Synthesis of Chromone-Related Pyrazole Compounds
Abstract
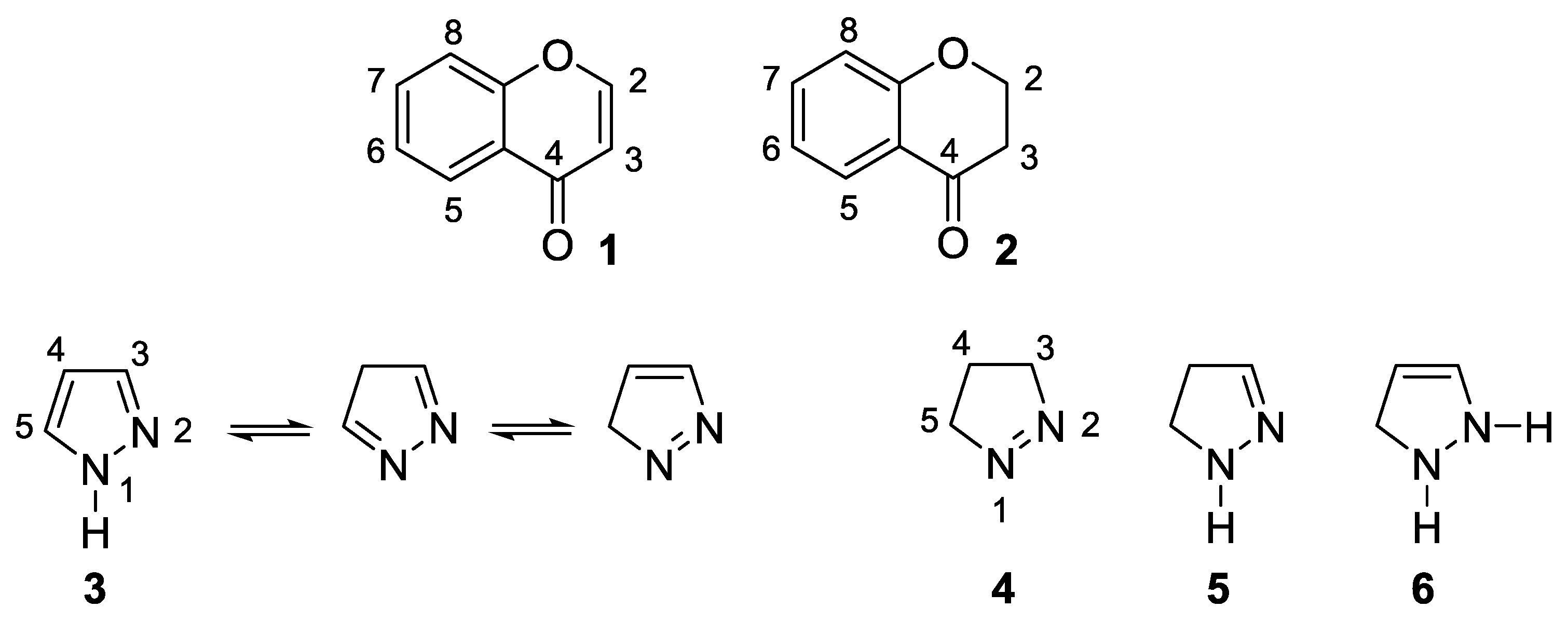
:1. Introduction
2. Synthesis of Chromone-Pyrazole Dyads
2.1. Pyrazole as Substituent
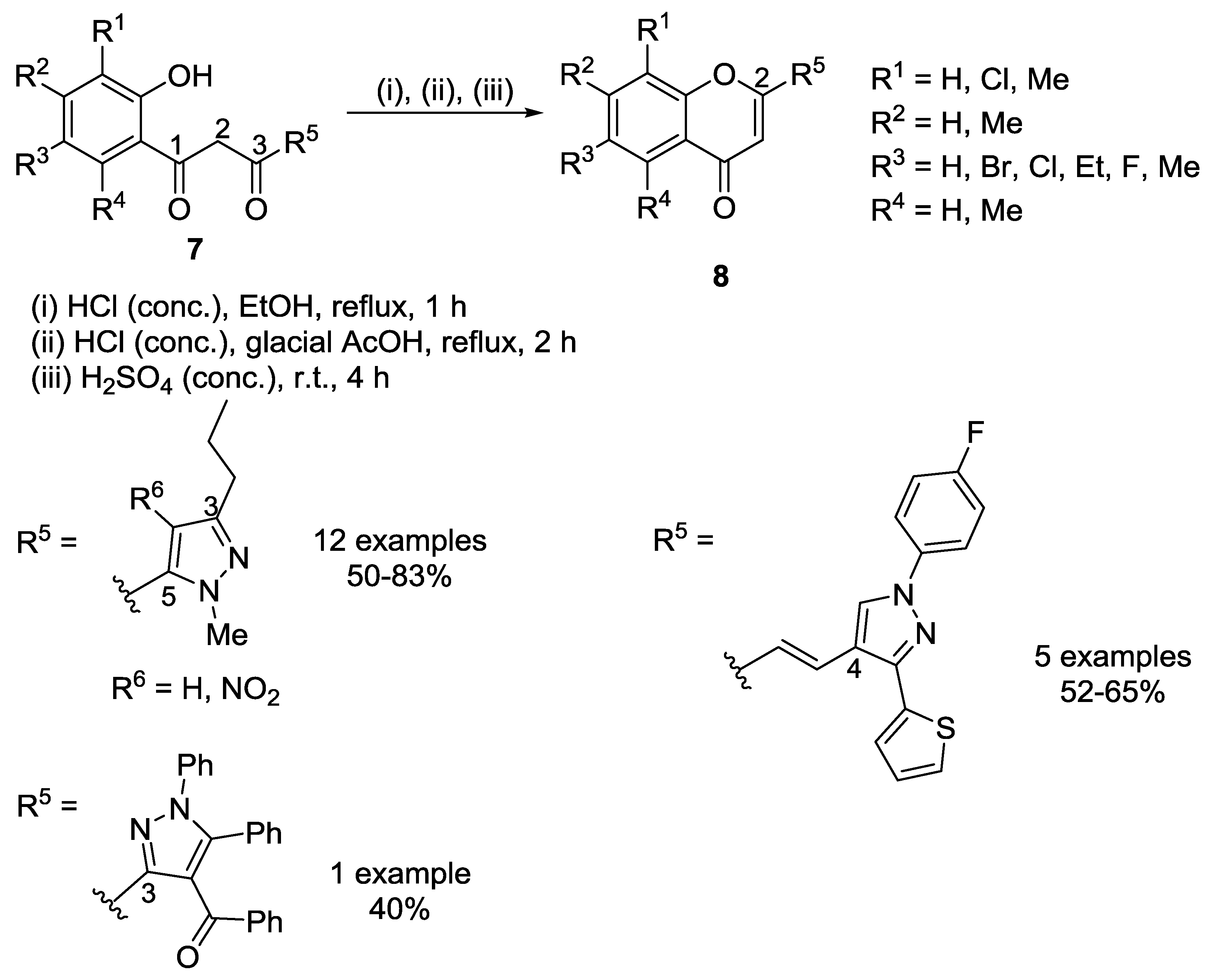
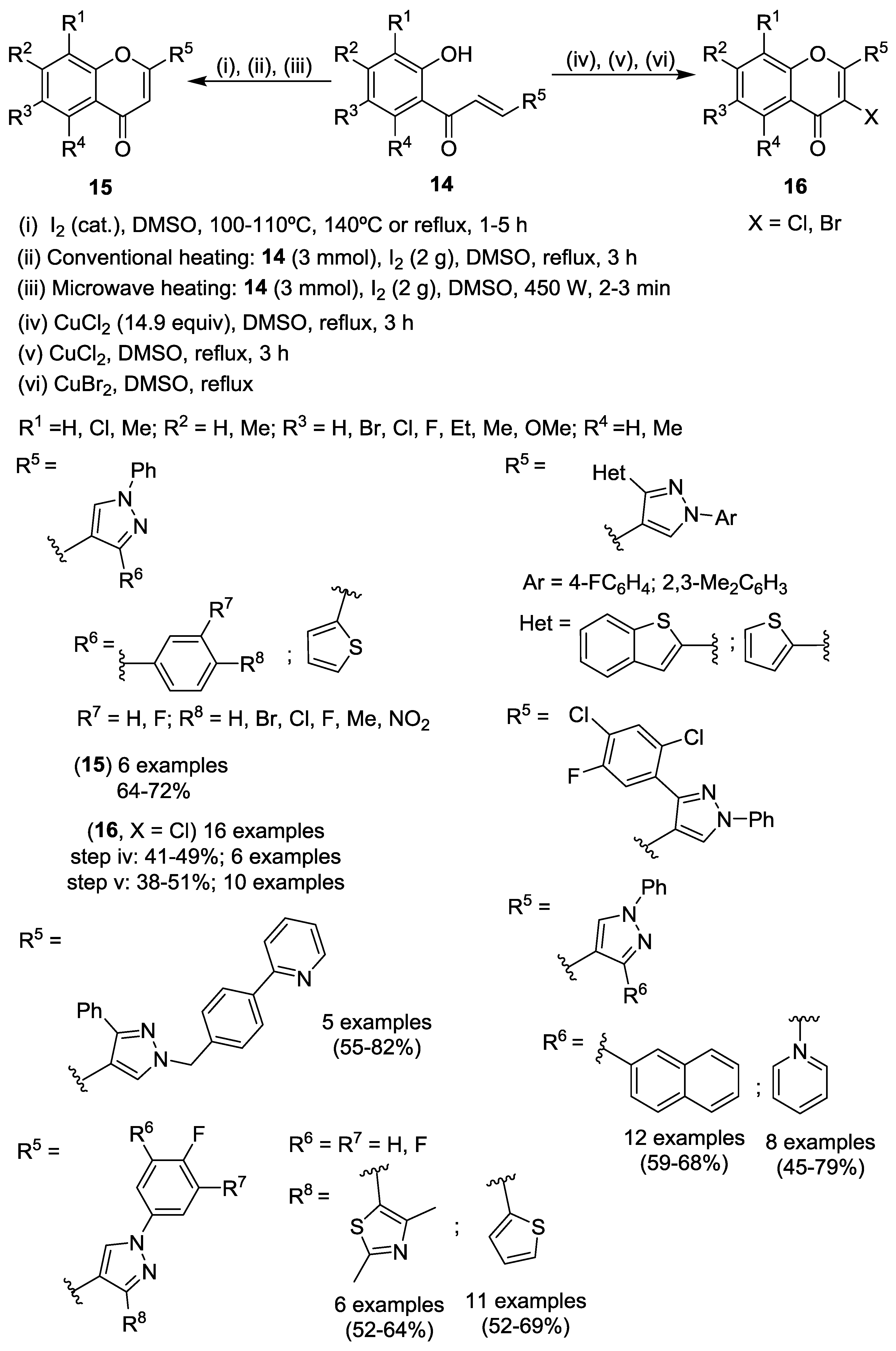
2.1.1. Cyclodehydration
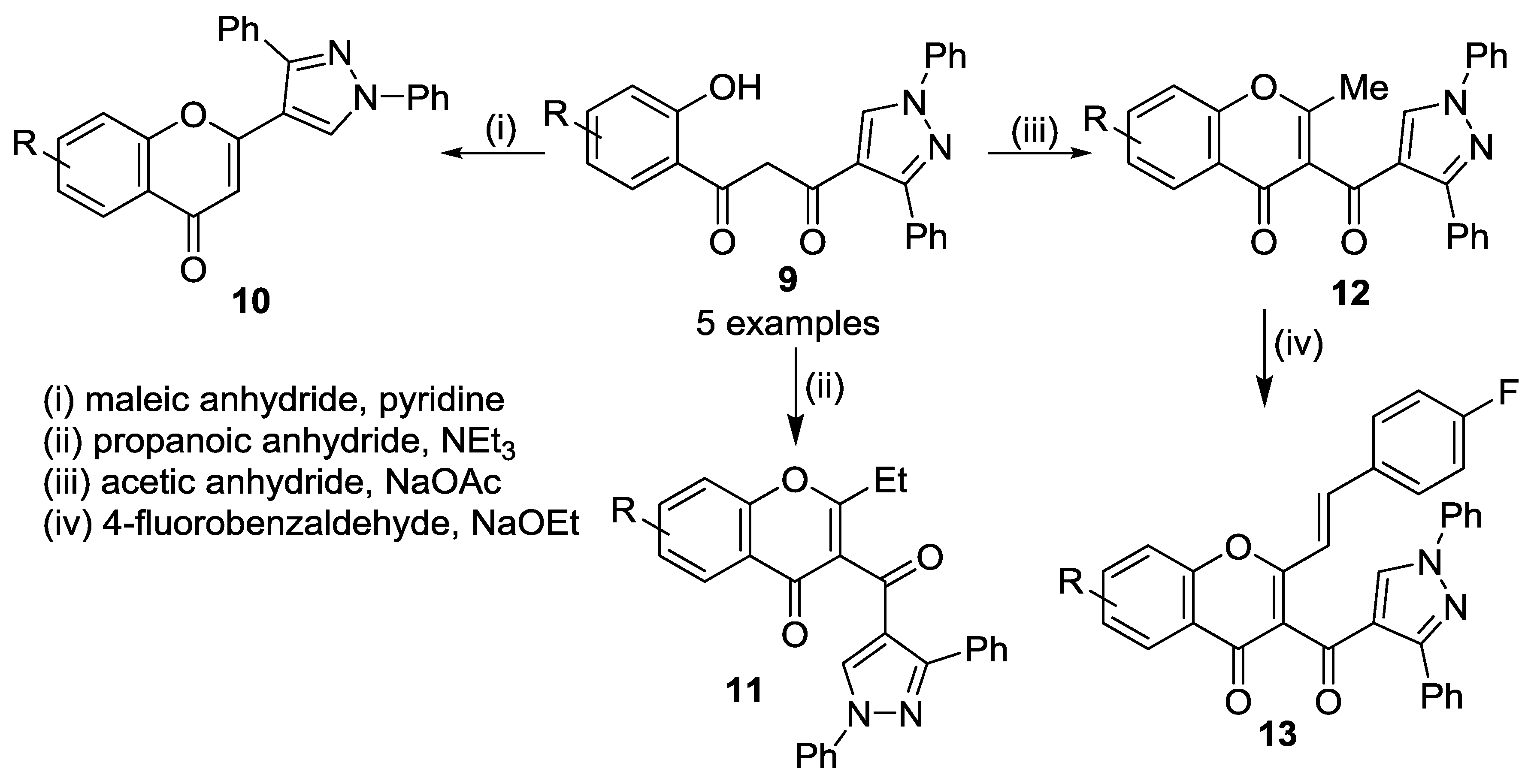
2.1.2. Oxidative Cyclization
2.2. Pyrazole Ring Formation
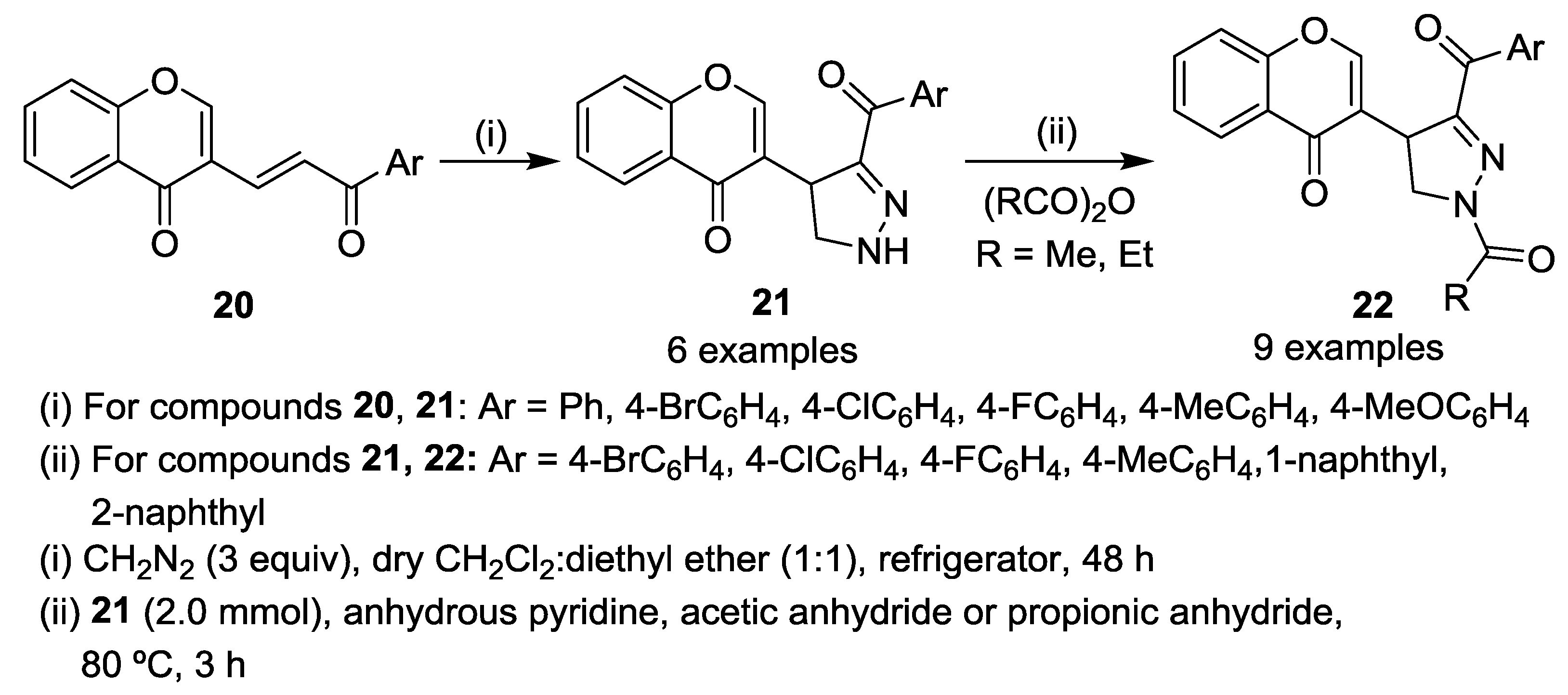
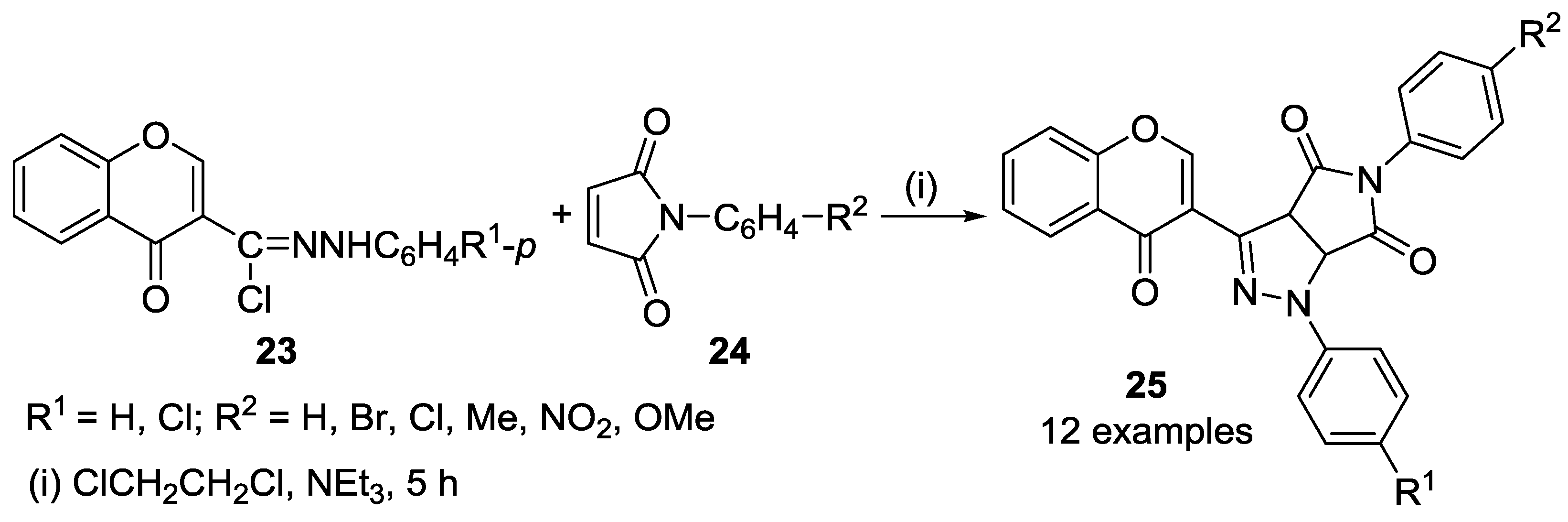
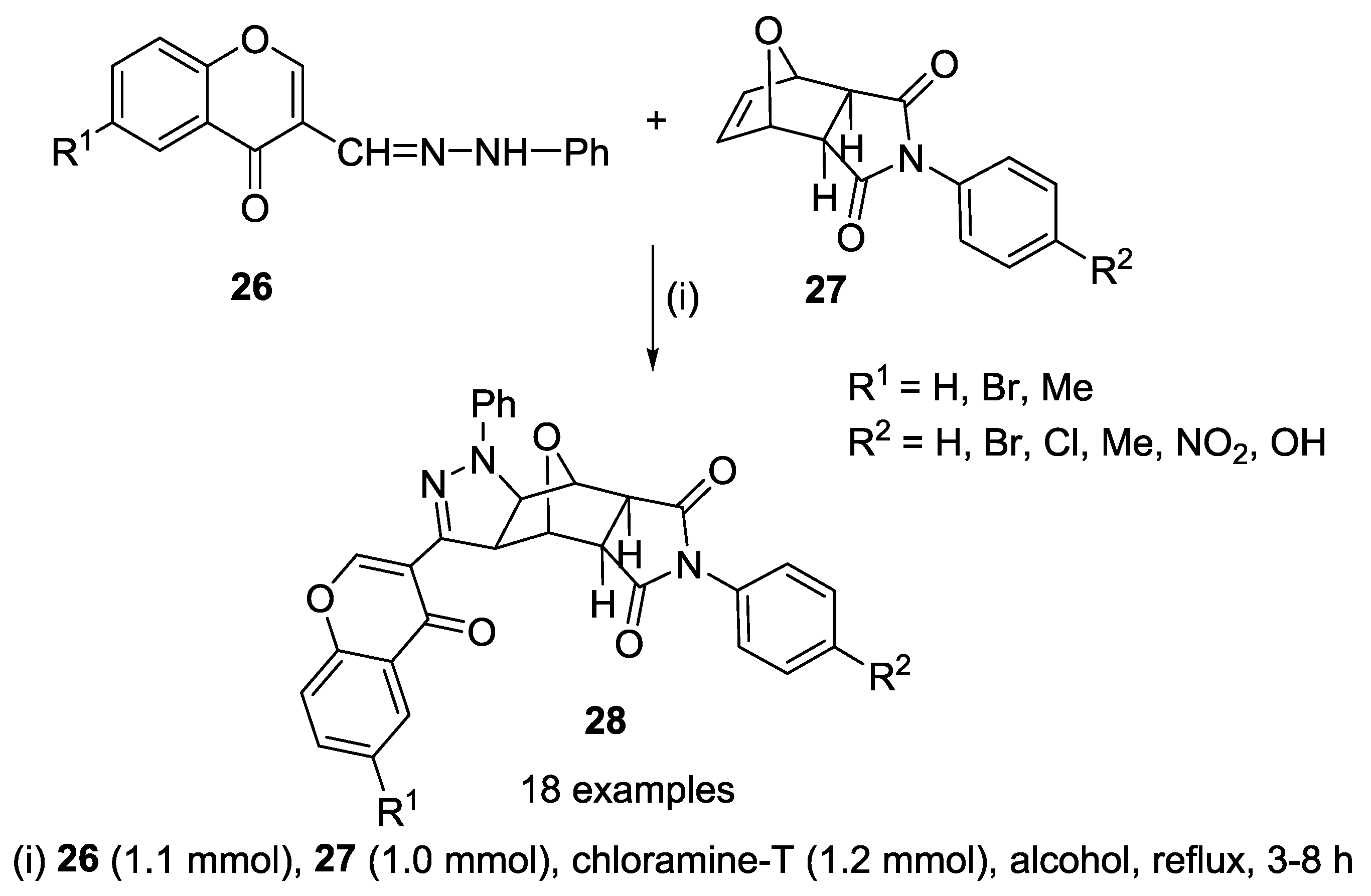
2.2.1. 1,3-Dipolar Cycloaddition
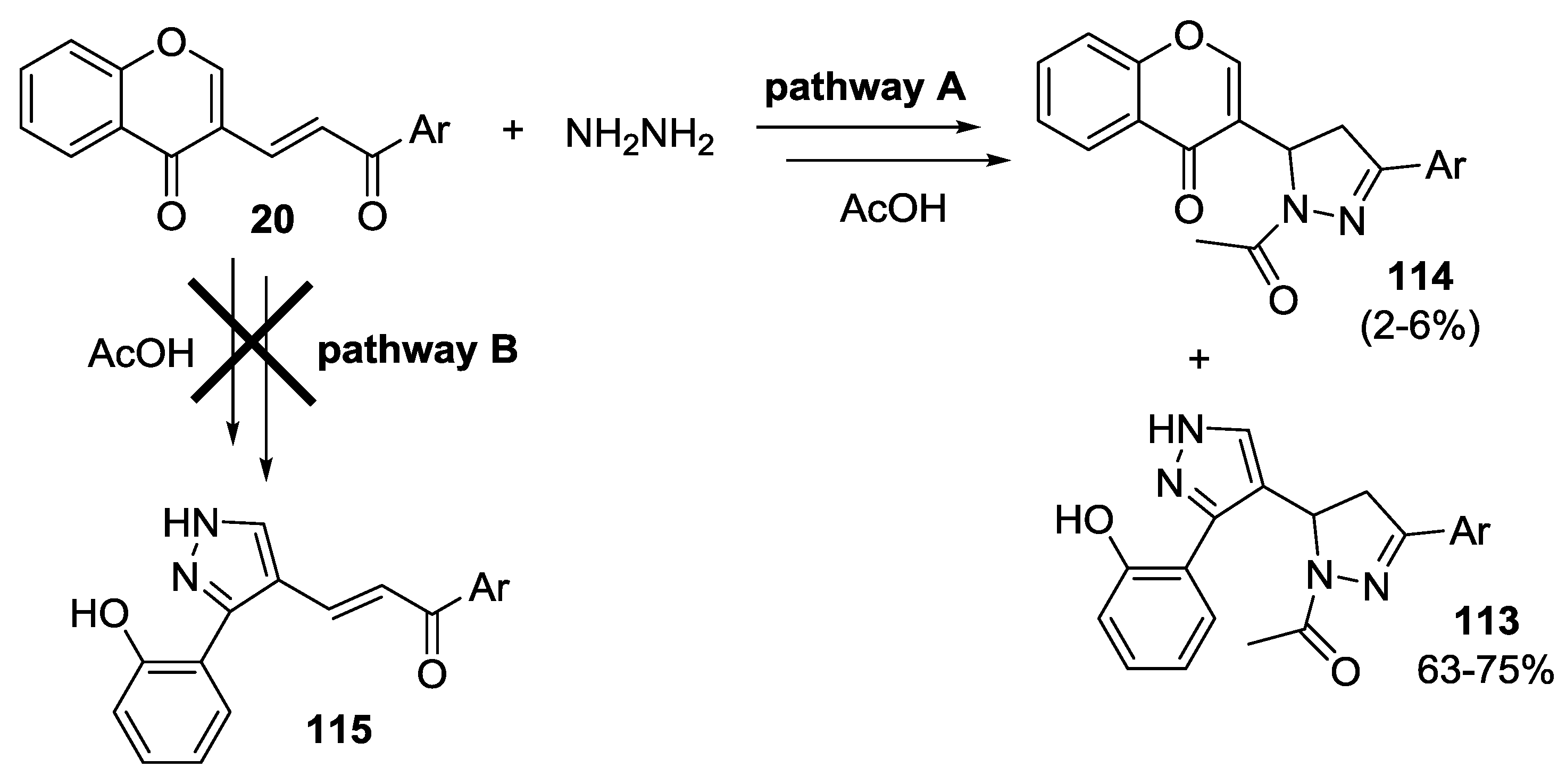
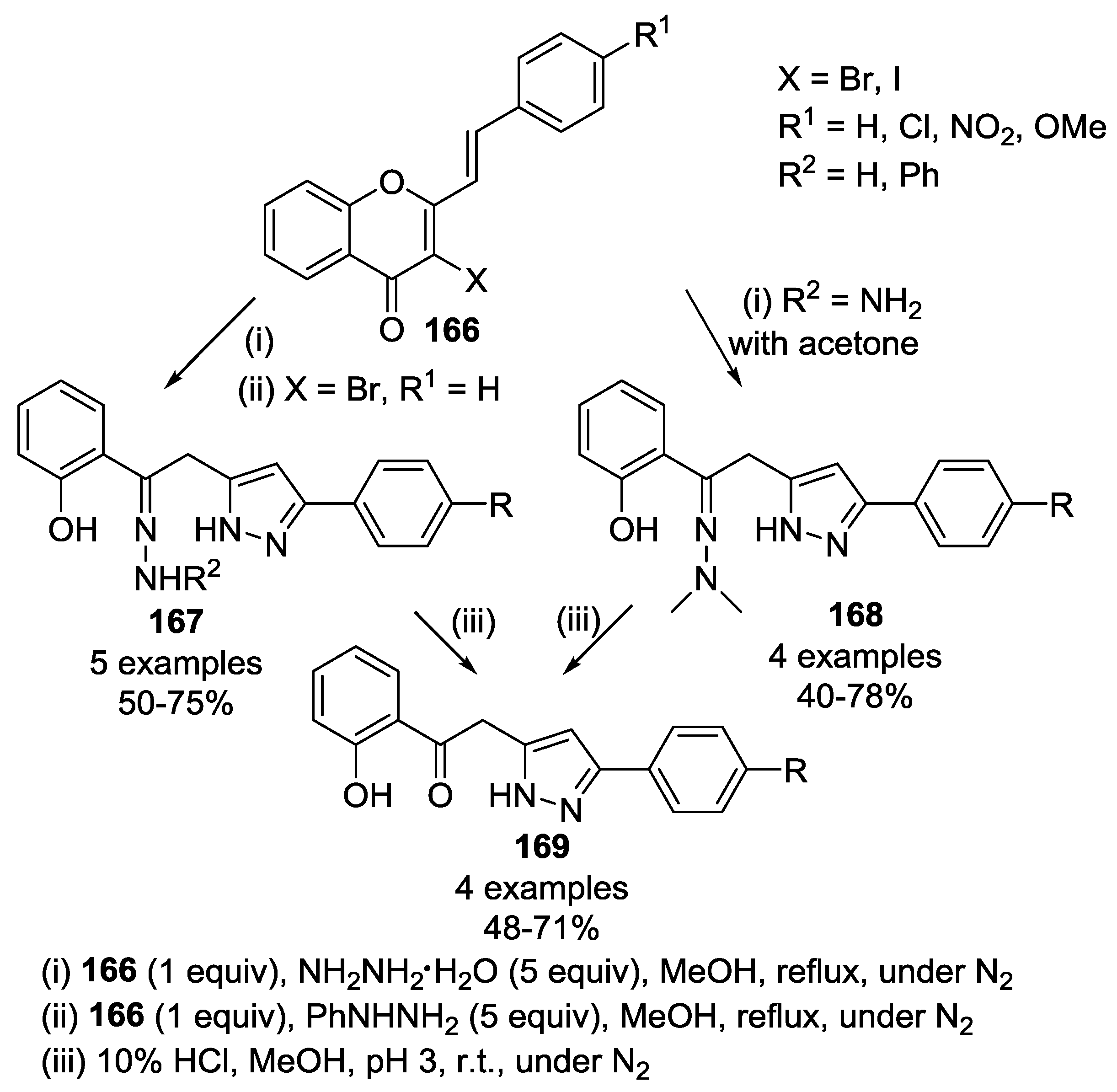
2.2.2. Condensation of Hydrazines with α,β-Unsaturated Ketones
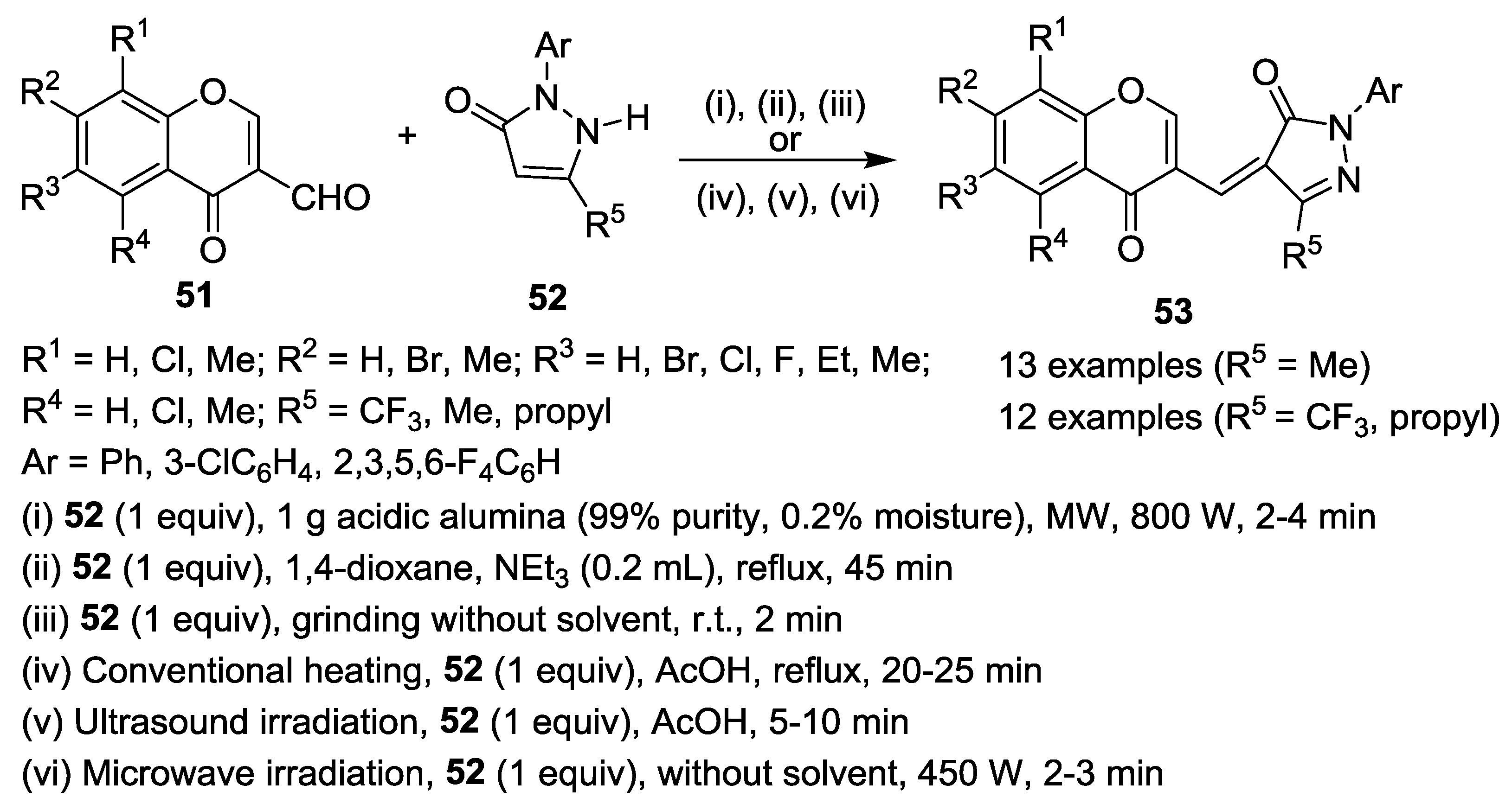
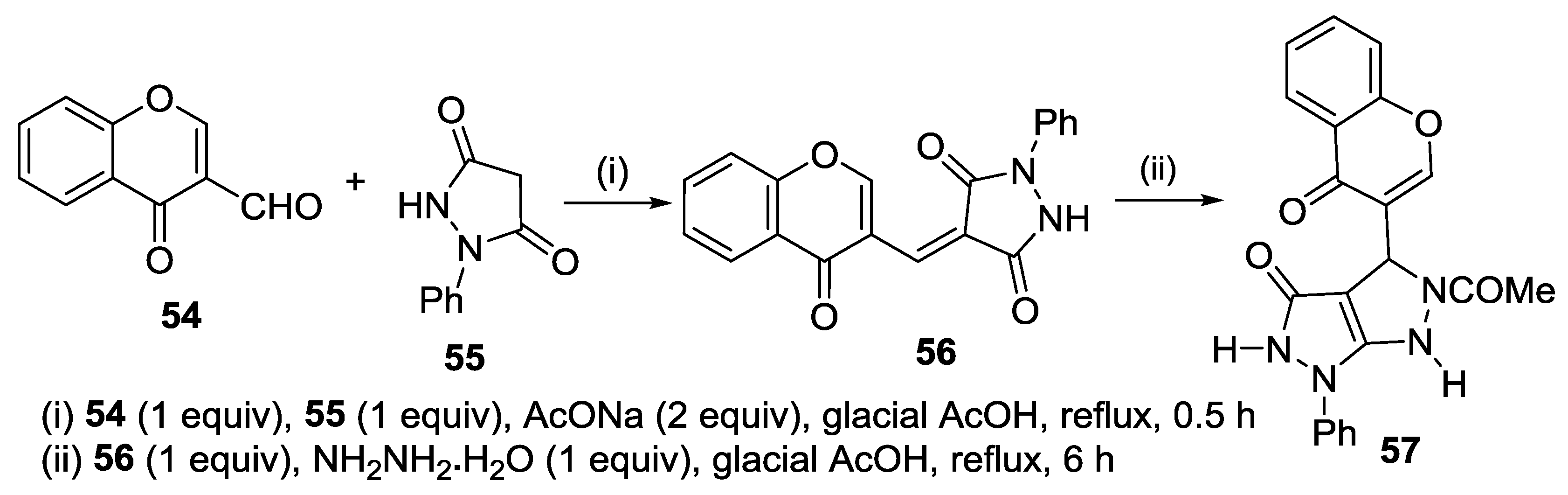
2.2.3. Knoevenagel Reaction
2.2.4. Other Reactions
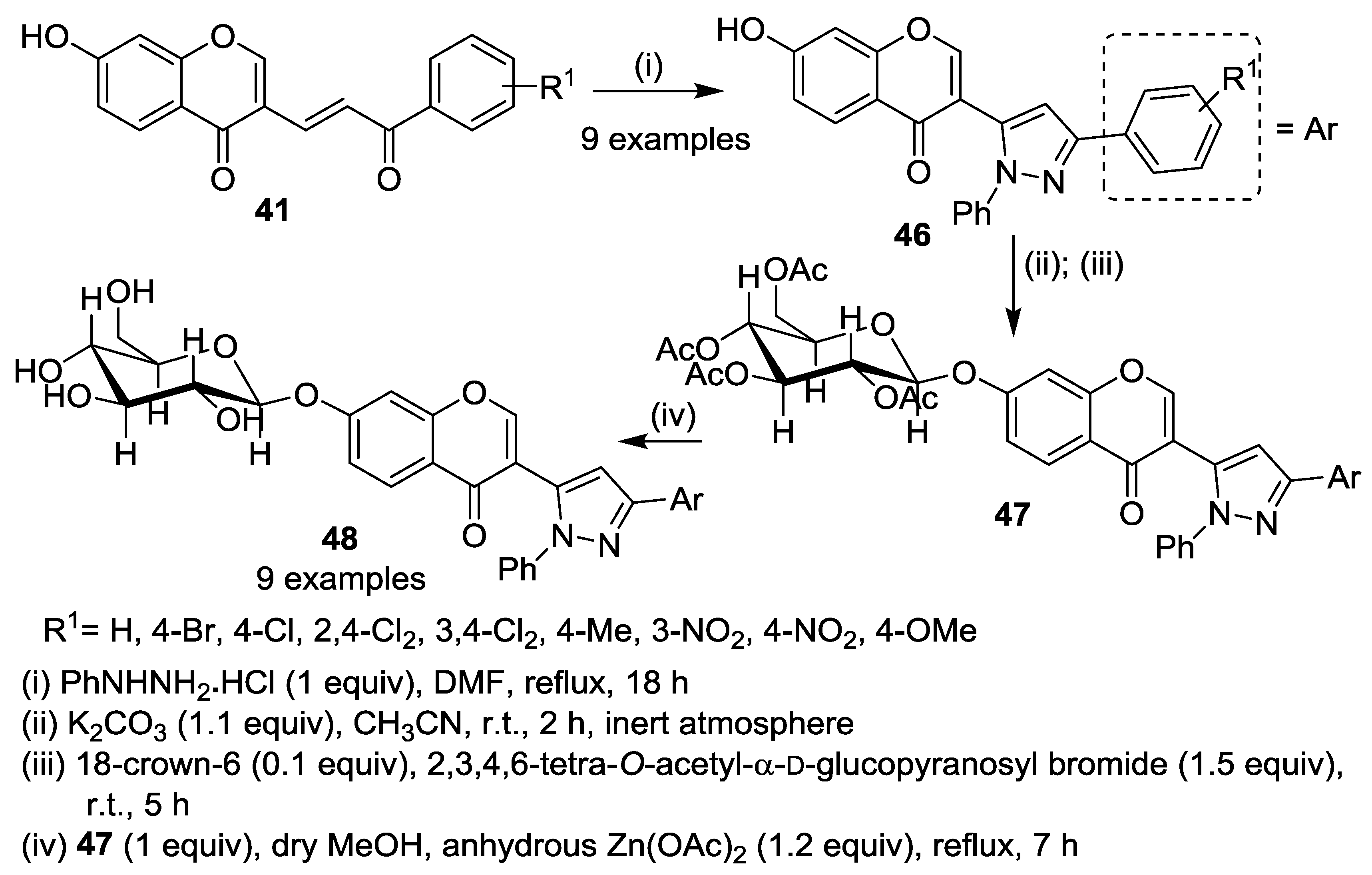
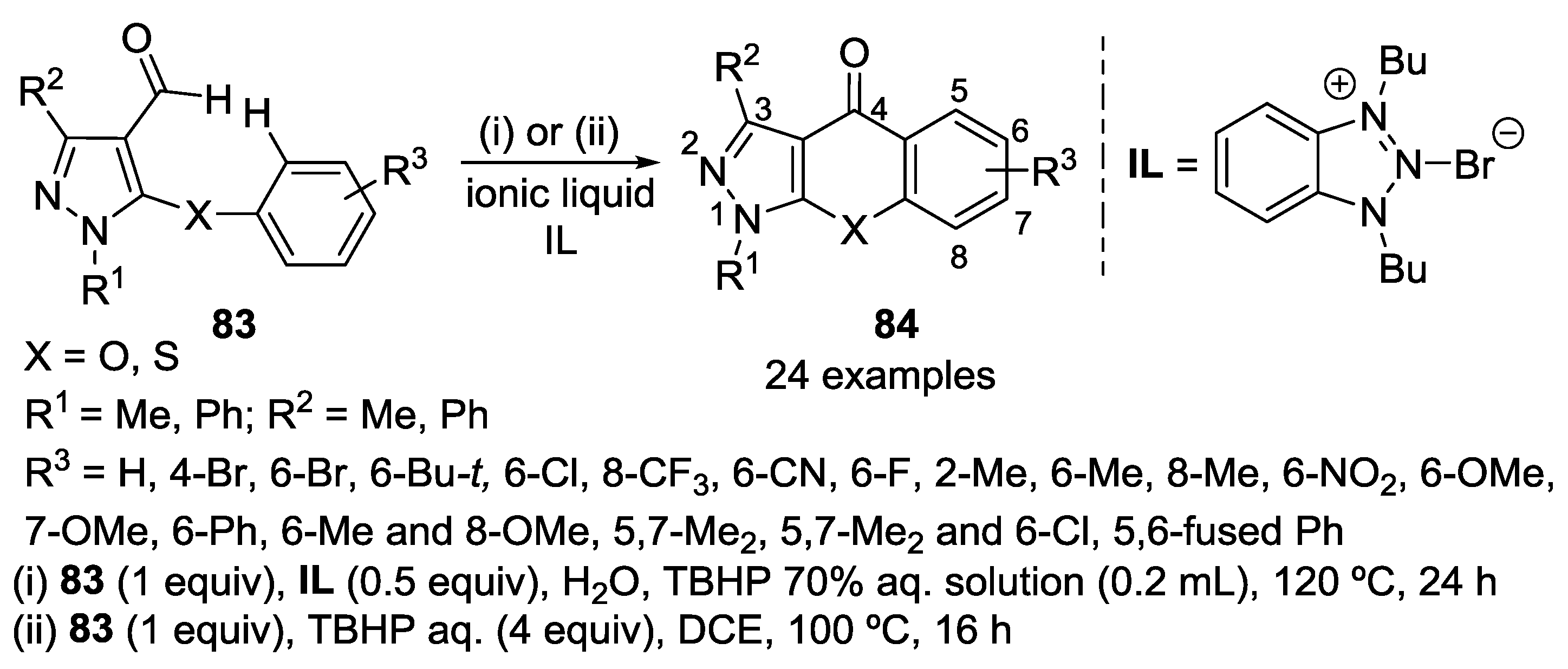
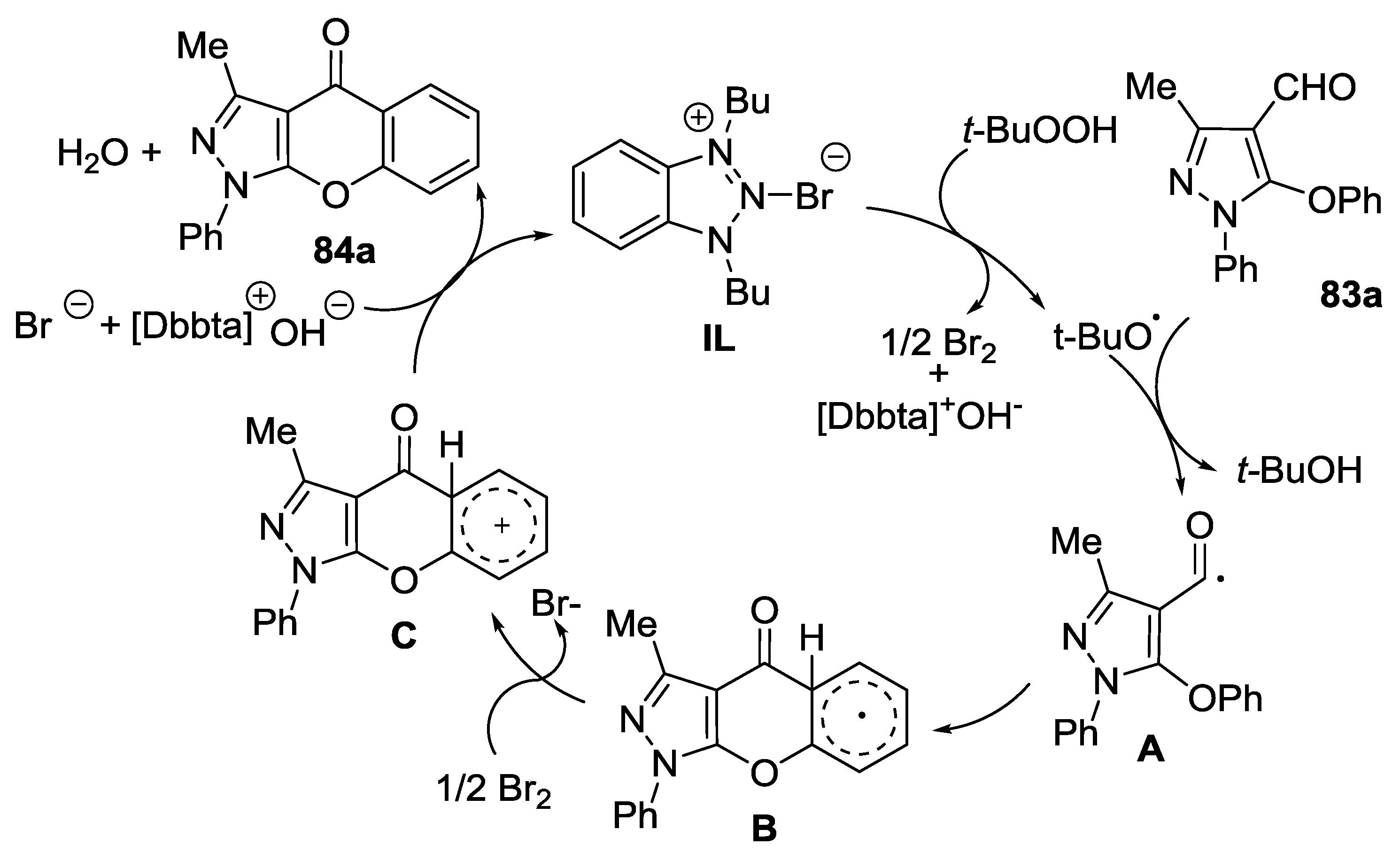
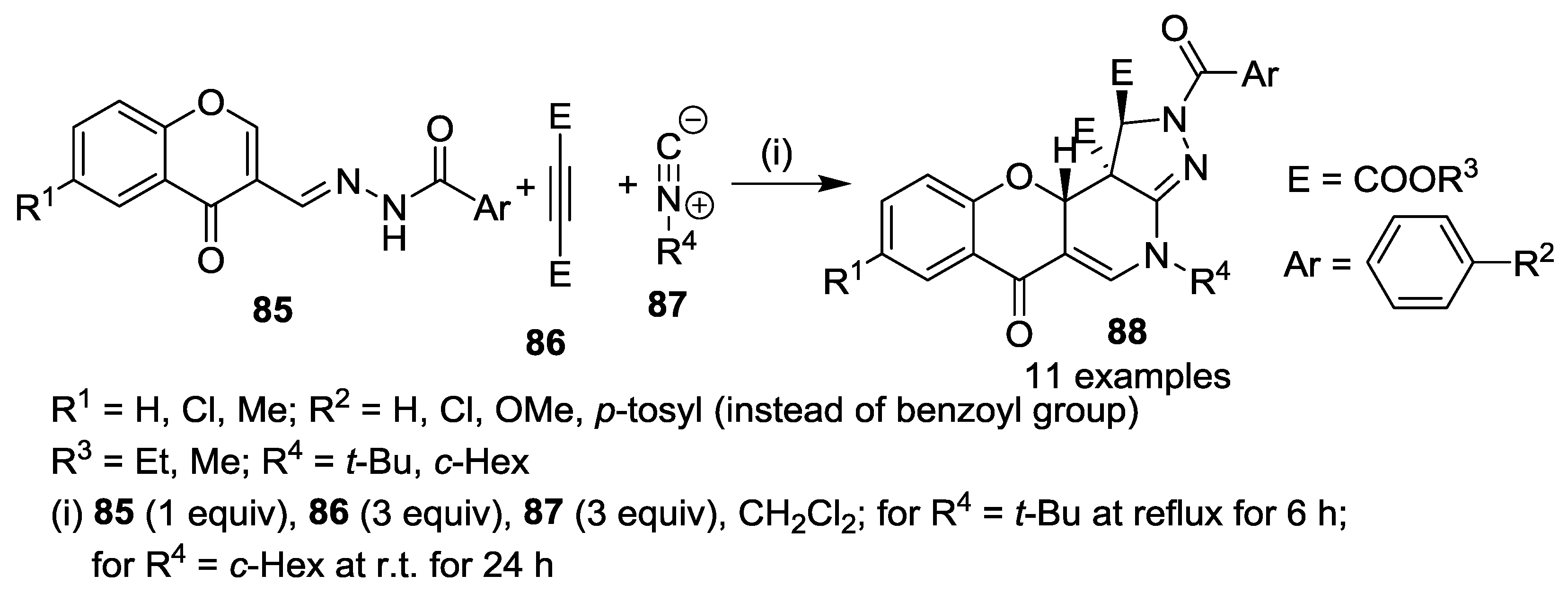
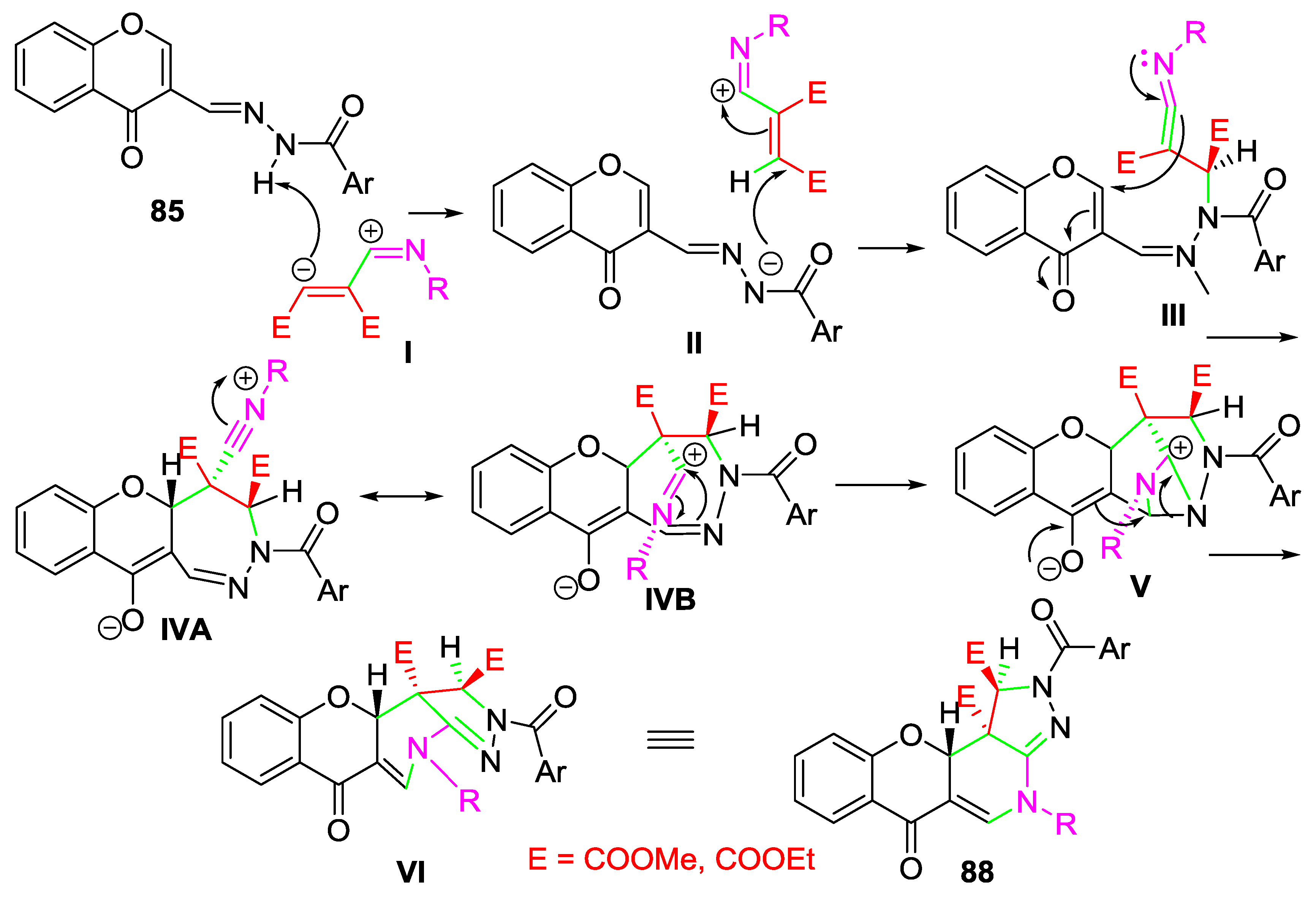
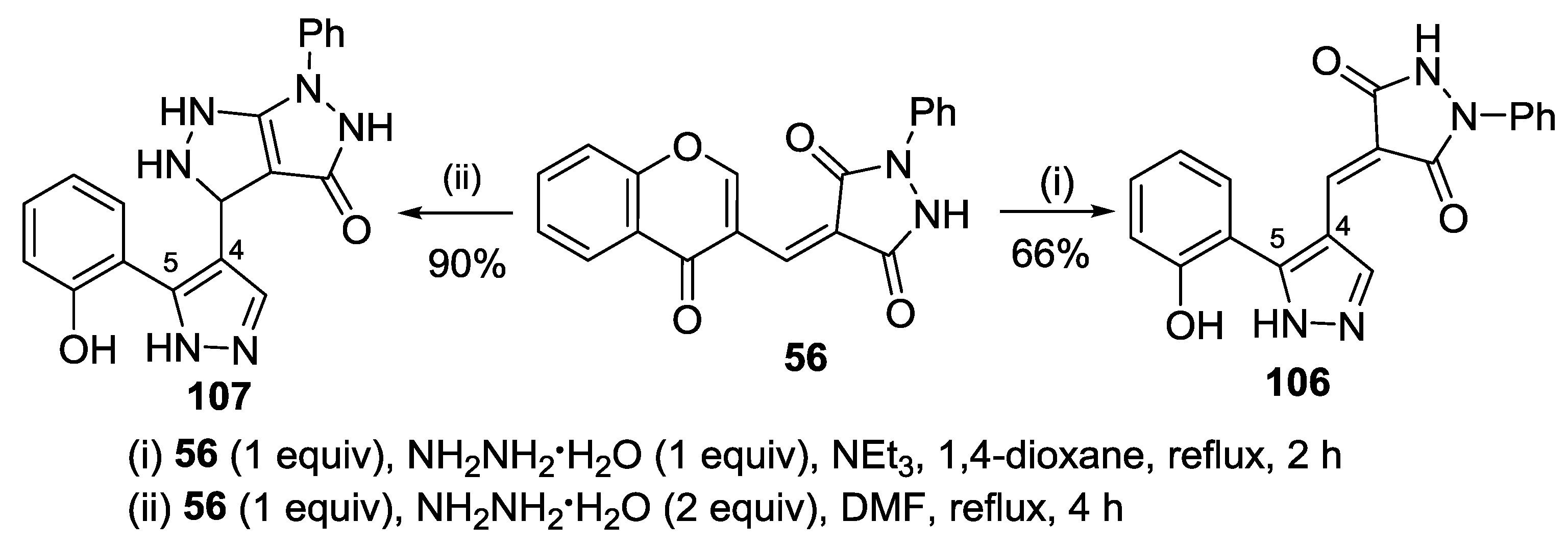
3. Synthesis of Chromone-Fused Pyrazoles
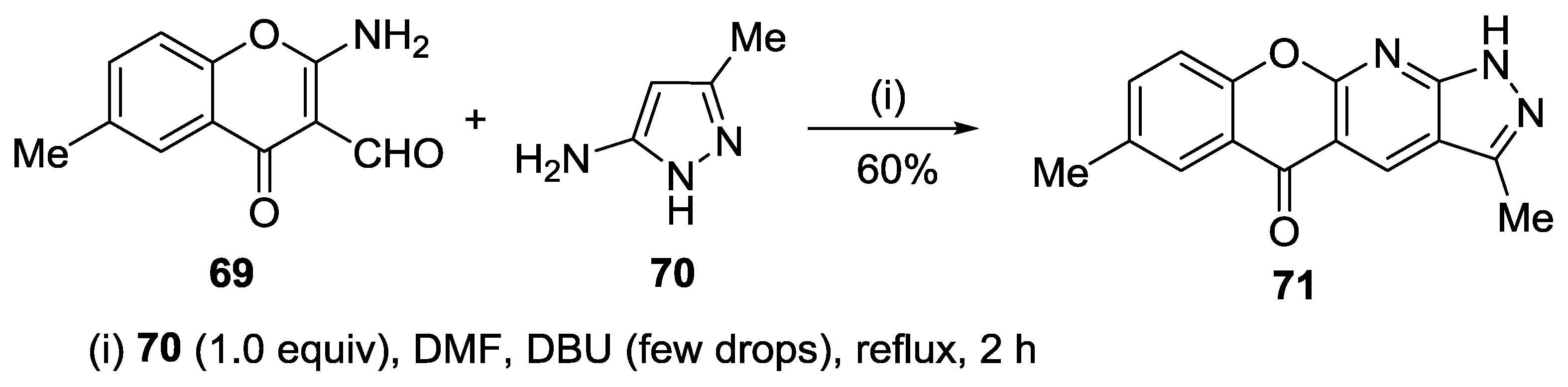
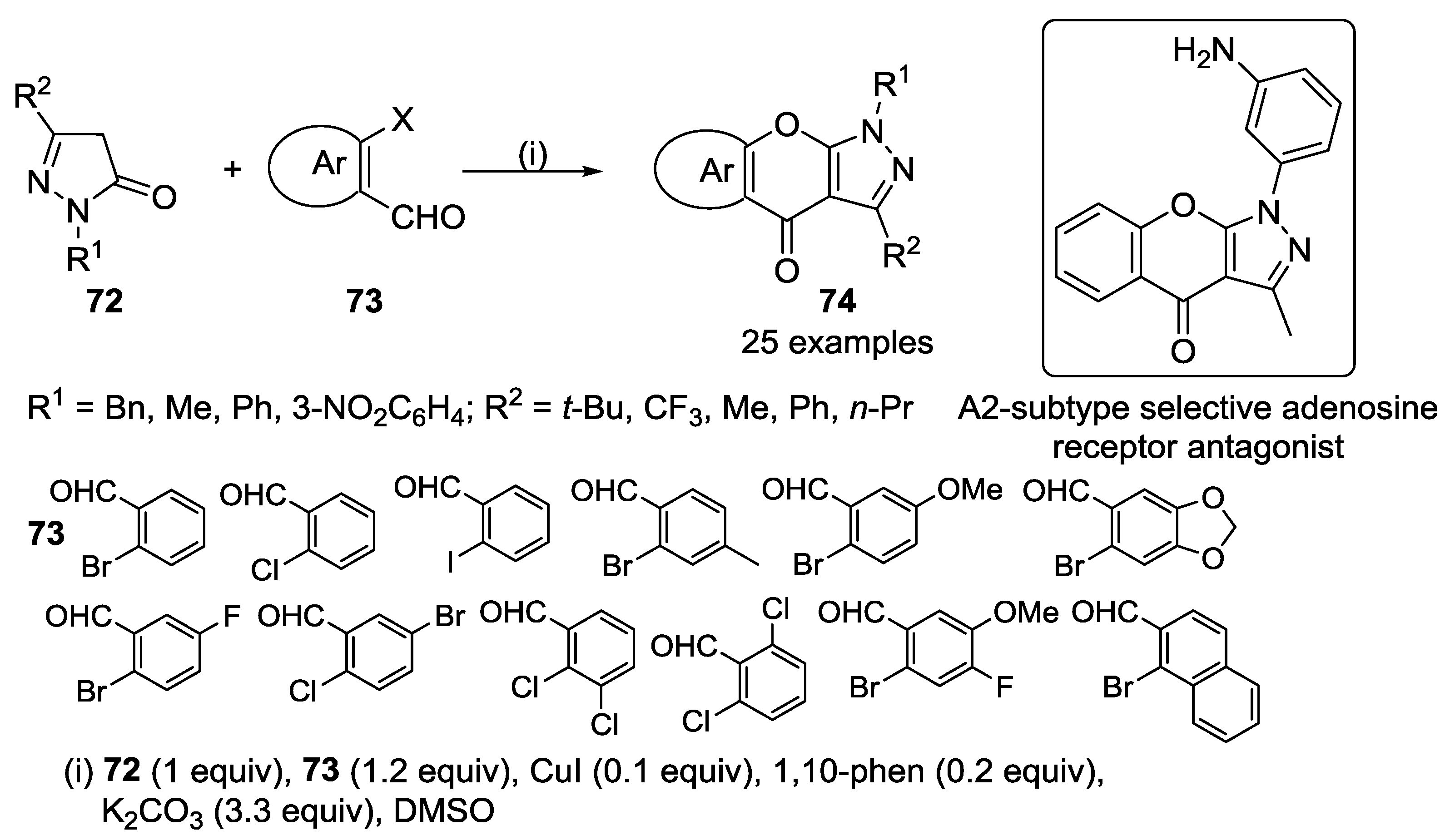
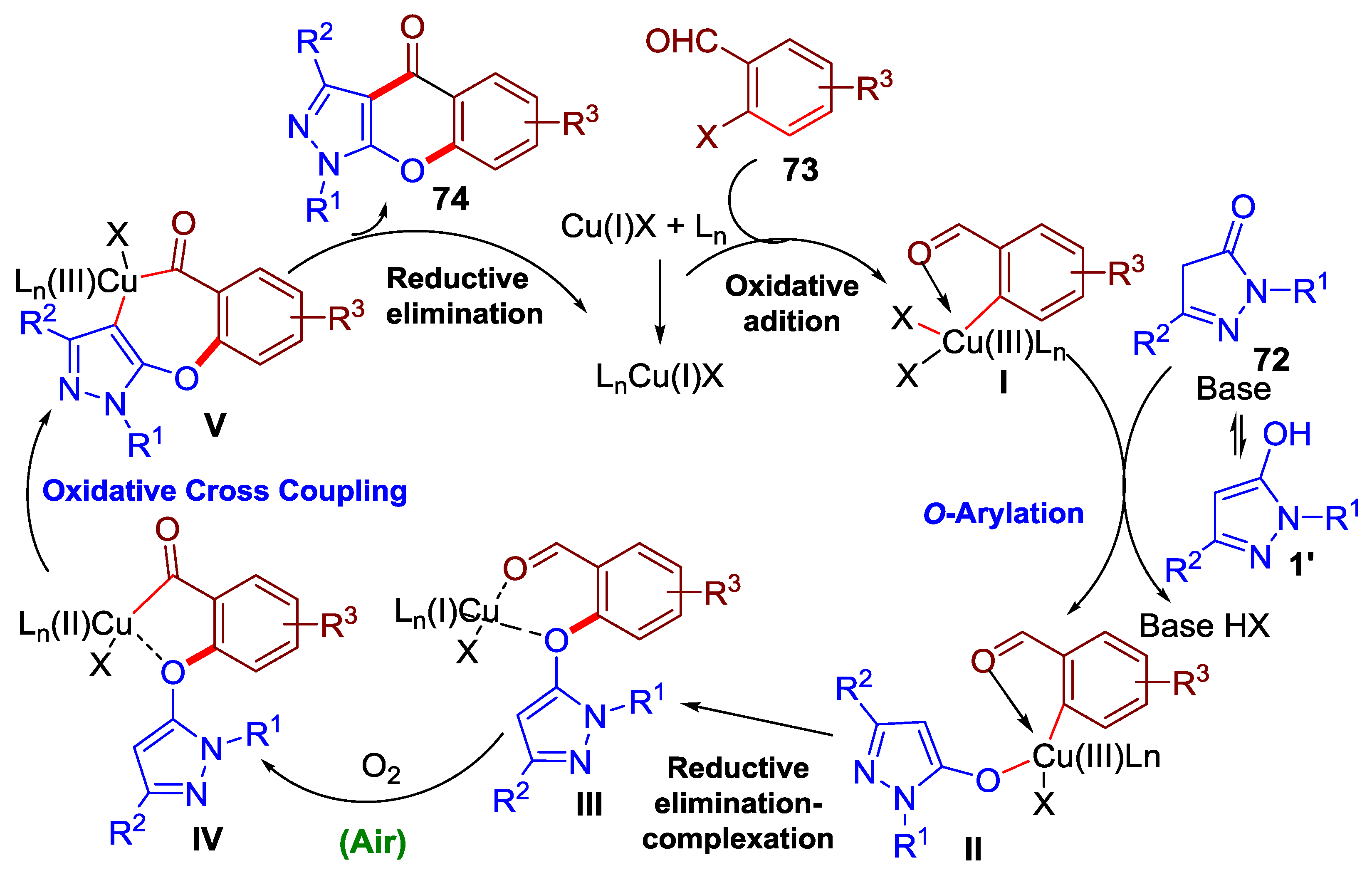
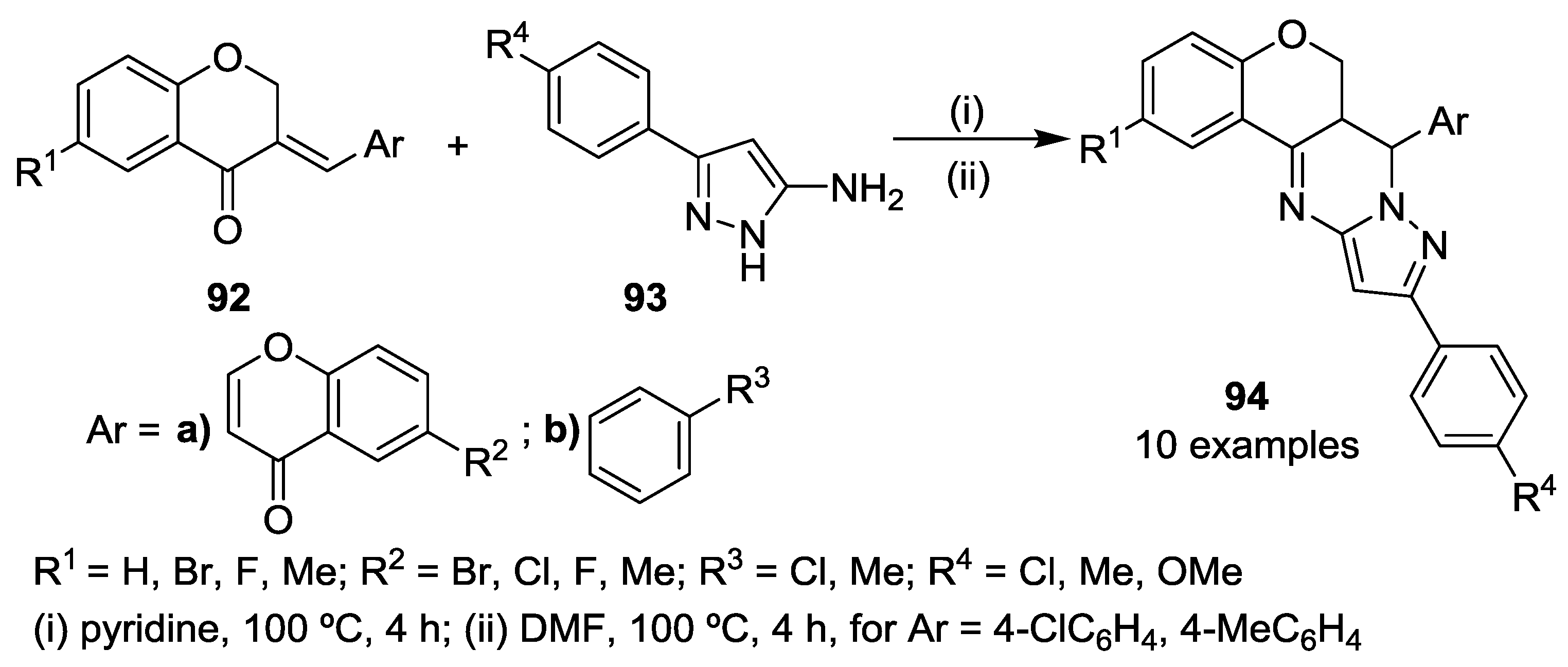
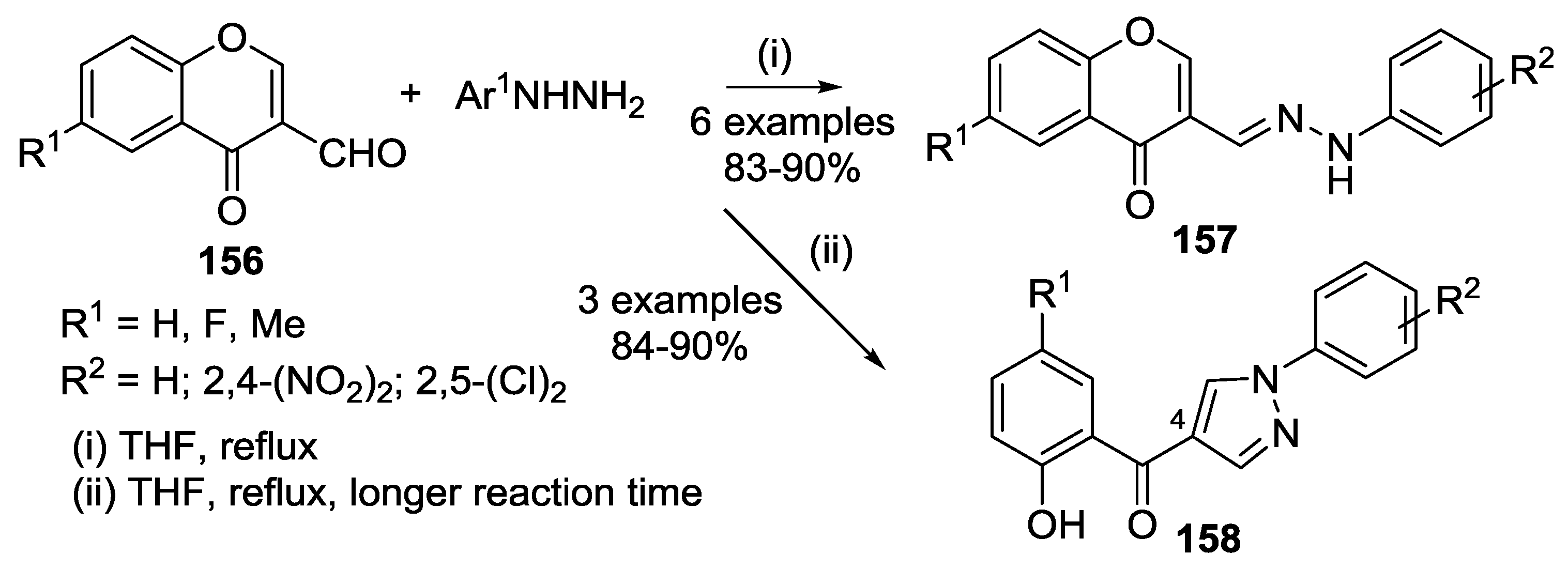
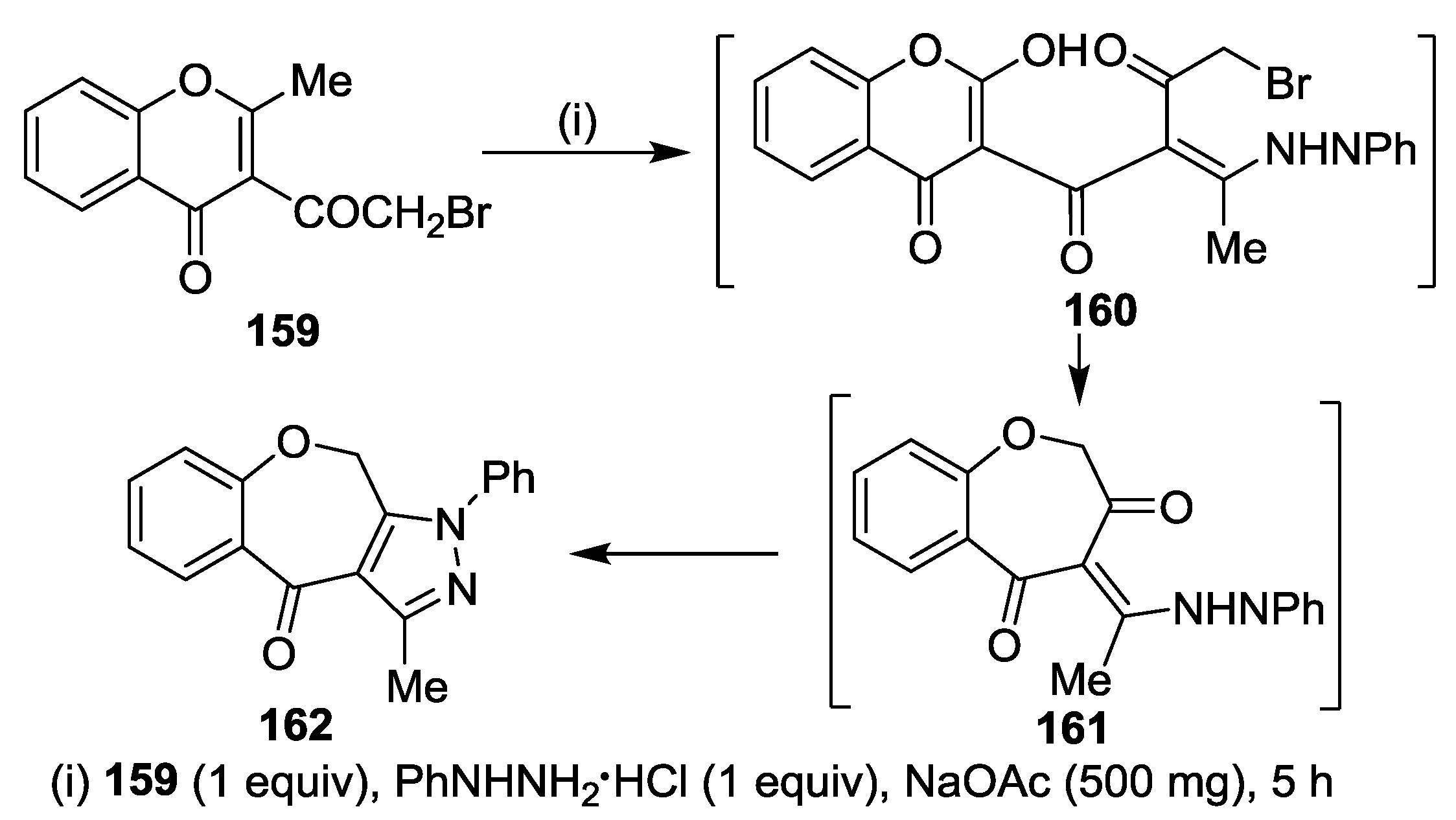
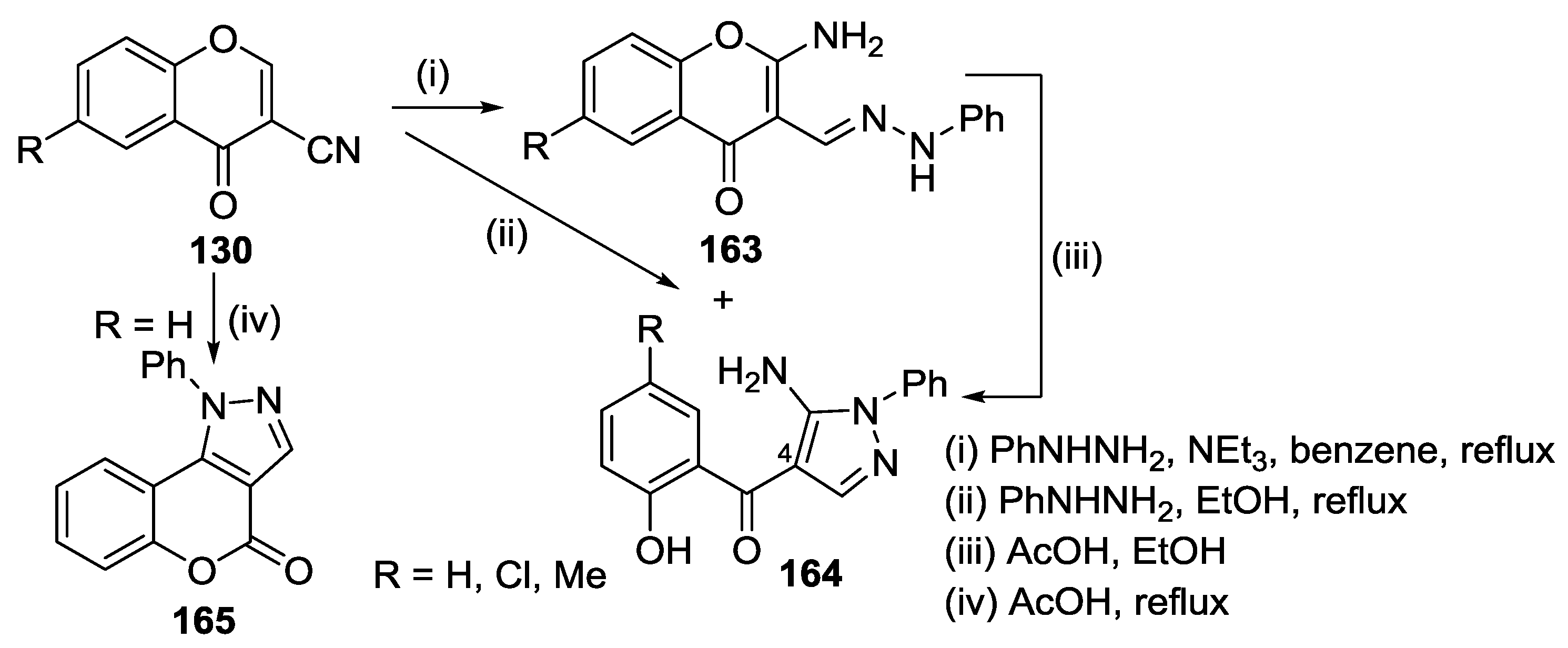
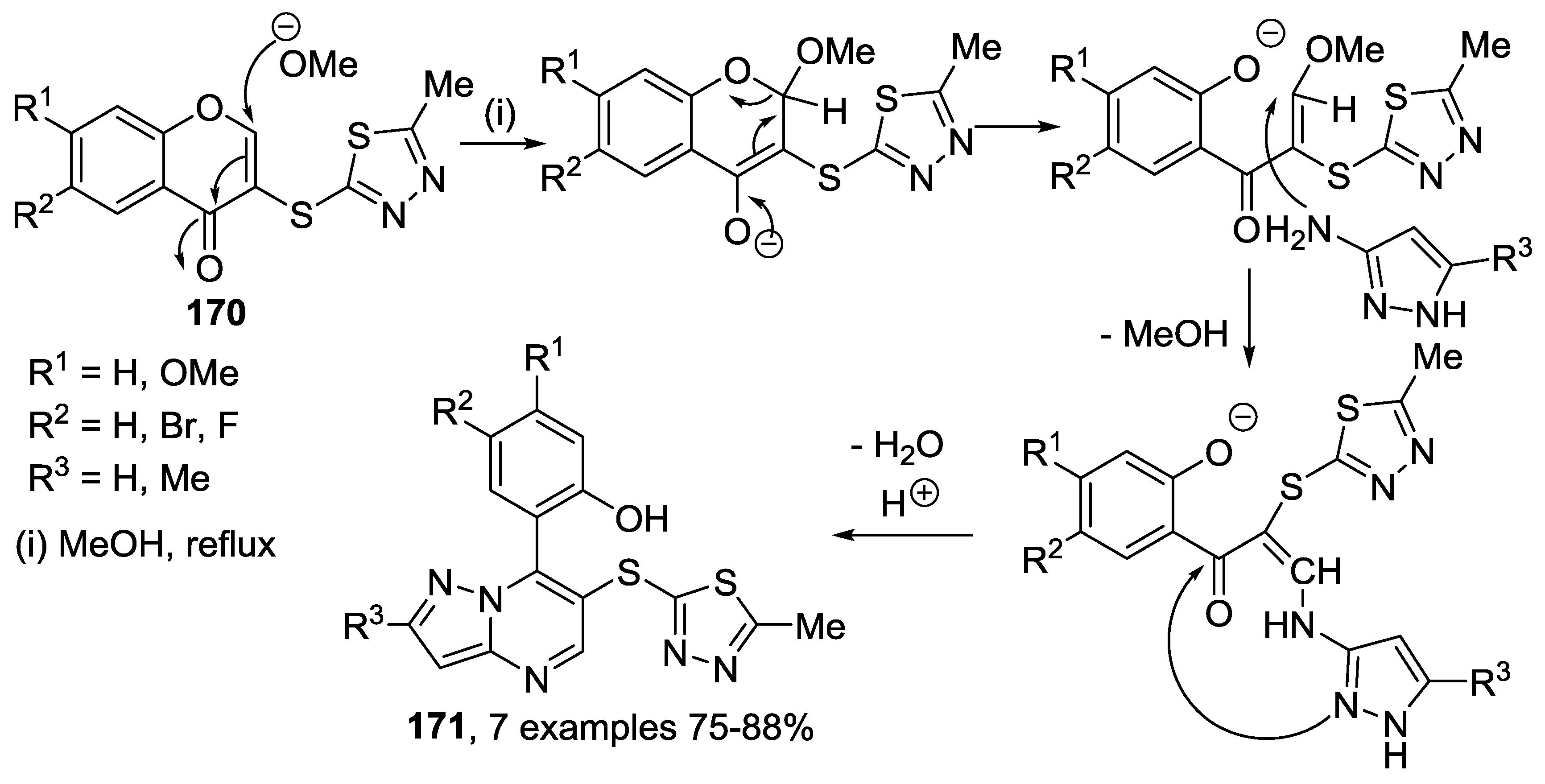
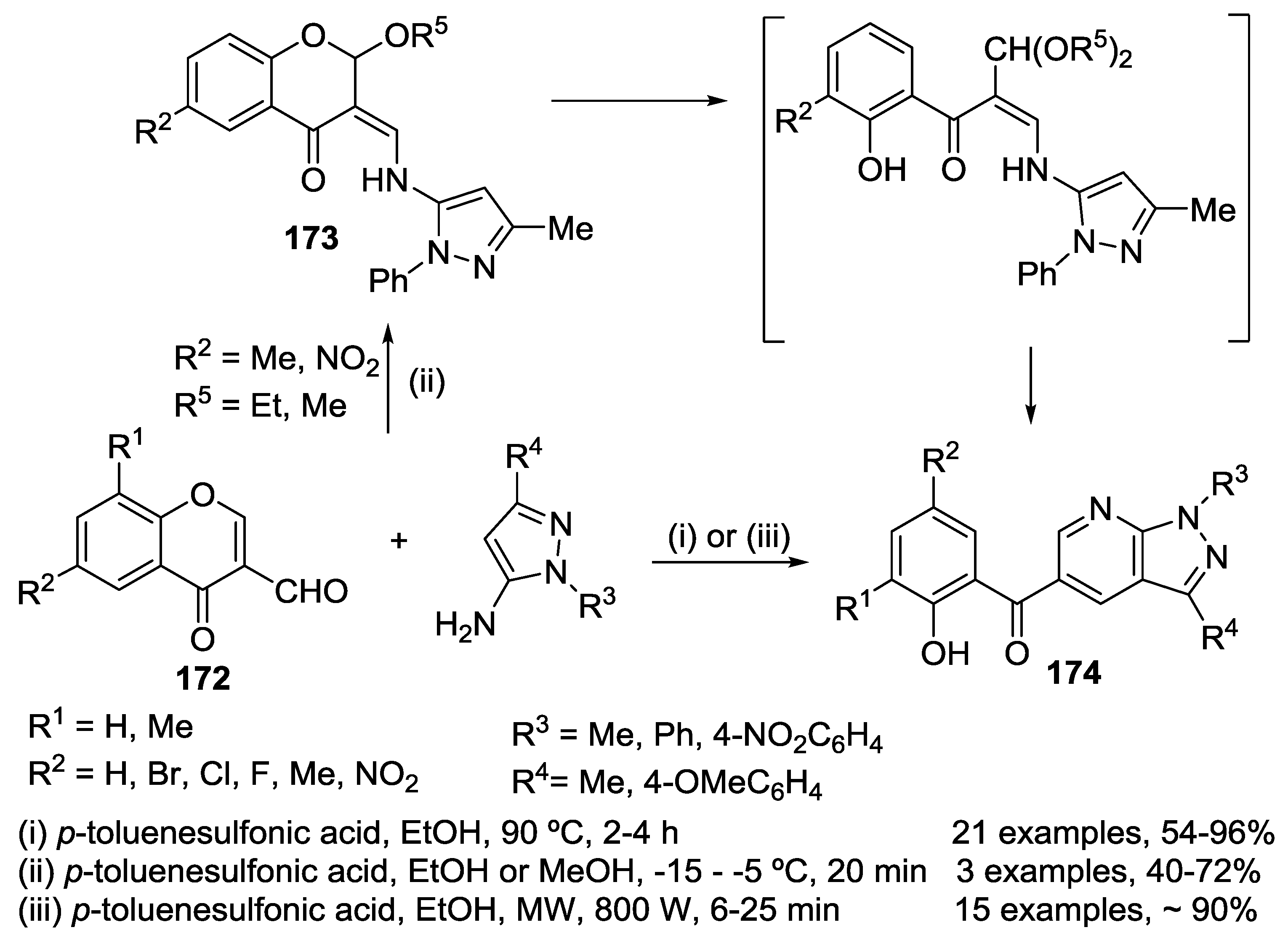
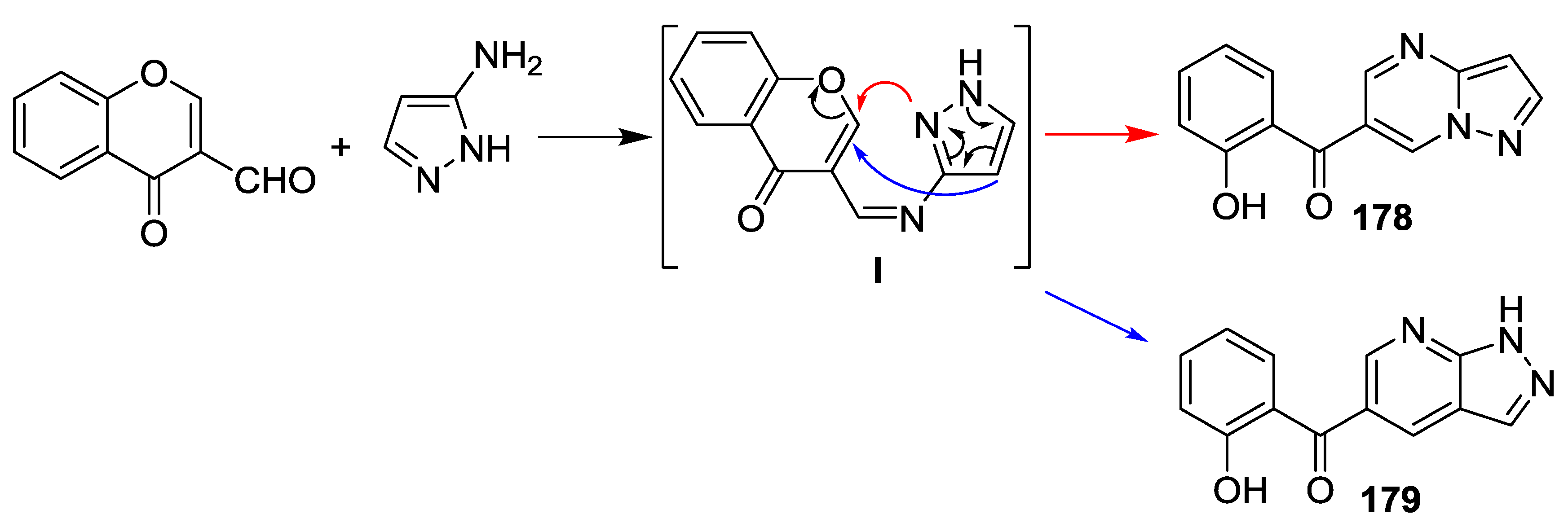
3.1. Tandem Reactions of 3-Formylchromones with Pyrazole Derivatives
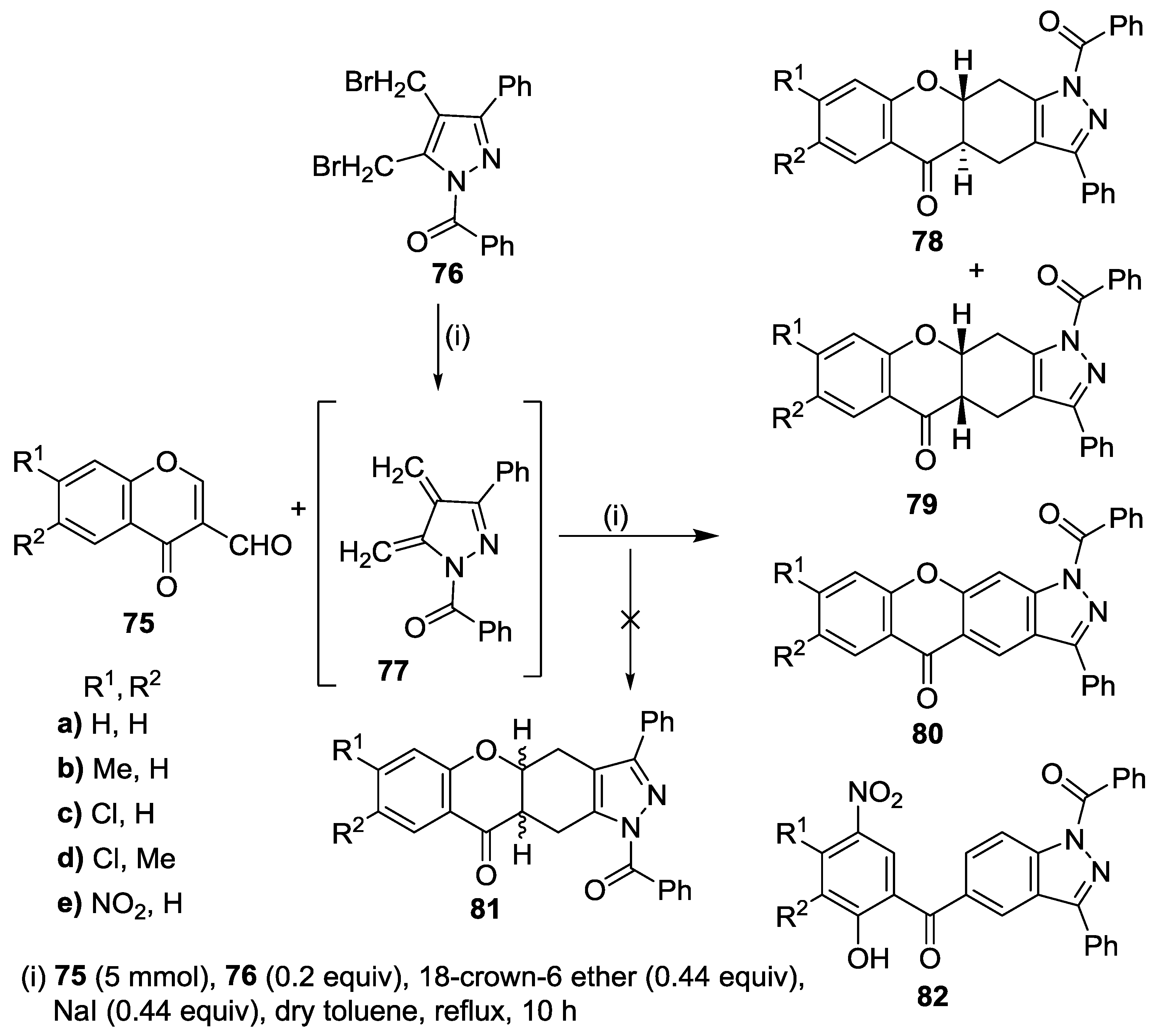
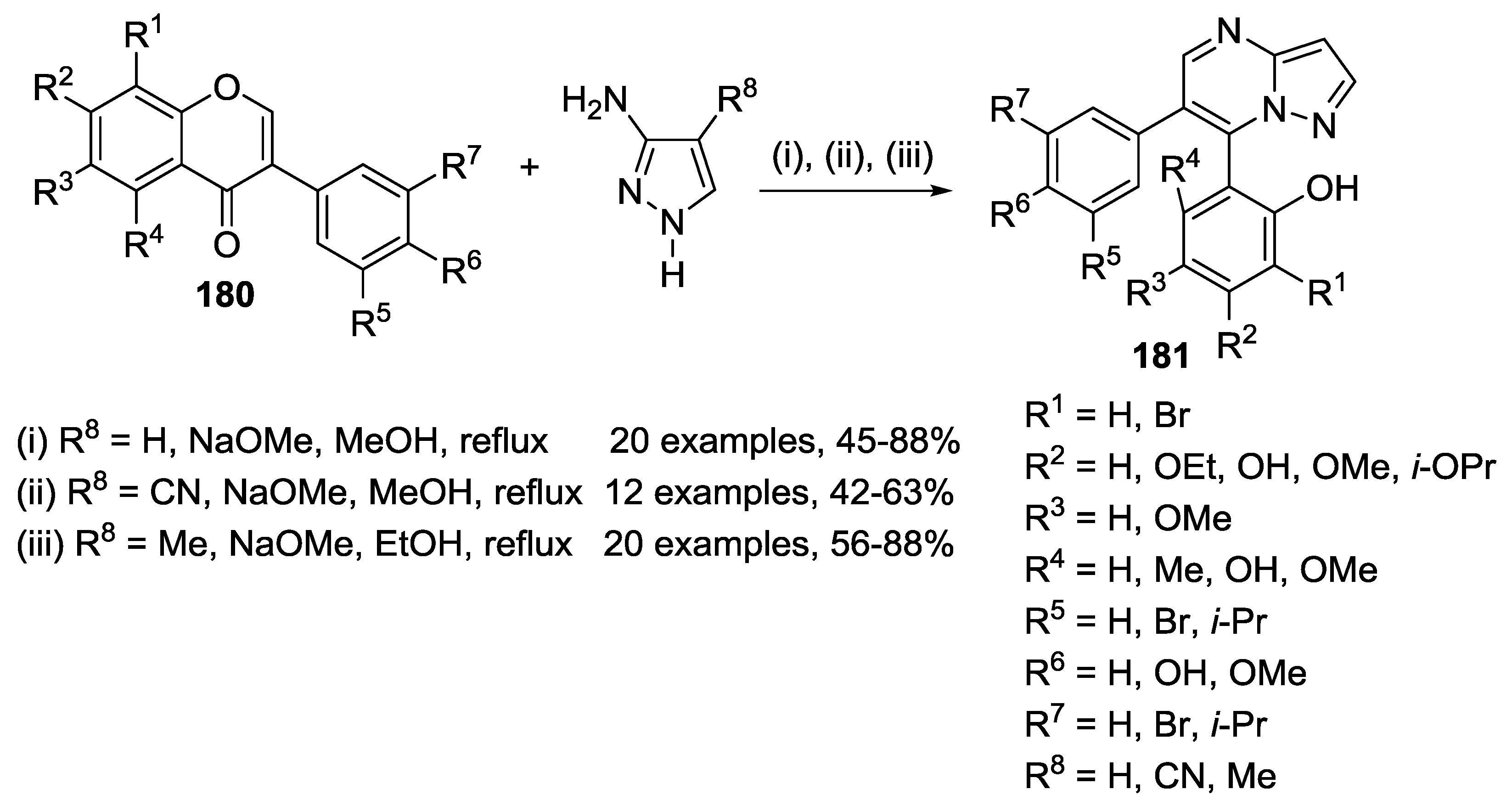
3.2. Other Transformations
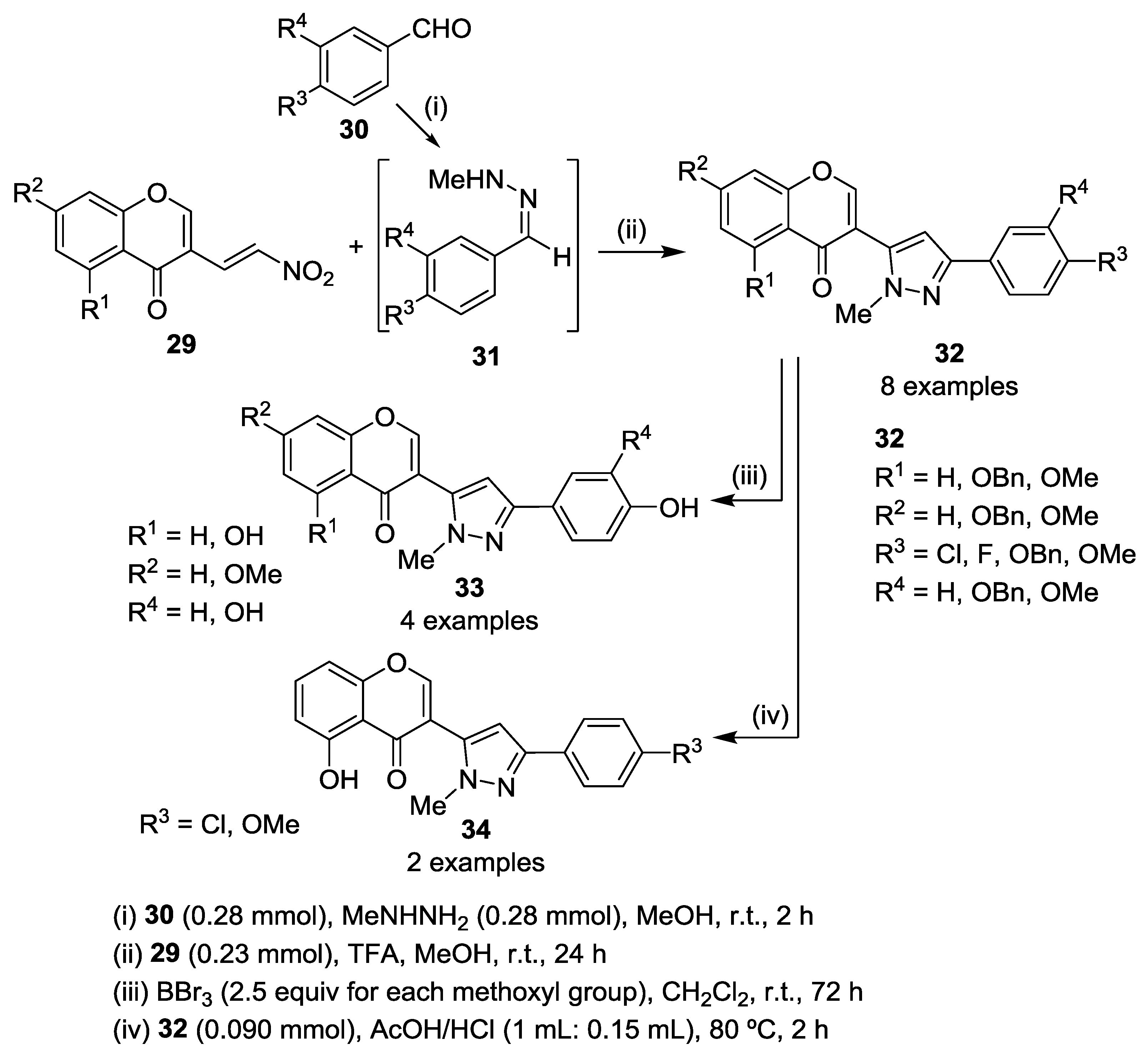
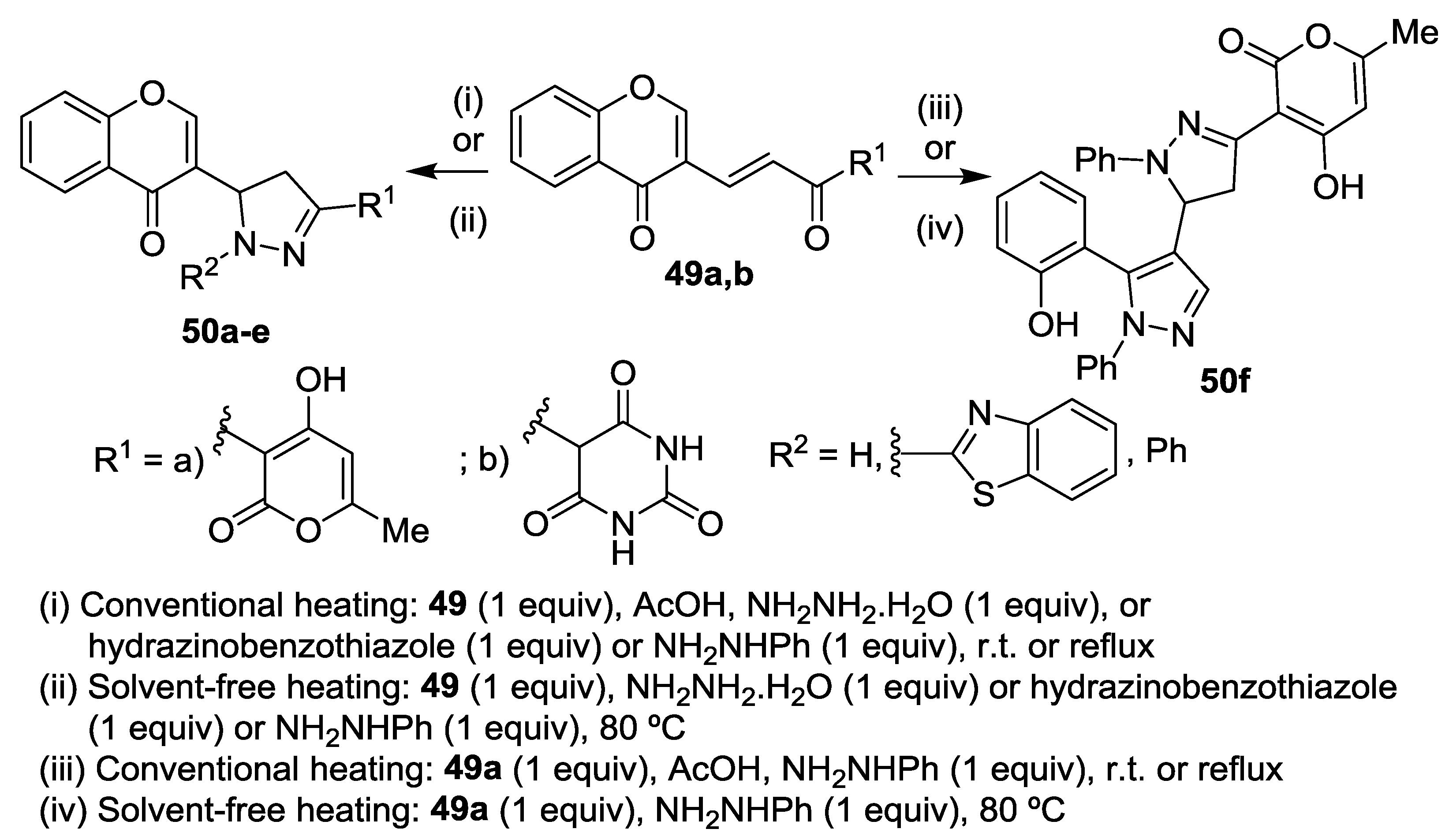
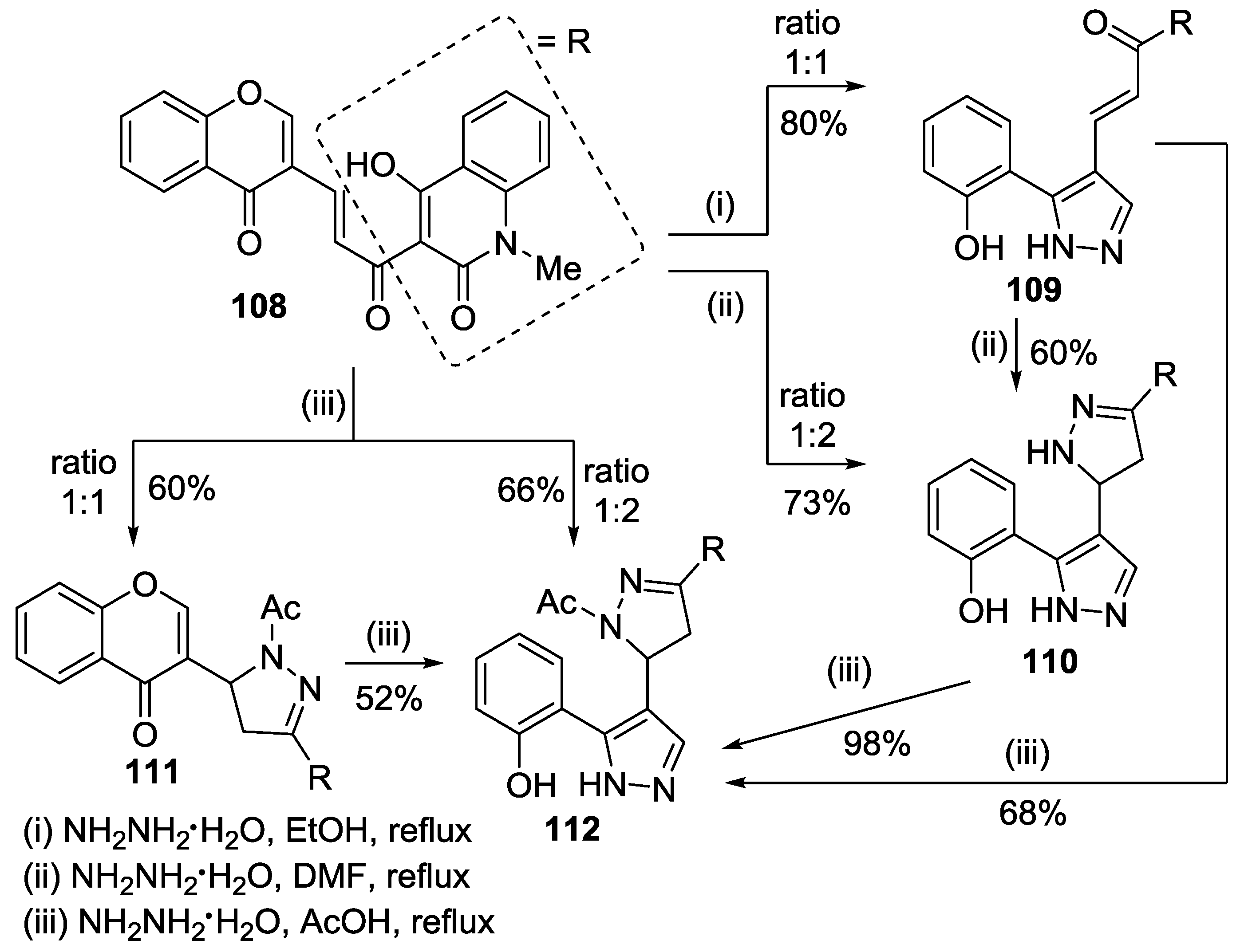
4. Chromones as Starting Materials in the Synthesis of 3(5)-(2-Hydroxyaryl)pyrazoles
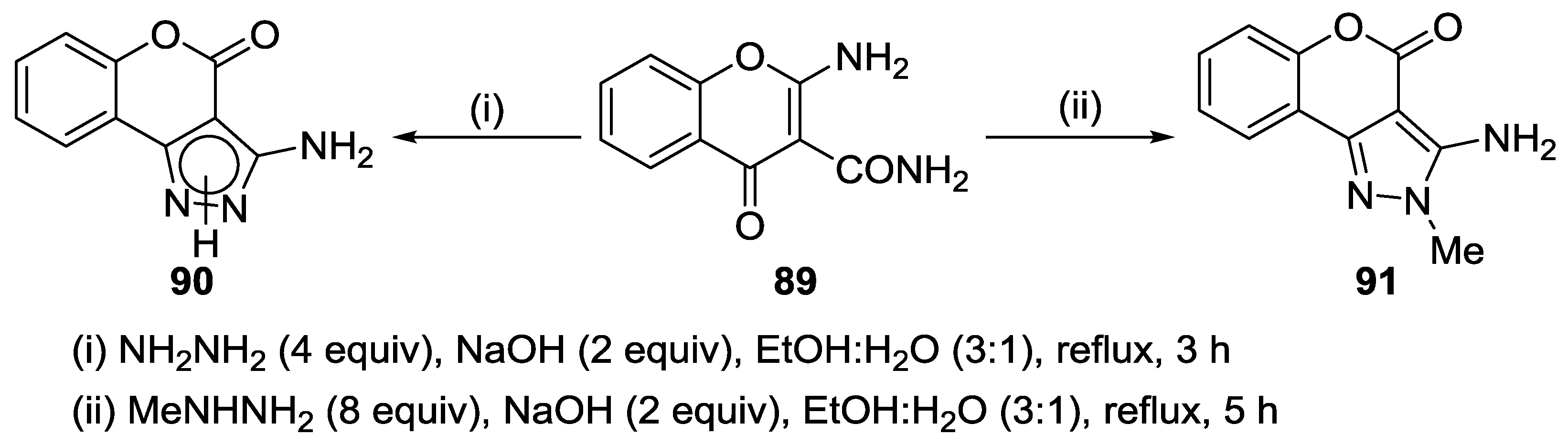
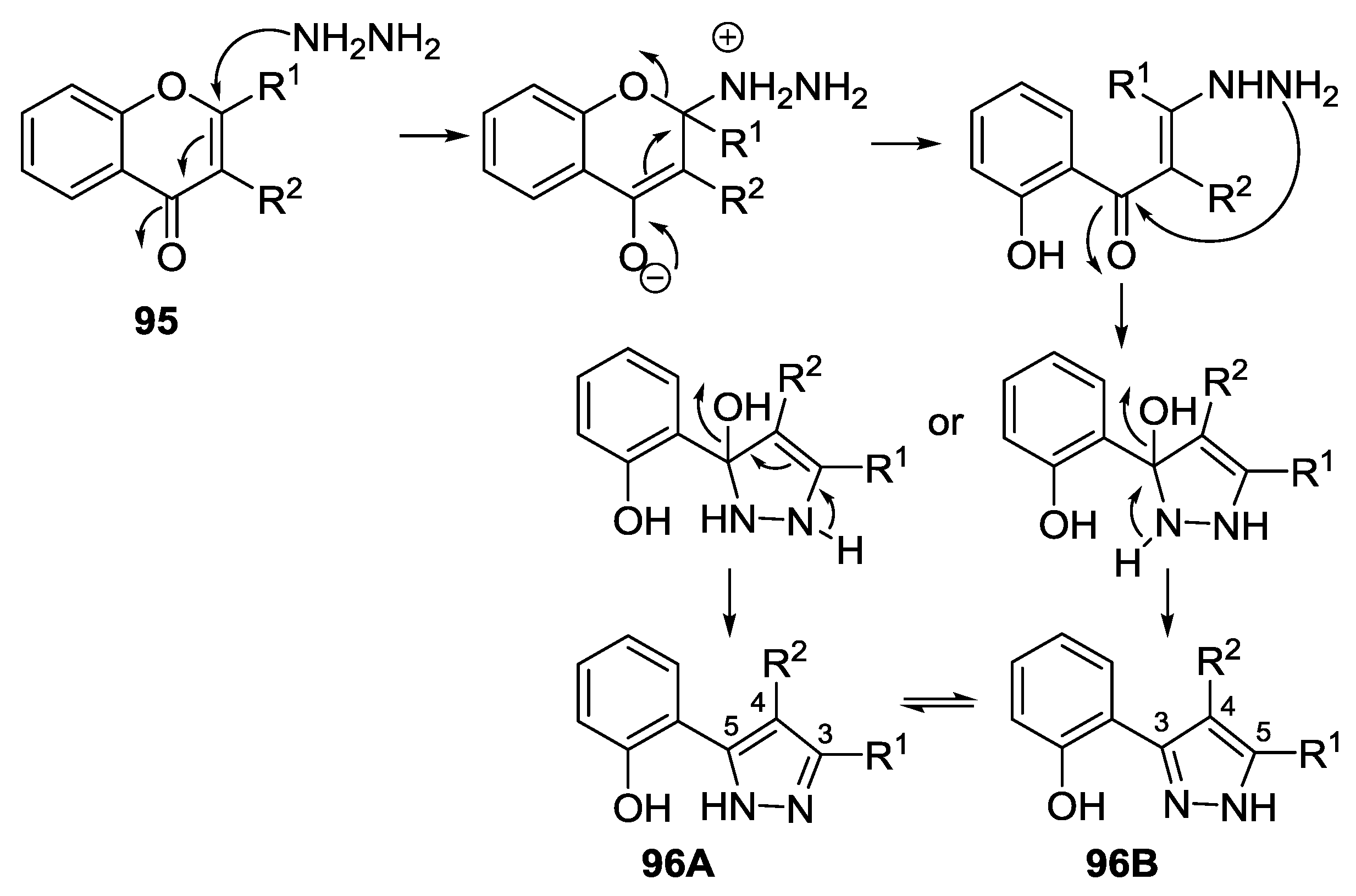
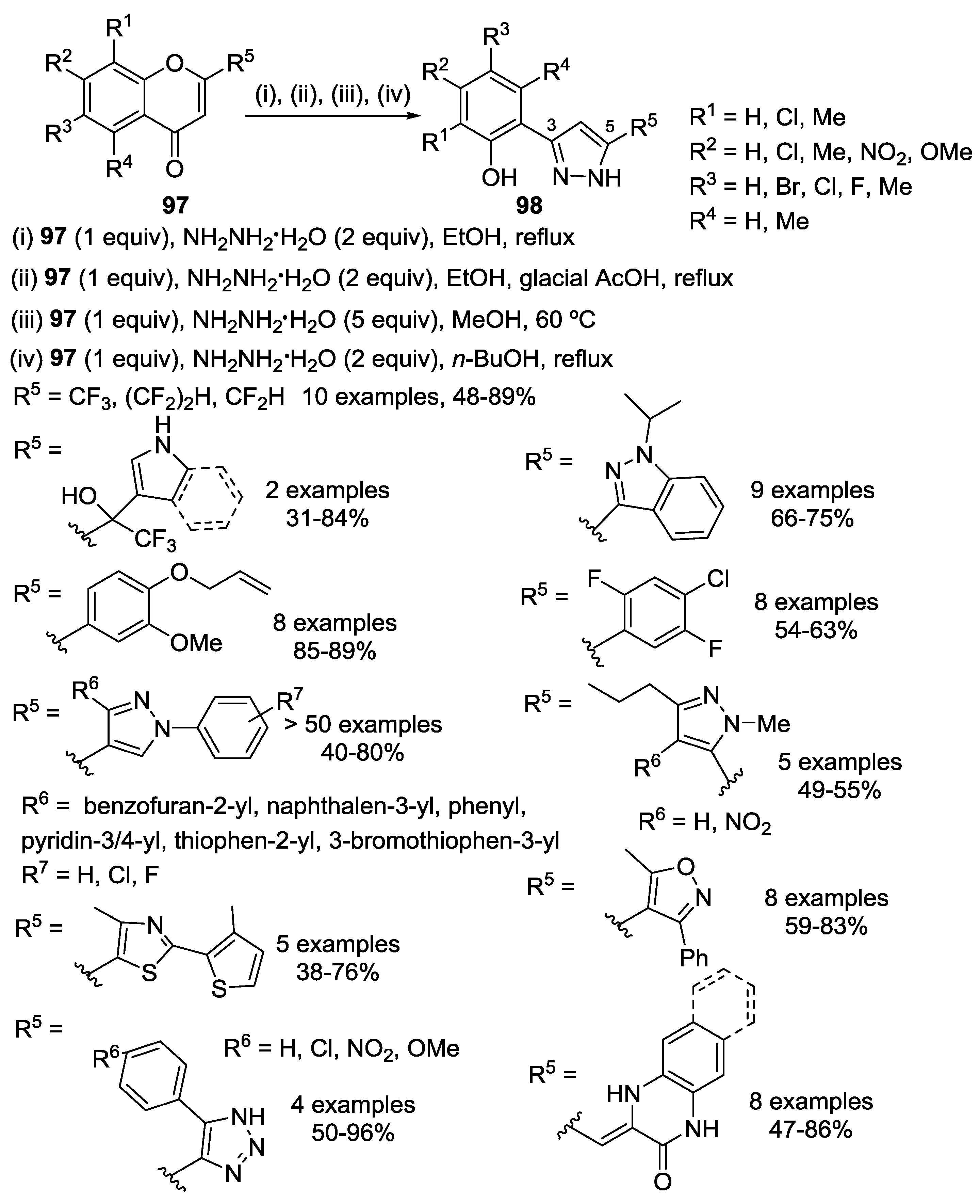
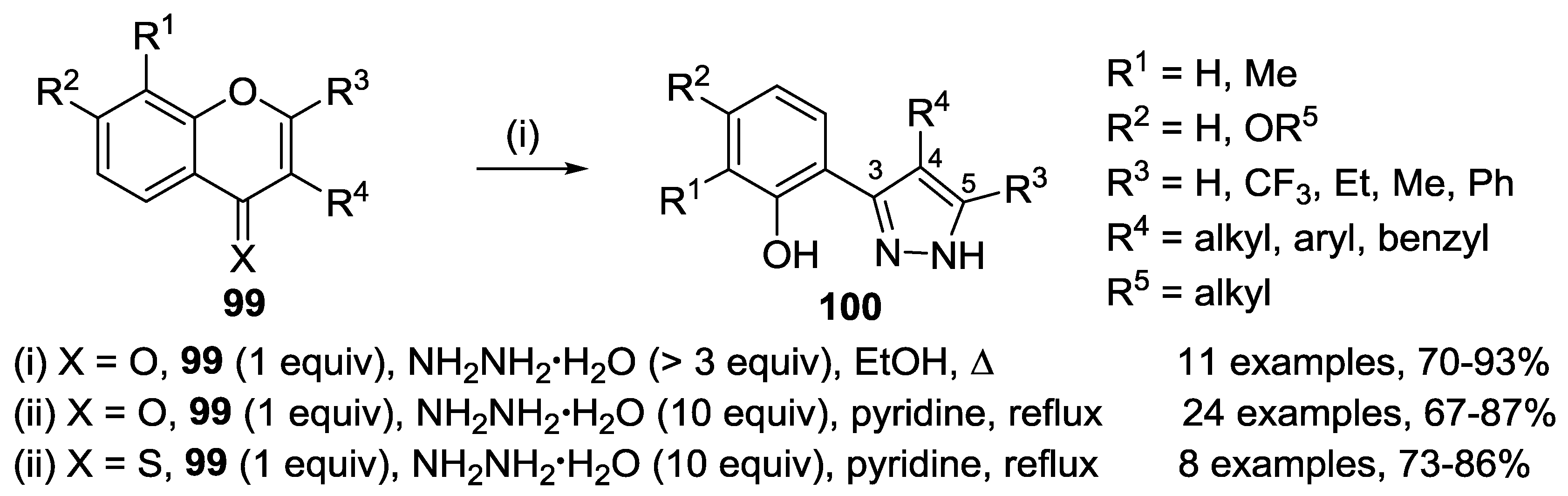
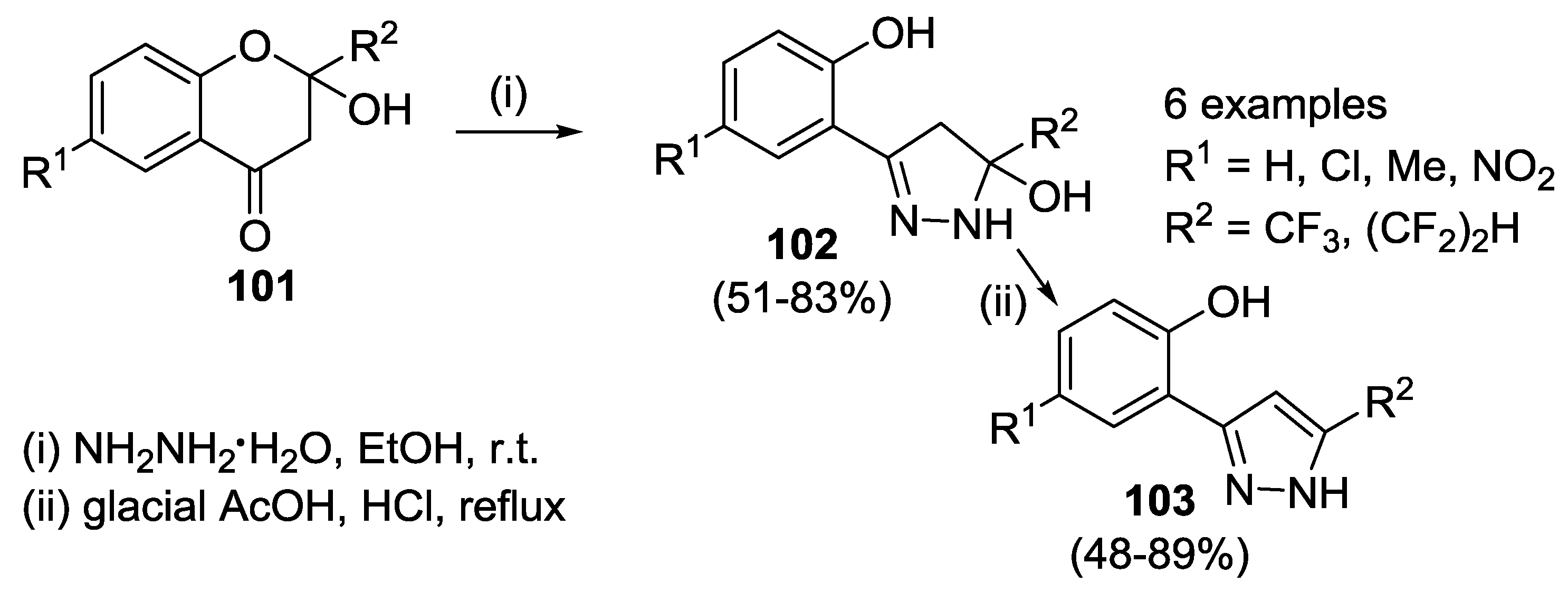
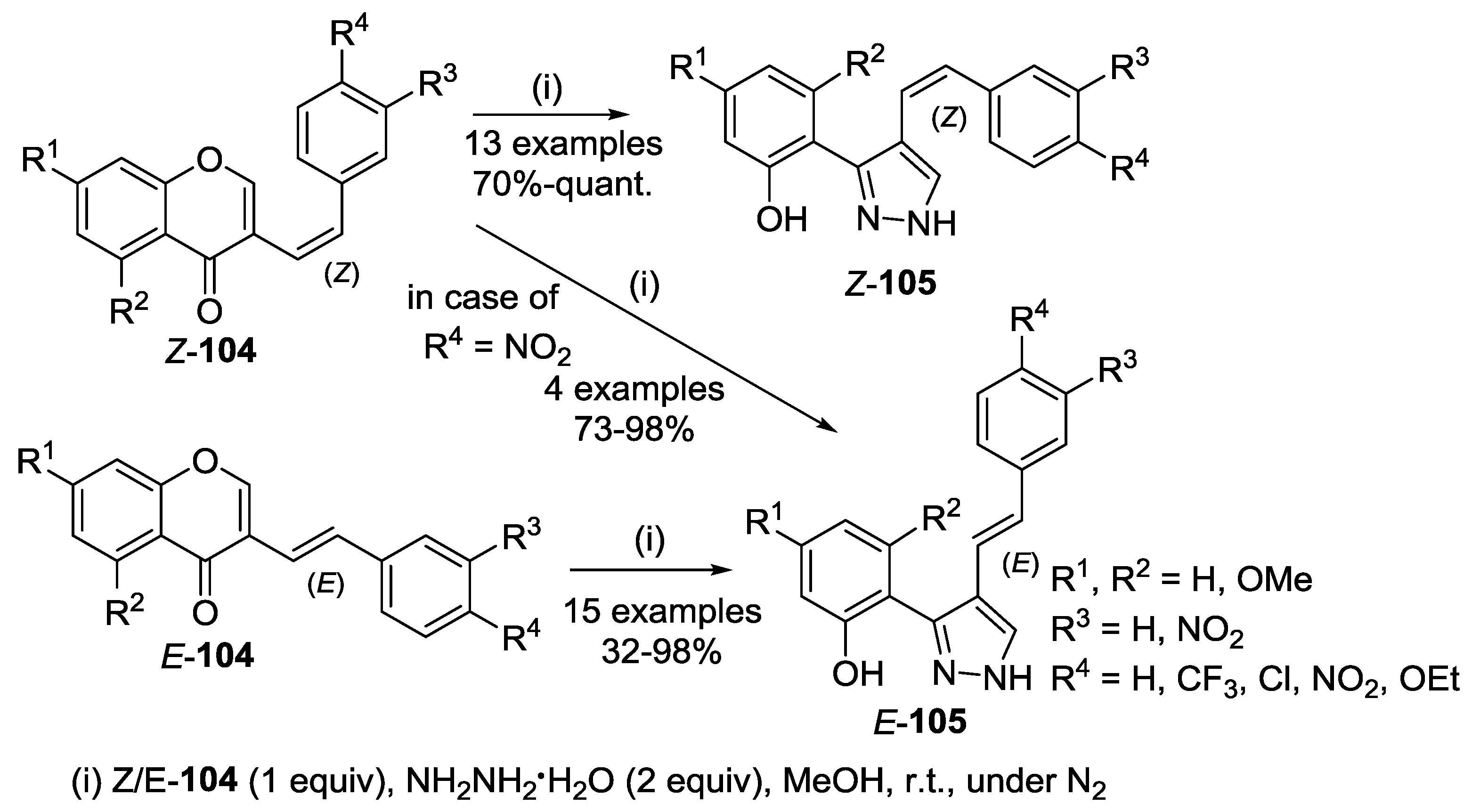
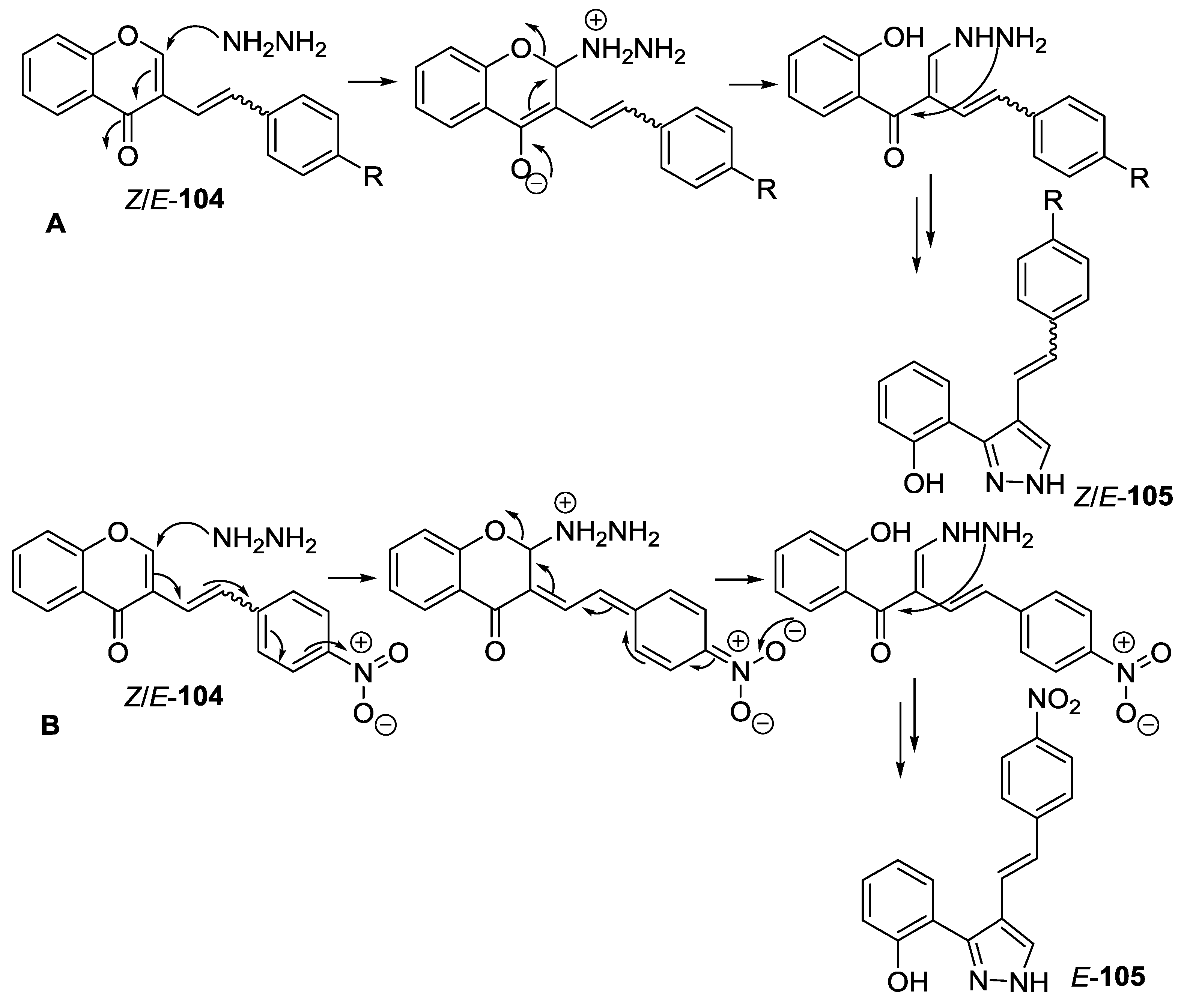
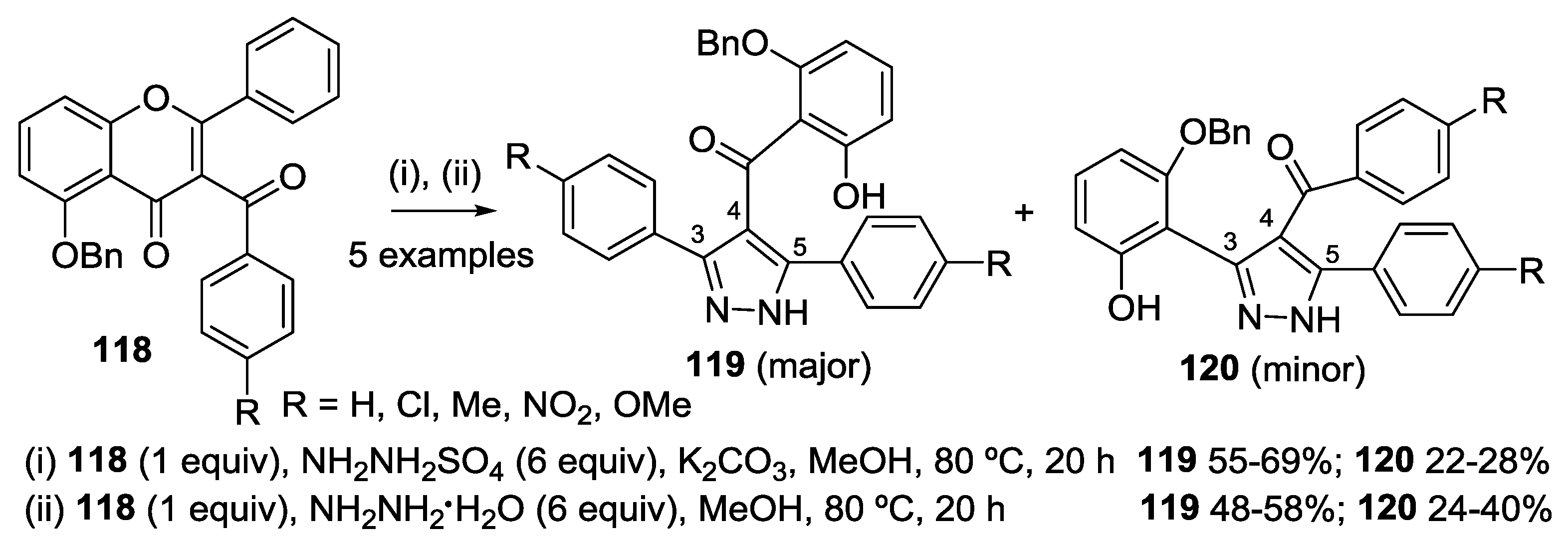
