3 (C-8), 23.5 (C-10), 22.3 (C-9); ESI-MS m/z: 270.75 [M + H]+. Anal. calcd. For C16H18N2O2: C, 71.09; H, 6.71; N, 10.36; Found: C, 70.83; H, 6.66; N, 10.33.