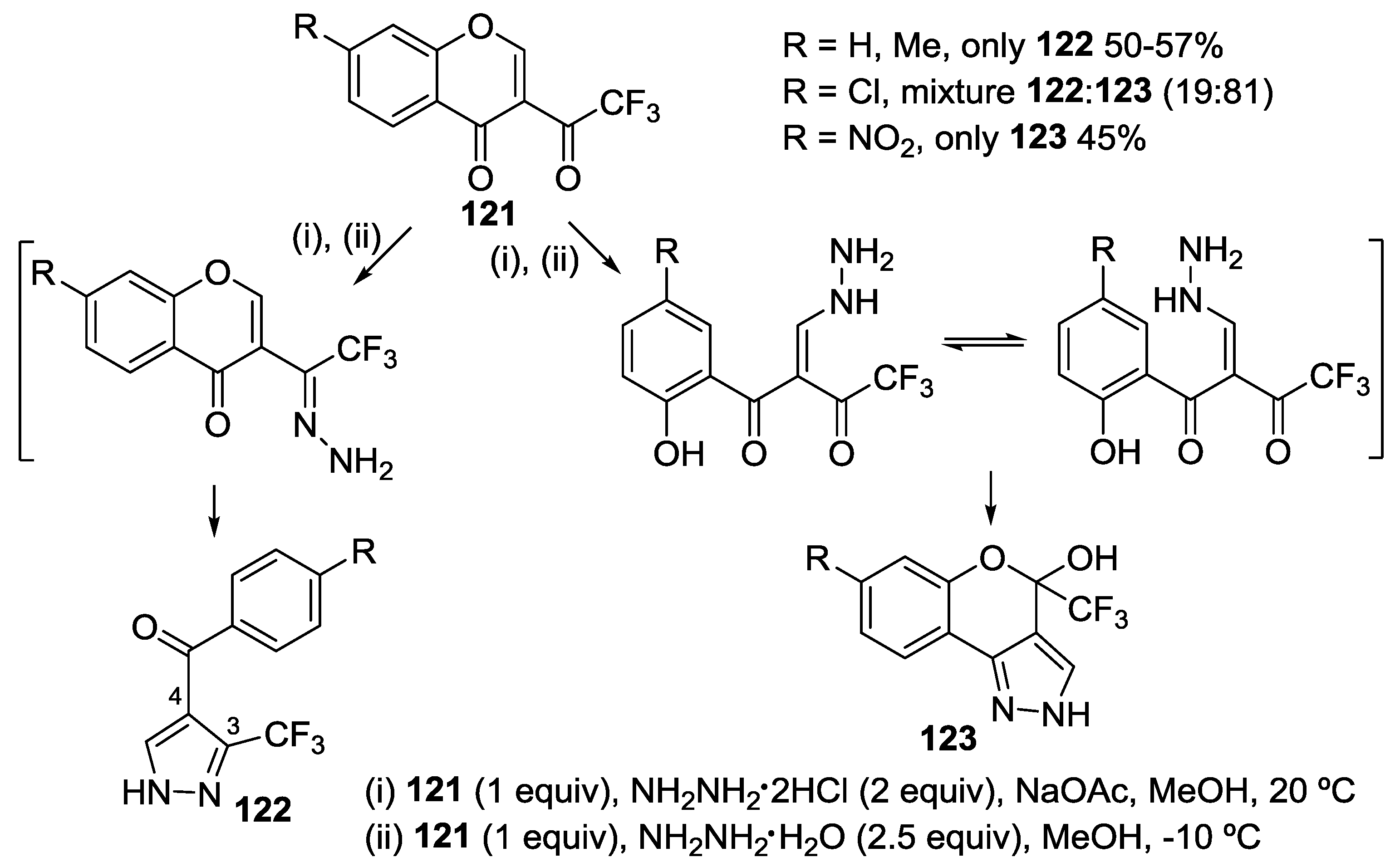
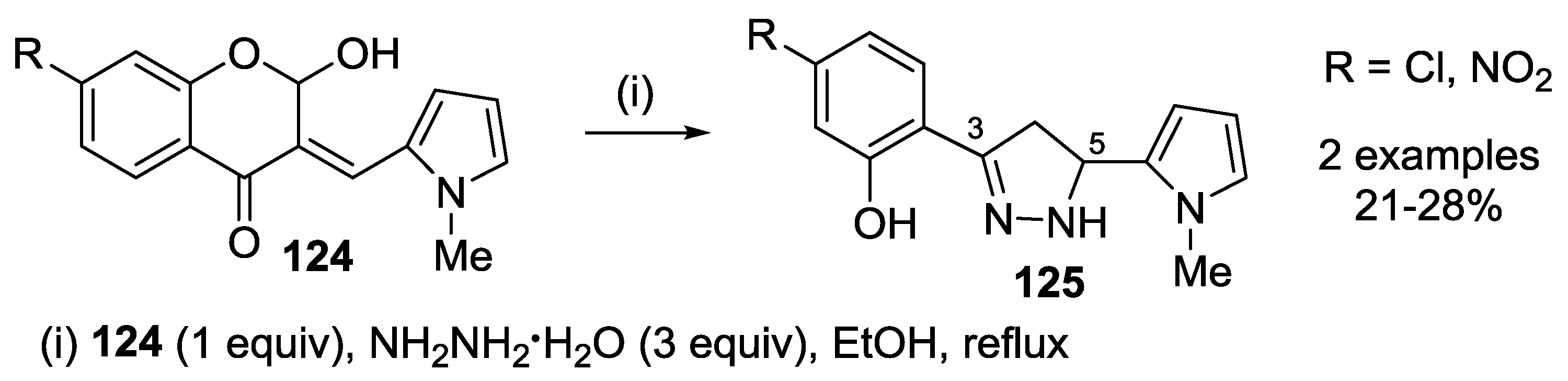
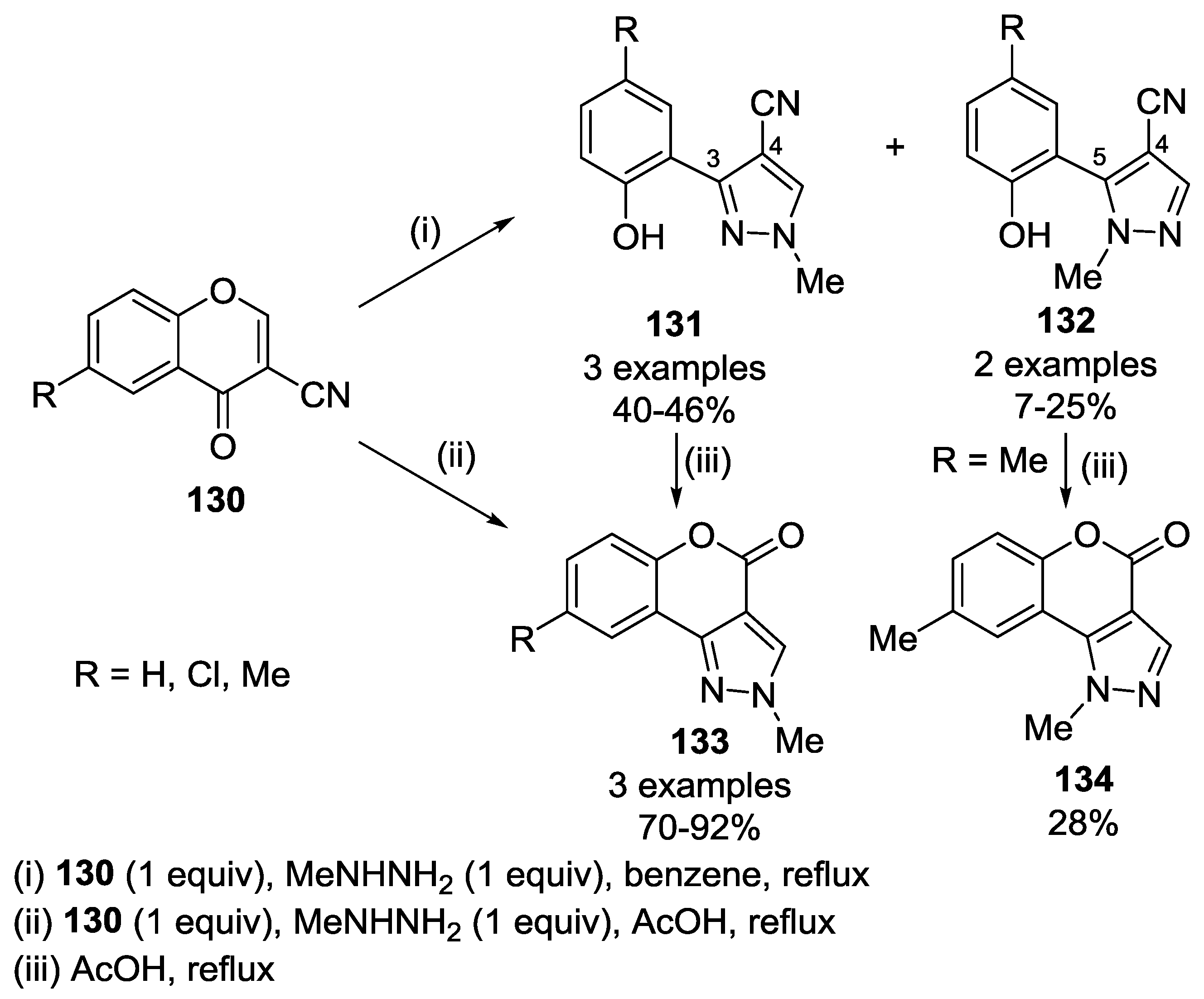
4.1. Reaction with Hydrazine
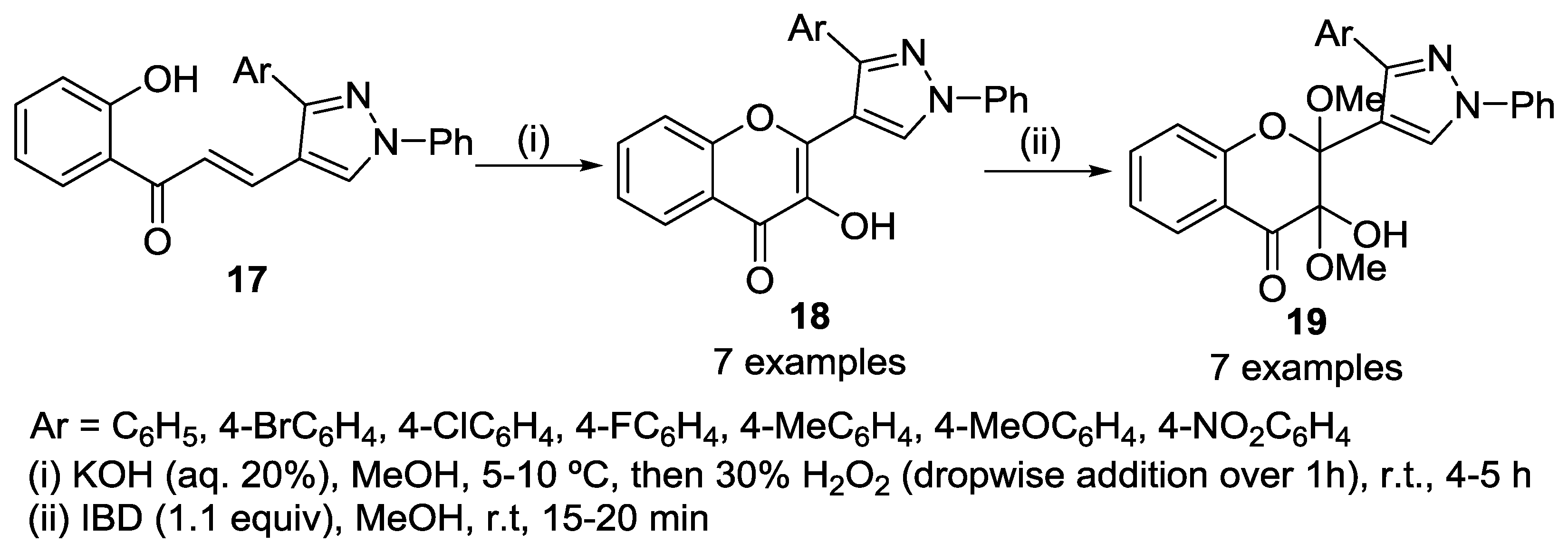
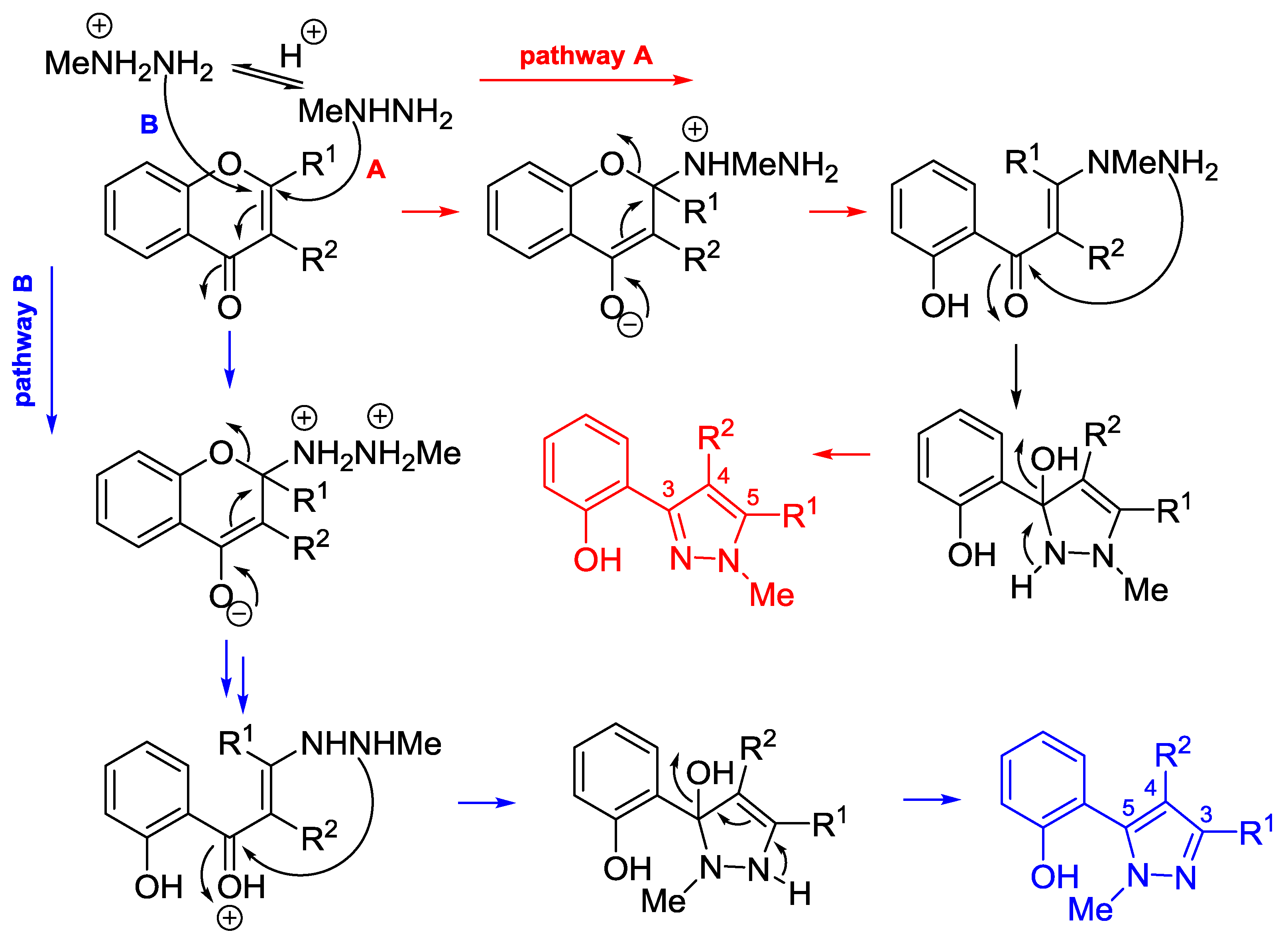
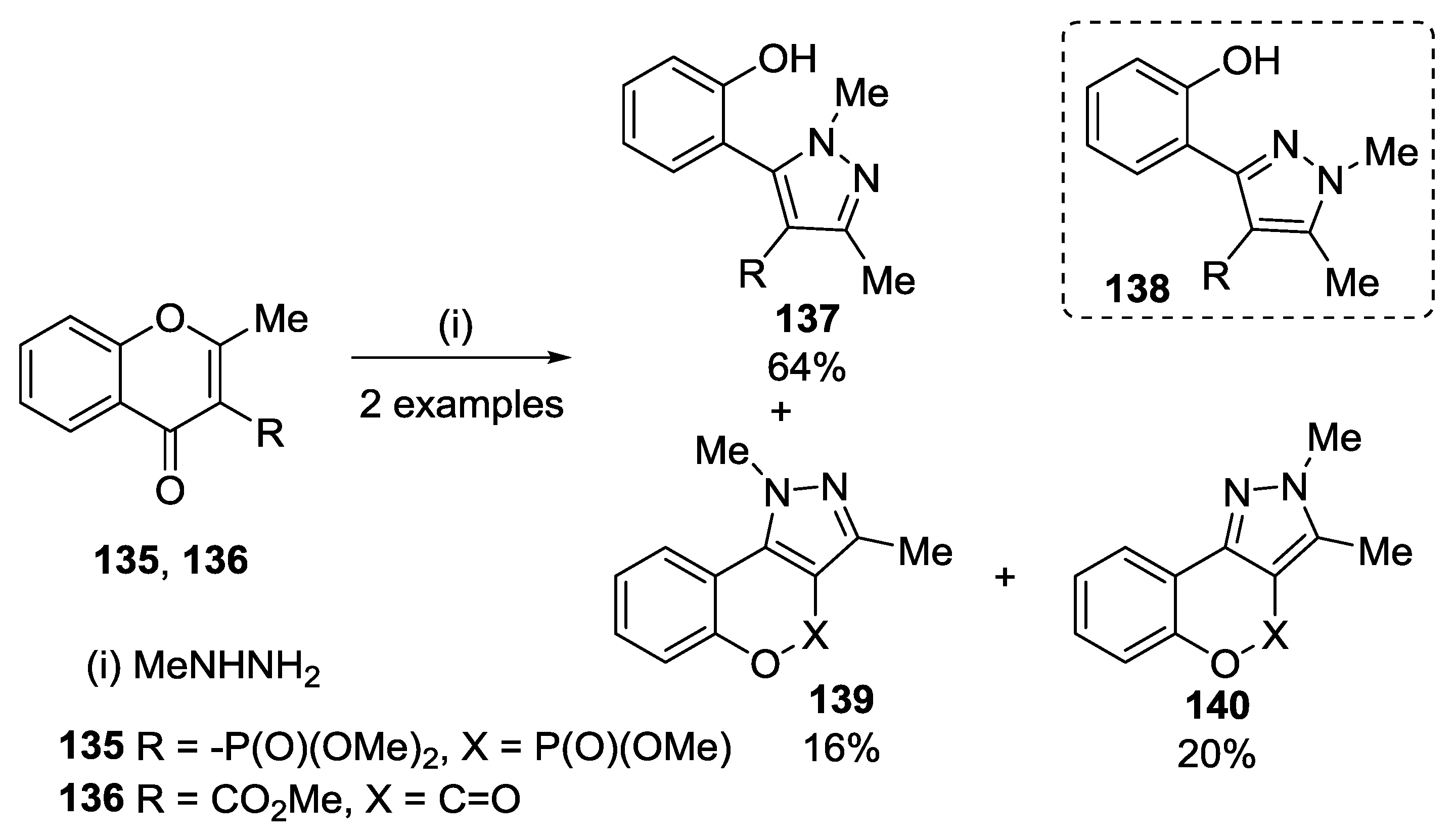
4.2. Reaction with Methylhydrazine
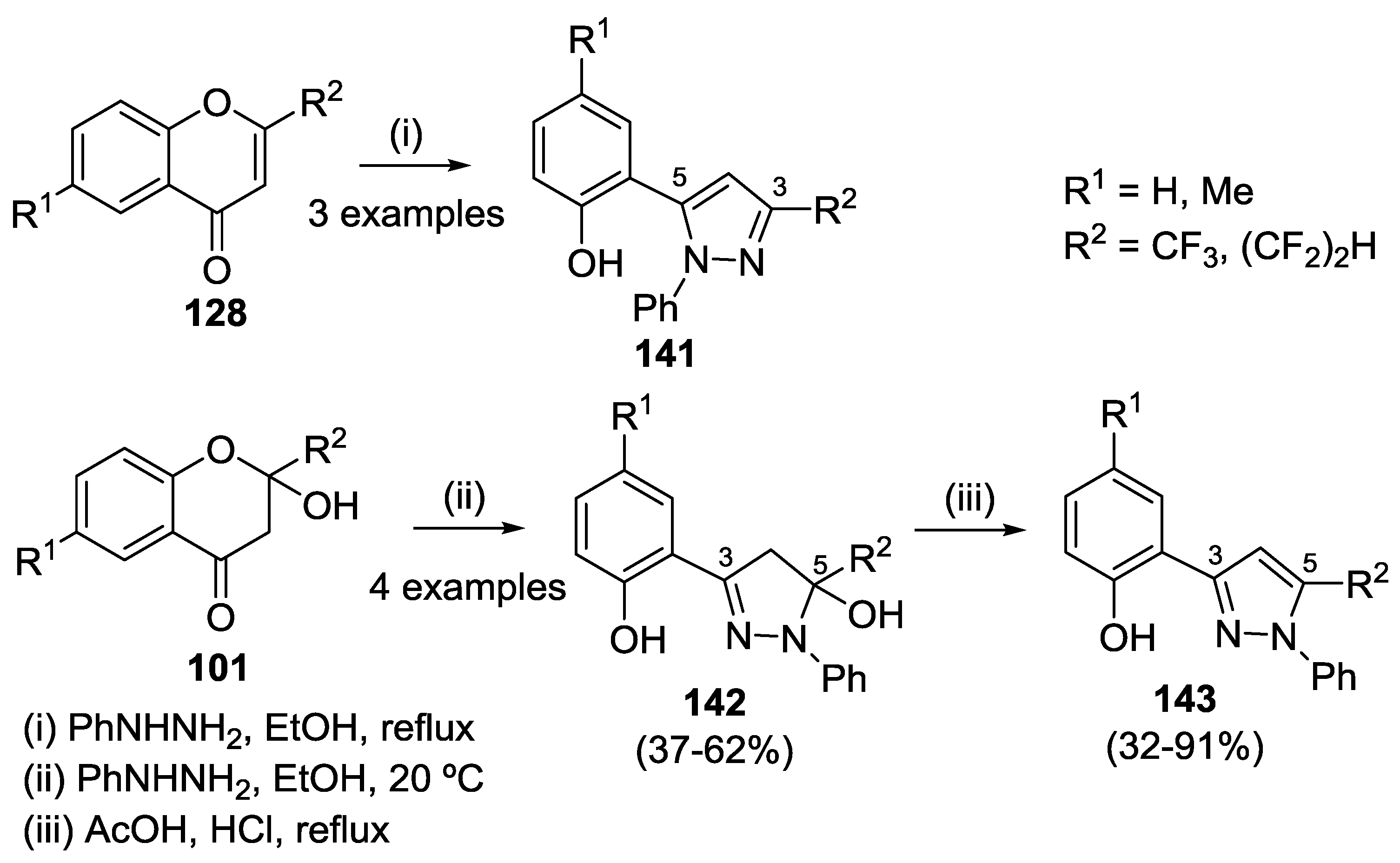
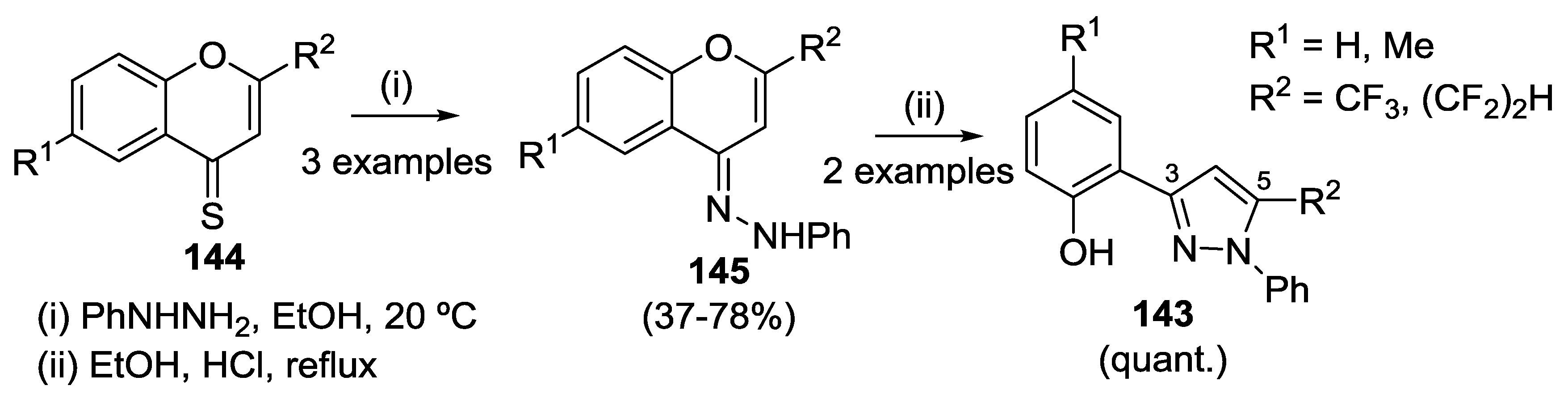
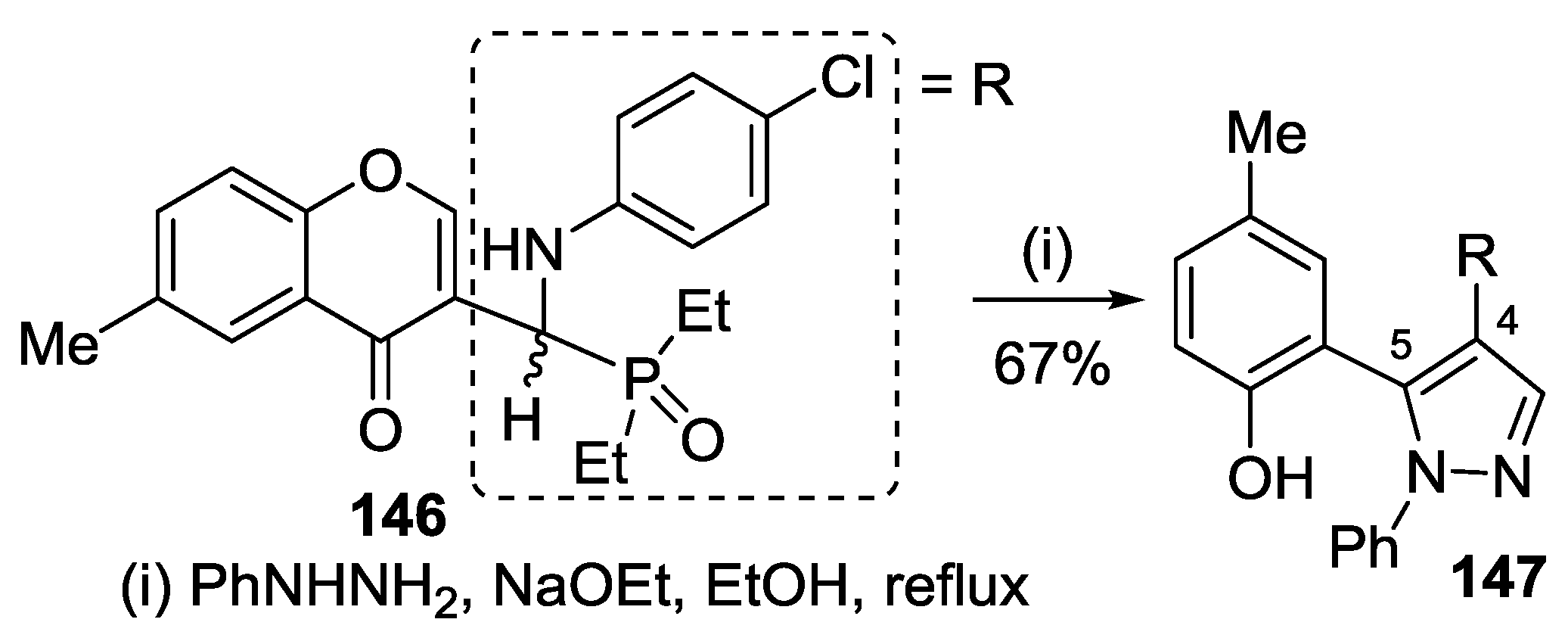
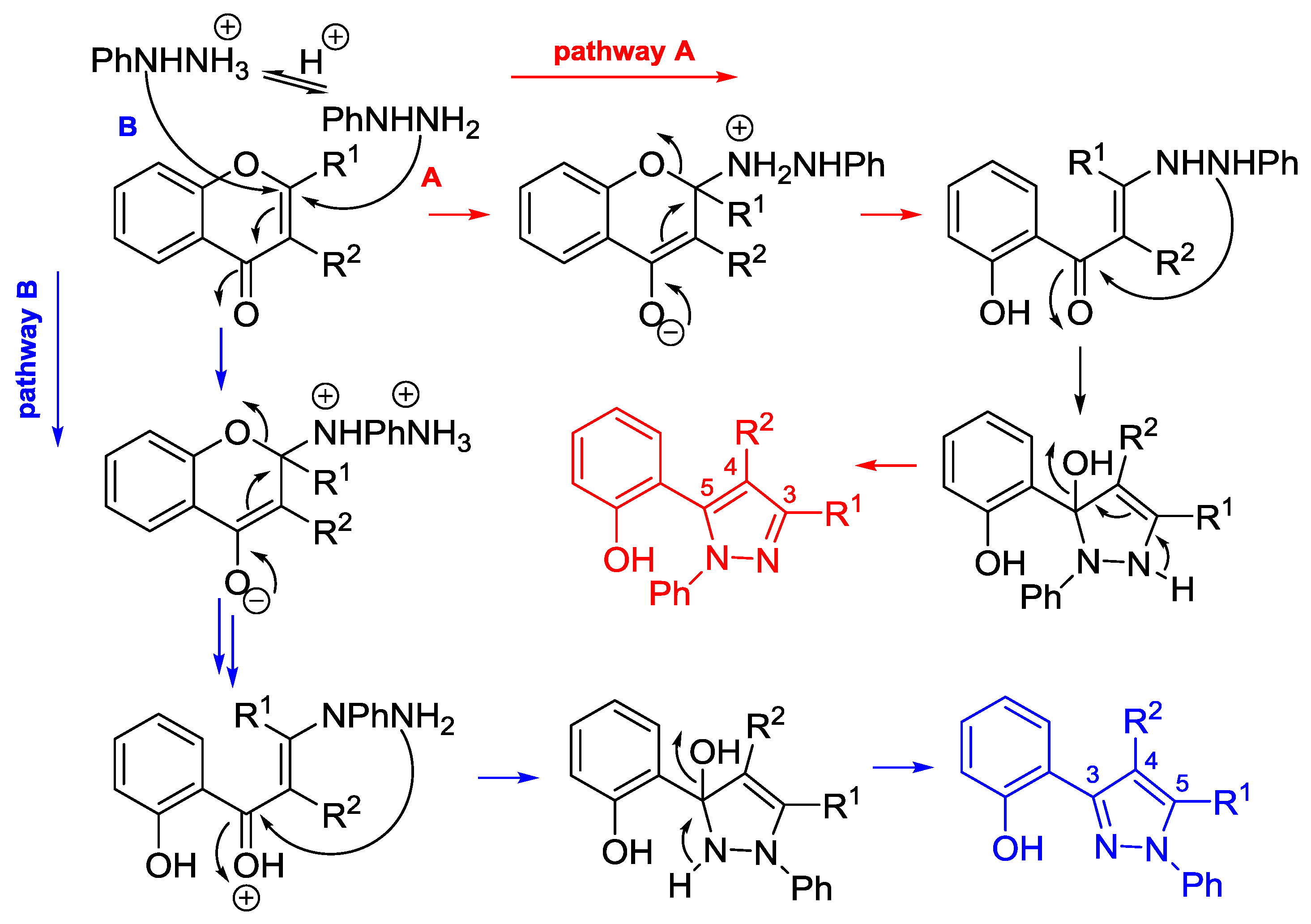
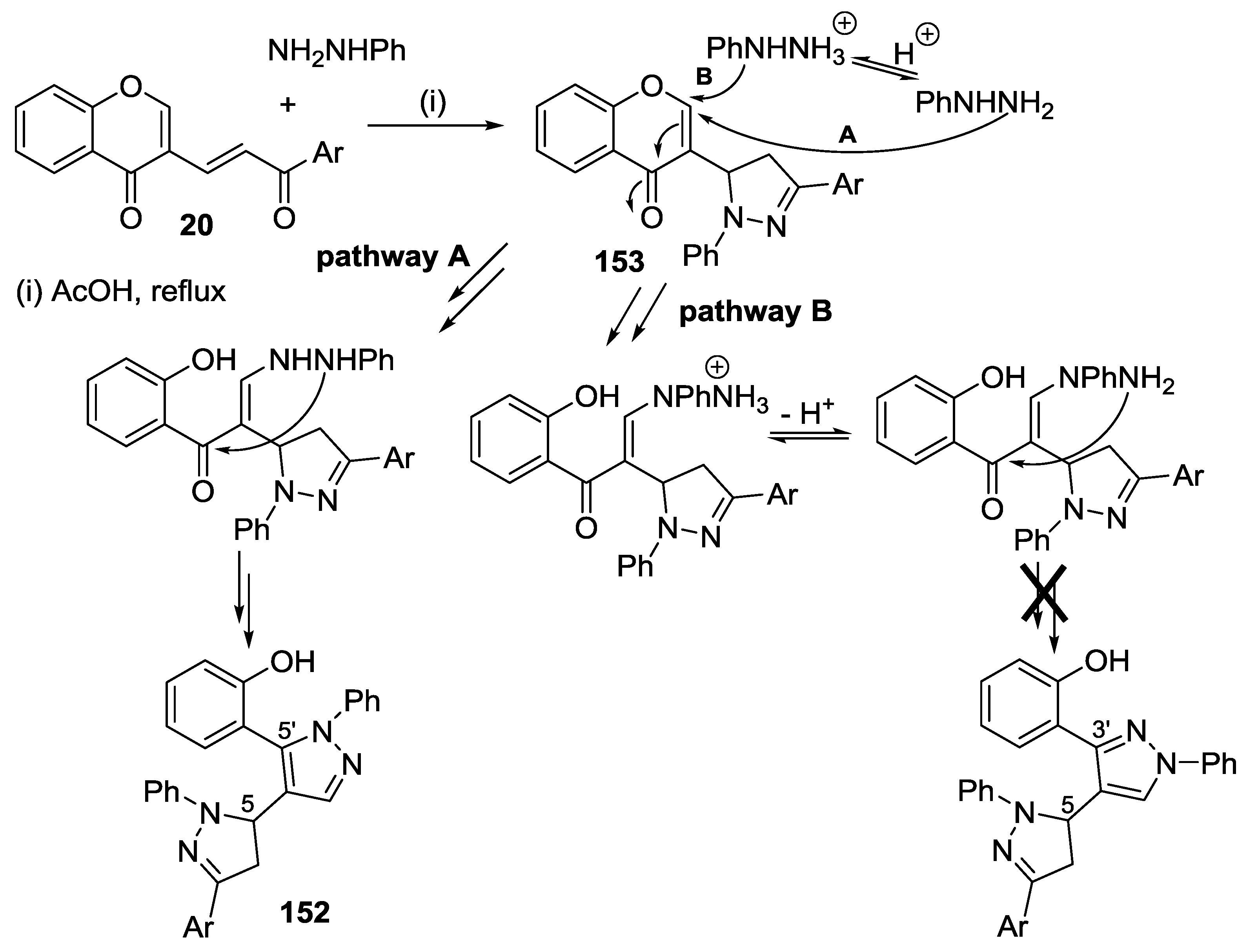
4.3. Reaction with Phenylhydrazine
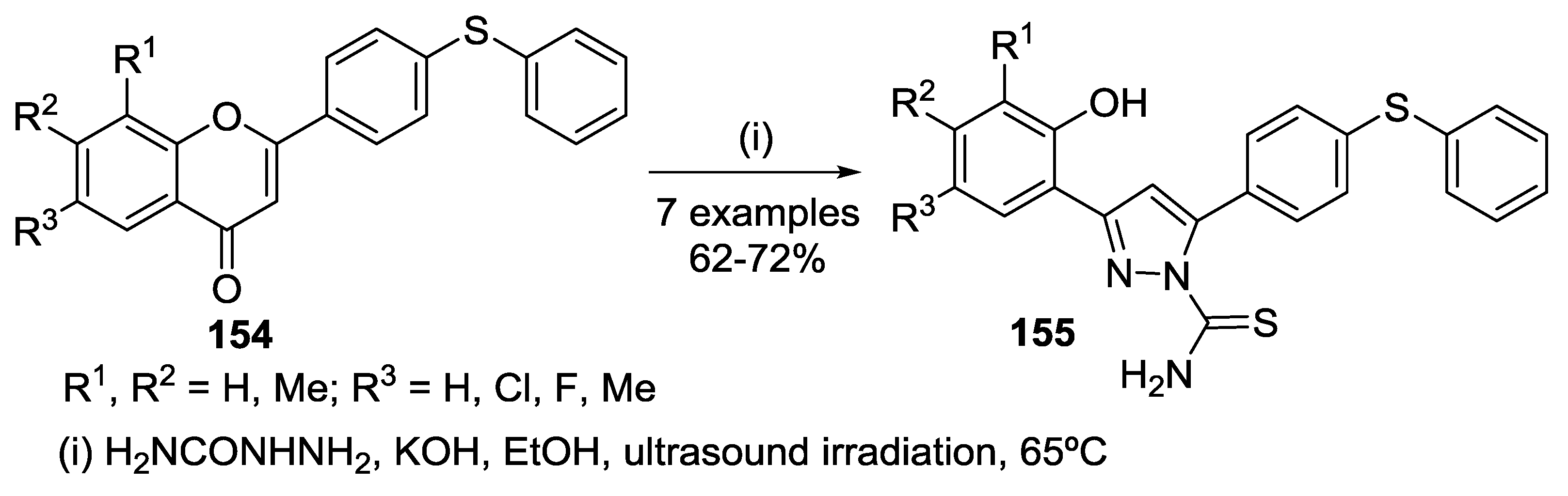
4.4. Reaction with Other Hydrazines
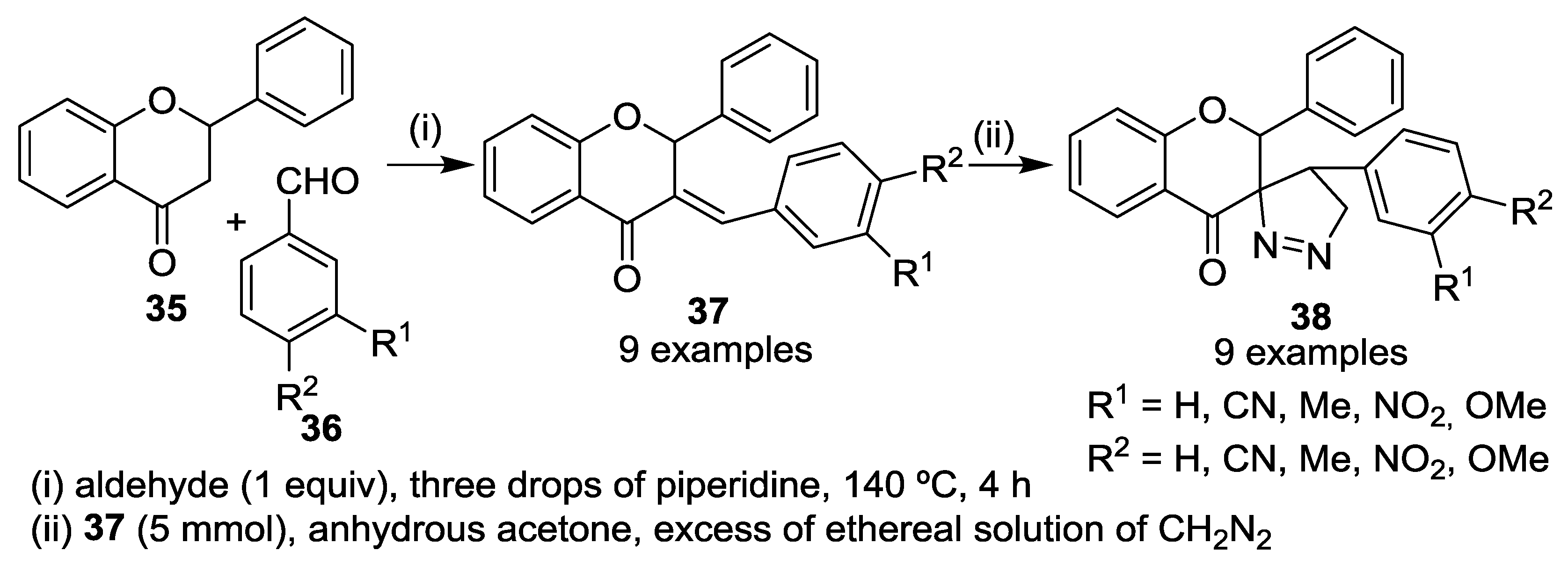
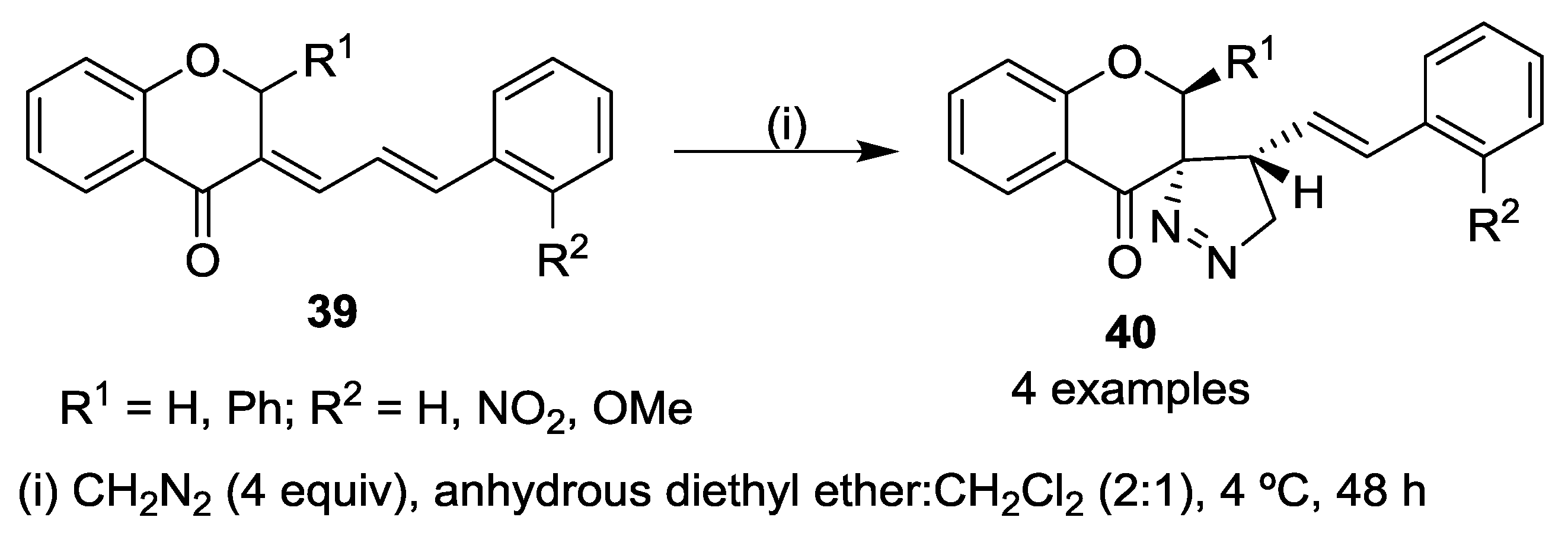
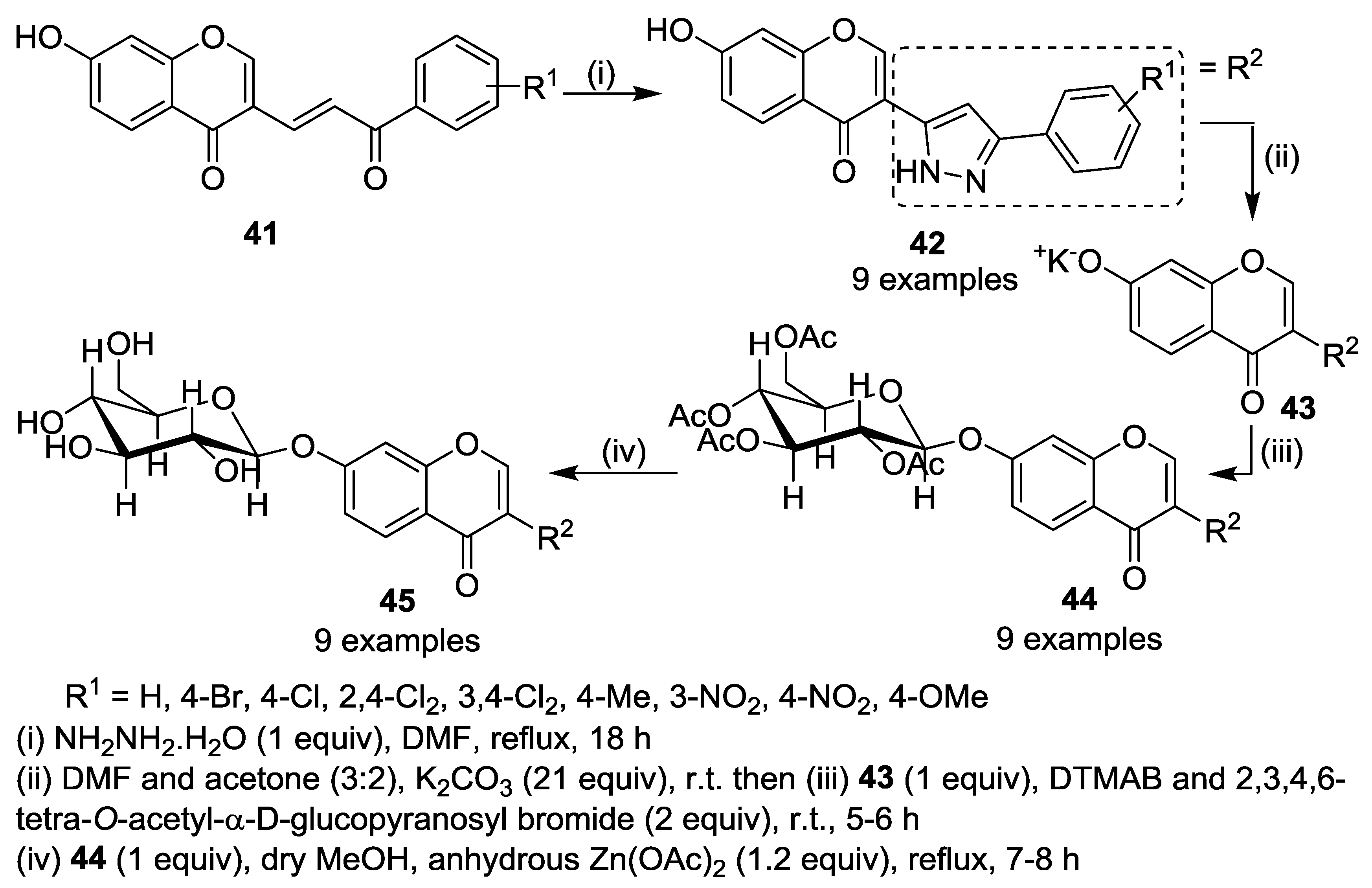
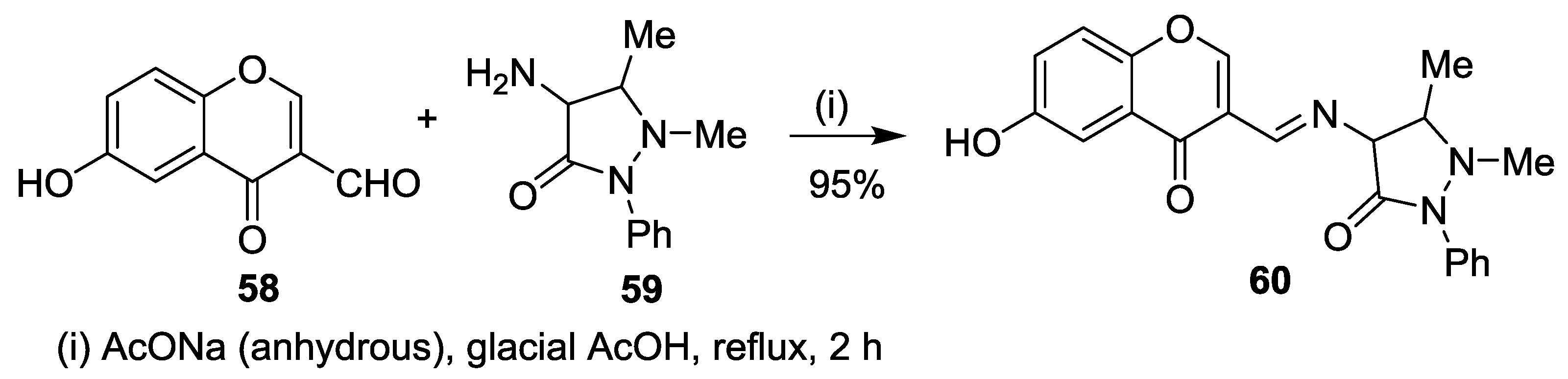
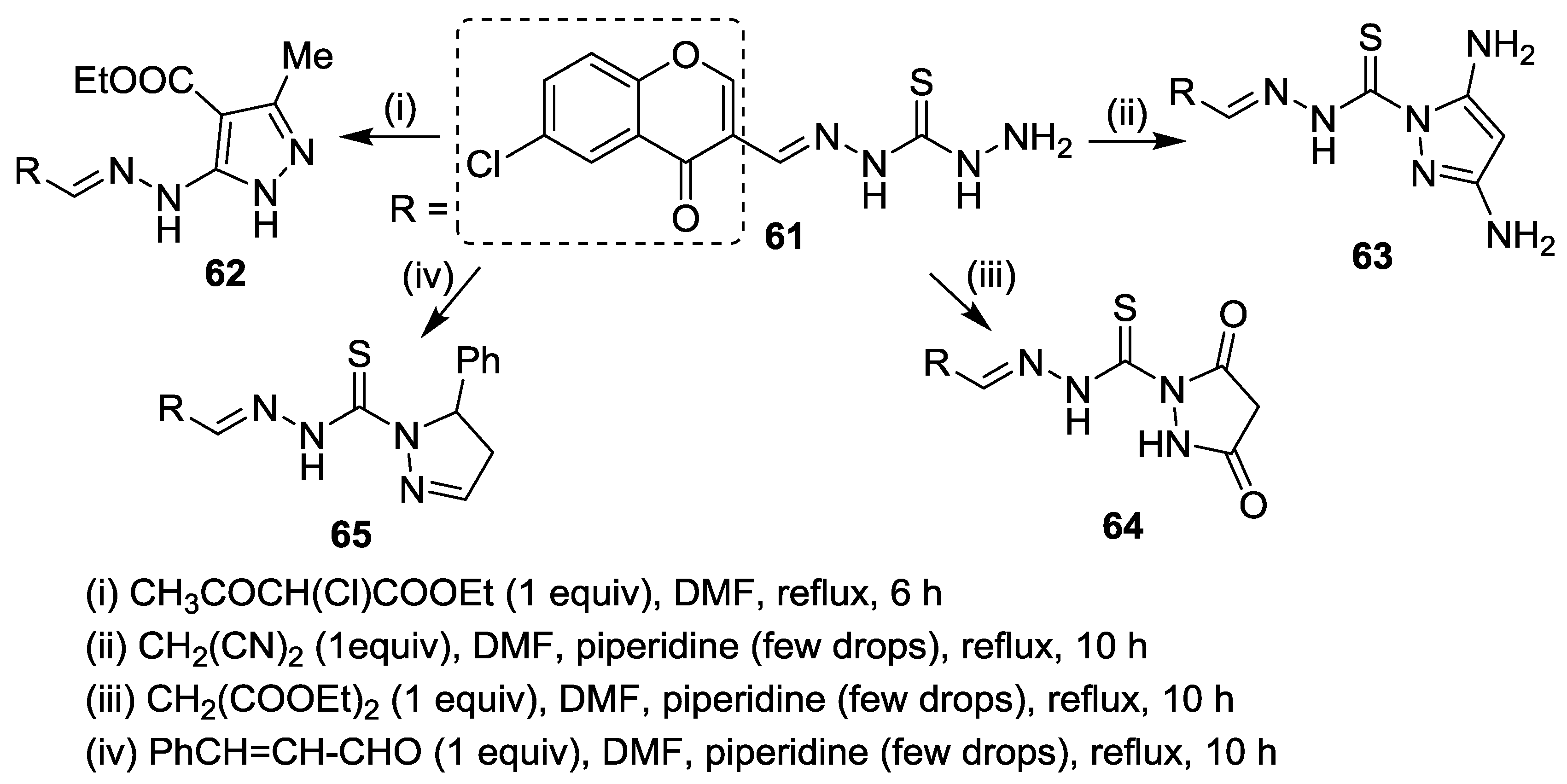
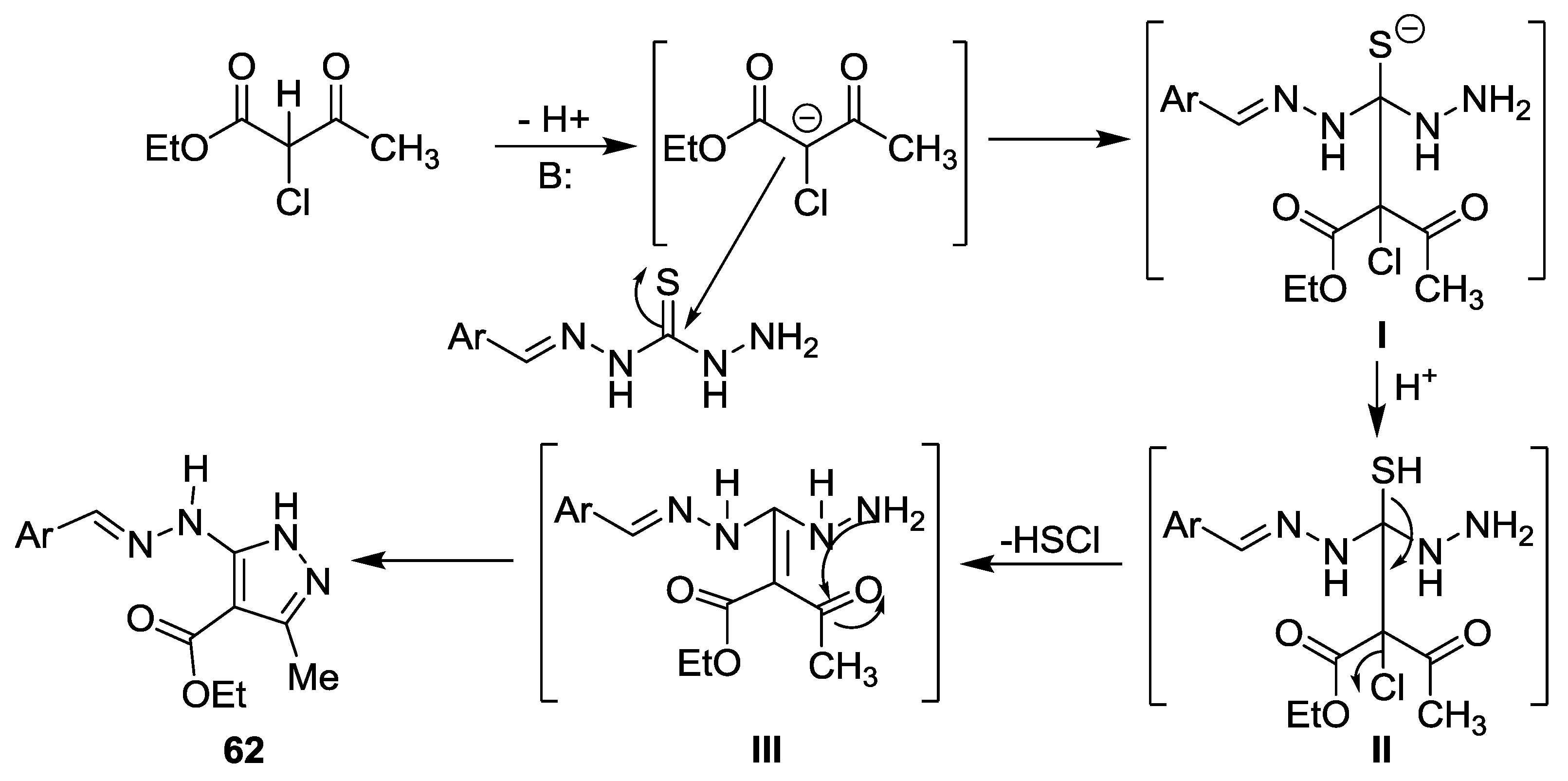
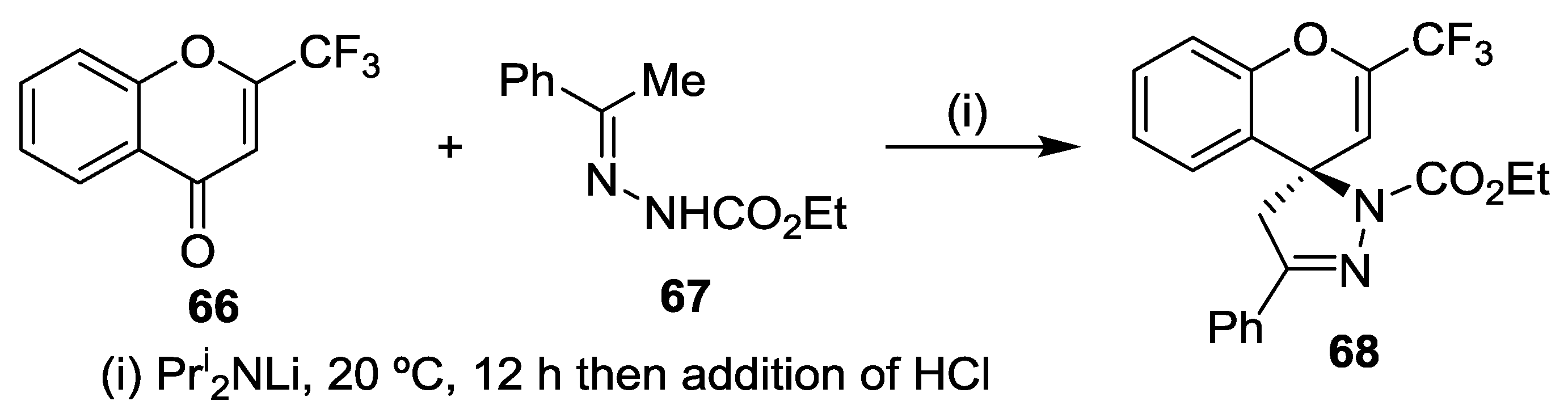
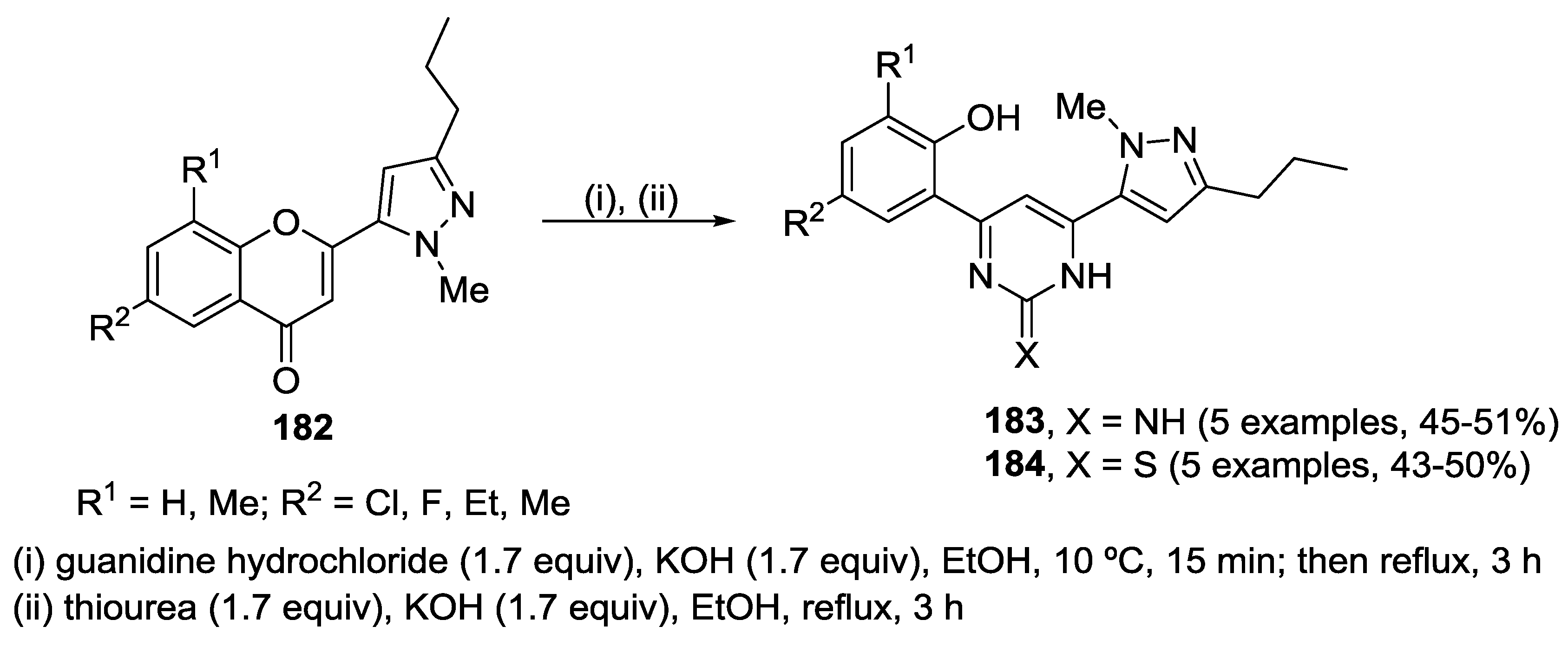
5. Miscellaneous
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Howell, J.B.L. The chromones: History, chemistry and clinical development. A tribute to the work of Dr R.E.C. Altounyan. Clin. Exp. Allergy 2000, 30, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Borges, F. Synthesis and pharmacological activities of non-flavonoid chromones: A patent review (from 2005 to 2015). Expert Opin Ther Pat. 2015, 25, 1285–1304. [Google Scholar] [CrossRef] [PubMed]
- Abrigach, F.; Touzani, R. Pyrazole derivatives with NCN junction and their biological activity: A review. Med. Chem. 2016, 6, 292–298. [Google Scholar] [CrossRef]
- Alam, M.J.; Alam, S.; Alam, P.; Naim, M.J. A review on pyrazole chemical entity and biological activity. Int. J. Pharm. Sci. Res. 2015, 6, 1433–1442. [Google Scholar]
- Joshi, N.S.; Karale, B.K.; Bhirud, S.B.; Gill, C.H. Synthesis and characterization of new biologically active 2-(1,3-disubstituted pyrazolyl)chromones and corresponding pyrimidine, thiopyrimidine, pyrazolyl derivatives. J. Heterocycl. Chem. 2004, 41, 541–548. [Google Scholar] [CrossRef]
- Karale, B.K.; Akolkar, H.N.; Burungale, A.S.; Mhaske, S.D.; Endait, R.S. Synthesis, characterization and biological evaluation of some novel thiophene anchored fluorinated heterocycles. Orient. J. Chem. 2015, 31, 453–464. [Google Scholar] [CrossRef]
- Çam, S.; Bildirici, I.; Menges, N.; Tan, M.; Sener, A. An entry into obtaining pyrazole-, chromone-, or oxadiazole-substituted 1H-pyrazoles via 2,3-furandiones. J. Heterocycl. Chem. 2013, 50, E211–E216. [Google Scholar] [CrossRef]
- Kale, S.B.; Dalvi, N.R.; More, M.S.; Shingare, M.S.; Karale, B.K. Synthesis of some pyrazolyl chromones. Indian J. Heterocycl. Chem. 2006, 15, 371–374. [Google Scholar]
- Joshi, N.S.; Shaikh, A.A.; Deshpande, A.P.; Karale, B.K.; Bhirud, S.B.; Gill, C.H. Synthesis, characterization antimicrobial activities of some fluorine containing 2-(1-phenyl-3-aryl-1H-pyrazol-4-yl)-3-chlorochromones, 2-(1-phenyl-3-aryl-1H-pyrazol-4-yl)chromones and 5-(1-phenyl-3-aryl-1H-pyrazol-4-yl)-3-(2-hydroxyphenyl)-4,5-dihydropyrazolines. Indian J. Chem. 2005, 44B, 422–425. [Google Scholar]
- Halnor, V.B.; Joshi, N.S.; Karale, B.K.; Gill, C.H. Synthesis, characterization of some 1-(2-hydroxy-phenyl)-3-(1-phenyl-3-thiophen-2-yl-1H-pyrazol-4-yl)-propenone, 3-chloro-2-(1-phenyl-3-thiophen-2-yl-1H-pyrazol-4-yl)-chromon-4-one and 2-(1′-phenyl-3′-thiophen-2-yl-3,4-dihydro-2H,1H′-[3,4]bipyrazol-5-yl)-phenol. Heterocycl. Commun. 2005, 11, 167–172. [Google Scholar]
- Karale, B.K.; Chaudhari, C.S.; Jadhav, R.K.; Akolkar, H.N. Synthesis and biological screening of new pyrazolyl chromones. Indian J. Heterocycl. Chem. 2011, 20, 381–384. [Google Scholar]
- Gaikar, R.B.; Gadhave, A.G.; Karale, B.K. Synthesis of fluorinated chromones. Indian J. Heterocycl. Chem. 2011, 20, 385–388. [Google Scholar]
- Akolkar, H.N.; Karale, B.K. Synthesis of some novel thiophene containing chromones and aurones. Indian J. Heterocycl. Chem. 2015, 24, 359–362. [Google Scholar]
- Gadhave, A.G.; Burungale, A.S.; Kuchekar, S.; Karale, B. Synthesis and antimicrobial screening of chromones, 3-chloro-chromones, benzothiazepines and pyrazolines. Indian J. Heterocycl. Chem. 2012, 22, 191–196. [Google Scholar]
- Narwade, S.K.; Kale, S.B.; Karale, B.K. Synthesis, and antimicrobial activities of some fluorinated substituted pyrazoles. Indian J. Heterocycl. Chem. 2007, 16, 275–278. [Google Scholar]
- Shelke, S.N.; Dalvi, N.R.; Gill, C.H.; Karale, B.K. Synthesis of various heterocycles from 3-(naphthylene-3-yl)-1H-pyrazol-4-carbaldehyde. Asian J. Chem. 2007, 19, 5068–5074. [Google Scholar]
- Badadhe, P.V.; Chavhan, N.M.; Nagargoje, D.R.; Gill, C.H. Synthesis and antimicrobial evaluation of some pyridine incorporated chromones and pyrazoles. Indian J. Heterocycl. Chem. 2009, 19, 175–178. [Google Scholar]
- Chavhan, N.M.; Badadhe, P.V.; Joshi, R.S.; Mandhane, P.G.; Gill, C.H. Synthesis and biological evaluation of some heterocycles from 1-phenyl-3-(pyridine-3-yl)-1H-pyrazole-4-carbaldehyde. Asian J. Chem. 2010, 22, 4255–4260. [Google Scholar]
- Jagadhani, S.G.; Shelke, S.N.; Karale, B.K. Synthesis and antimicrobial screening of some chromones and thiazepines by conventional and microwave irradiation. Heterocycl. Commun. 2009, 15, 159–166. [Google Scholar] [CrossRef]
- Karale, B.K.; Takate, S.J.; Salve, S.P.; Zaware, B.H.; Jadhav, S.S. Synthesis and antibacterial screening of novel fluorine containing heterocycles. Orient. J. Chem. 2015, 31, 307–315. [Google Scholar] [CrossRef]
- Jadhav, R.K.; Nikumbh, A.B.; Karale, B.K. Synthesis and screening of fluoro substituted pyrazolyl benzoxazoles. Orient. J. Chem. 2015, 31, 967–972. [Google Scholar] [CrossRef]
- Karale, B.K.; Nirmal, P.R.; Akolkar, H.N. Synthesis and biological screening of some novel fluorinated chromones and aurones. Indian J. Chem. 2015, 54B, 434–438. [Google Scholar]
- Prakash, O.; Kumar, R.; Parkash, V. Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl) chromones. Eur. J. Med. Chem. 2008, 43, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, R. Synthesis and antibacterial activity of some new 2,3-dimethoxy-3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl)chromanones. Eur. J. Med. Chem. 2009, 44, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Lévai, A.; Jekő, J. Synthesis of 3-aroyl-4-(3-chromonyl)-2-pyrazolines. J. Heterocycl. Chem. 2002, 39, 1333–1336. [Google Scholar] [CrossRef]
- Xie, Z.F.; Liu, F.M.; Mo, X.X.; Hui, Y.H.; Sun, Y.D. Synthesis of new 3a,6a-dihydro-4,6-dioxopyrrolo[3,4-d]pyrazole derivatives by 1,3-dipolar cycloaddition reaction. Chin. J. Org. Chem. 2005, 25, 696–699. [Google Scholar]
- Wang, W.; Deng, L.P.; Tang, S.L.; Qian, Q. Synthesis of pyrazole-linked norcantharidin analogues of substituted chromones. J. Heterocycl. Chem. 2016, 53, 1631–1634. [Google Scholar] [CrossRef]
- Soengas, R.G.; Silva, V.L.M.; Ide, D.; Kato, A.; Cardoso, S.M.; Paz, F.A.A.; Silva, A.M.S. Synthesis of 3-(2-nitrovinyl)-4H-chromones: Useful scaffolds for the construction of biologically relevant 3-(pyrazol-5-yl)chromones. Tetrahedron 2016, 72, 3198–3203. [Google Scholar] [CrossRef]
- Budzisz, E.; Paneth, P.; Geromino, I.; Muzioł, T.; Rozalski, M.; Krajewska, U.; Pipiak, P.; Ponczek, M.B.; Małecka, M.; Kupcewicz, B. The cytotoxic effect of spiroflavanone derivatives, their binding ability to human serum albumin (HSA) and a DFT study on the mechanism of their synthesis. J. Mol. Struct. 2017, 1137, 267–276. [Google Scholar] [CrossRef]
- Lévai, A.; Simon, A.; Jenei, A.; Kálmán, G.; Jekő, J.; Tóth, G. Synthesis of spiro-1-pyrazolines by the reaction of exocyclic α,β,γ,δ-unsaturated ketones with diazomethane. Arkivoc 2009, xii, 161–172. [Google Scholar] [CrossRef]
- Hatzade, K.; Taile, V.; Gaidhane, P.; Ingle, V. Synthesis, structural determination, and biological activity of new 7-hydroxy-3-pyrazolyl-4H-chromen-4-ones and their O-beta-d-glucosides. Turk. J. Chem. 2010, 34, 241–254. [Google Scholar] [CrossRef]
- Sheikh, J.; Hatzade, K.; Bader, A.; Shaheen, U.; Sander, T.; Ben Hadda, T. Computational evaluation and experimental verification of antibacterial and antioxidant activity of 7-hydroxy-3-pyrazolyl-4H-chromen-4-ones and their O-glucosides: Identification of pharmacophore sites. Med. Chem. Res. 2014, 23, 243–251. [Google Scholar] [CrossRef]
- Hatzade, K.M.; Taile, V.S.; Gaidhane, P.K.; Haldar, A.G.M.; Ingle, V.N. Synthesis and biological activities of new hydroxy-3-pyrazolyl-4H-chromen-4-ones and their O-glucosides. Indian J. Chem. B 2008, 47, 1260–1270. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Praveen, S.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Thermal solvent-free synthesis of chromonyl chalcones, pyrazolines and their in vitro antibacterial, antifungal activities. J. Enzym. Inhib. Med. Chem. 2012, 27, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Karale, B.K.; Chavan, V.P.; Mane, A.S.; Hangarge, R.V.; Gill, C.H.; Shingare, M.S. Microwave induced synthesis of 3-methyl-4-[(chromon-3-yl)methylene]-1-phenyl pyrazolin-5-(4H)-ones with alumina support and in solvent free conditions. Synth. Commun. 2002, 32, 497–503. [Google Scholar] [CrossRef]
- Madje, B.R.; Shindalkar, S.S.; Ware, M.N.; Shingare, M.S. An efficient condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one. Arkivoc 2005, xiv, 82–86. [Google Scholar] [CrossRef]
- Shelke, S.N.; Dalvi, N.R.; Kale, S.B.; More, M.S.; Gill, C.H.; Karale, B.K. Environmentally benign synthesis of fluorinated pyrazolone derivatives and their antimicrobial activity. Indian. J. Chem. 2007, 46B, 1174–1178. [Google Scholar] [CrossRef]
- Jagadhani, S.G.; Kale, S.B.; Chaudhari, C.S.; Sangle, M.D.; Randhavane, P.V.; Karale, B.V. Knoevenagel reactions of 3-formyl chromones and 4-formyl pyrazoles with pyrazolone by conventional and non conventional methods. Indian J. Heterocycl. Chem. 2007, 16, 255–258. [Google Scholar]
- Dalvi, R.N.; Karale, B.K.; Gill, C.H. Ultrasound induced synthesis of 3-methyl-4-[(chromon-3-yl)methylene]-1-(4-nitrophenyl)pyrazolin-5-(4H)-ones and 3-methyl-4[(1,3-diphenyl-1H-pyrazol-4-yl)methylene]-1-(4-nitrophenyl)pyrazolin-5-(4H)-ones. Indian J. Heterocycl. Chem. 2005, 14, 263–264. [Google Scholar]
- Singh, P.; Kaur, M.; Holzer, W. Synthesis and evaluation of indole, pyrazole, chromone and pyrimidine based conjugates for tumor growth inhibitory activities—Development of highly efficacious cytotoxic agents. Eur. J. Med. Chem. 2010, 45, 4968–4982. [Google Scholar] [CrossRef] [PubMed]
- Amrinder Singh, S.; Kaur, M.; Sharma, S.; Bhatti, R.; Singh, P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: Identification of a lead for anti-inflammatory drug. Eur. J. Med. Chem. 2014, 77, 185–192. [Google Scholar] [CrossRef]
- Gadhave, A.; Kuchekar, S.; Karale, B. Ultrasonication-induced synthesis and antimicrobial evaluation of some multifluorinated pyrazolone derivatives. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.H.; Hammouda, M.A.A.; El-Desoky, S.I. Synthesis of some new azole, azepine, pyridine, and pyrimidine derivatives using 6-hydroxy-4H-4-oxo[1]-benzopyran-3-carboxaldehyde as a versatile starting material. Heteroat. Chem. 2005, 16, 20–27. [Google Scholar] [CrossRef]
- Khodairy, A. Synthetic studies on the synthesis of some new fused heterocyclic compounds derived from 3,5-pyrazolidinedione. J. Chin. Chem. Soc. 2007, 54, 93–102. [Google Scholar] [CrossRef]
- Ali, T.E.; Abdel-Aziz, S.A.; El-Shaaer, H.M.; Hanafy, F.I.; El-Fauomy, A.Z. Synthesis of bioactive 4-oxo-4H-chromenes bearing heterocyclic systems from hydrazonecarbodithioic acid and thiocarbohydrazone. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2139–2160. [Google Scholar] [CrossRef]
- Mahajan, P.S.; Nikam, M.D.; Khedkar, V.M.; Jha, P.C.; Sarkar, D.; Gill, C.H. Synthesis, biological evaluation and molecular docking studies of N-acylheteroaryl hydrazone derivatives as antioxidant and anti-inflammatory agents. Res. Chem. Intermed. 2016, 42, 2707–2729. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Sizov, A.Y.; Usachev, B.I.; Kodess, M.I.; Anufriev, V.A. Spiro [4H-chromene-4,5′-isoxazolines] and related compounds: Synthesis and reactivities. Russ. Chem. Bull. 2006, 55, 535–542. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M.; Said, S. Synthesis of heteroannulated chromeno[2,3-b]pyridines: DBU catalyzed reactions of 2-amino-6-methylchromone-3-carboxaldehyde with some heterocyclic enols and enamines. J. Heterocycl. Chem. 2016, 53, 117–120. [Google Scholar] [CrossRef]
- Obulesu, O.; Babu, K.H.; Nanubolu, J.B.; Suresh, S. Copper-catalyzed tandem O-arylation-oxidative cross coupling: Synthesis of chromone fused pyrazoles. J. Org. Chem. 2017, 82, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Terzidis, M.A.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J. Synthesis of chromeno[2,3-b]carbazole and chromeno[3,2-f]indazole derivatives. A new class of indole- and pyrazole-fused polycyclic compounds using o-quinodimethane chemistry. A reactivity and regioselectivity computational study. Arkivoc 2008, xiv, 132–157. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.J.; Zhang, Y.H.; Sun, Y.D.; Wang, B.; Liu, W.B. Green method for the synthesis of chromeno[2,3-c]pyrazol-4(1H)-ones through ionic liquid promoted directed annulation of 5-(aryloxy)-1H-pyrazole-4-carbaldehydes in aqueous media. Org. Lett. 2015, 17, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.B.; Mishra, K.; Gupta, T.; Singh, R.M. TBHP promoted cross-dehydrogenative coupling (CDC) reaction: Metal/additive-free synthesis of chromone-fused quinolones. ChemistrySelect 2017, 2, 1207–1210. [Google Scholar] [CrossRef]
- Dimitriadou, E.; Stephanidou-Stephanatou, J.; Tsoleridis, C.A.; Hadjipavlou-Litina, D.J.; Kontogiorgis, C.; Kostakis, G.E. Diastereoselective one-pot synthesis of novel ABCD-fused chromeno[2,3-d]pyrazolo[3,4-b]pyridines. Tetrahedron 2014, 70, 2938–2943. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Kodess, M.I.; Moshkin, V.S. Structure and reactivity of 2-amino-3-carbamoylchromone. Russ. Chem. Bull. 2009, 58, 1253–1258. [Google Scholar] [CrossRef]
- Reddy, G.J.; Latha, D.; Sailaja, S.; Rao, K.S. Synthesis of 7-aryl/chromonyl substituted benzopyrano[4,3-d]pyrazolo[1,5-a]pyrimidines by reaction of 5(3)-aminopyrazoles. Heterocycl. Commun. 2003, 9, 615–619. [Google Scholar] [CrossRef]
- Koenigs, E.; Freund, J. Über die einwirkung von hydrazinen auf 4-chlor-chinaldin. Chem. Ber. 1947, 80, 143–149. [Google Scholar] [CrossRef]
- Baker, W.; Harbone, J.B.; Ollis, W.D. Some properties of 4-thionflavone and its methiodide, and of 4-thionchromones. J. Chem. Soc. 1952, 1303–1309. [Google Scholar] [CrossRef]
- Schönberg, A.; Sidky, M.M. Furochromones and -Coumarins. VIII. Action of hydrazine hydrate and hydroxylamine on Khellin, Khellol and Visnagin. J. Am. Chem. Soc. 1953, 75, 5128–5130. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Barabanov, M.A.; Sizov, A.Y. 2-Polyfluoroalkylchromones. 11. Synthesis and structures of 5-hydroxy-3-(2-hydroxyaryl)-5-polyfluoroalkyl-∆2-pyrazolines and 3(5)-(2-hydroxyaryl)-5(3)-polyfluoroalkylpyrazoles. Russ. Chem. Bull. 2002, 51, 1280–1291. [Google Scholar] [CrossRef]
- Safrygin, A.V.; Irgashev, R.A.; Barabanov, M.A.; Sosnovskikh, V.Y. Synthesis of a new class of carbinol linked bis-heterocycles via the reaction of 2-(trifluoroacetyl)chromones with indoles and pyrroles. Tetrahedron 2016, 72, 227–233. [Google Scholar] [CrossRef]
- Kale, S.B.; Karale, B.K. Synthesis and characterization of some important indazolyl derivatives. J. Heterocycl. Chem. 2007, 44, 289–301. [Google Scholar] [CrossRef]
- Chate, A.V.; Nikam, M.D.; Mahajan, P.S.; Mohekar, S.R.; Gill, C.H. Synthesis and antimicrobial screening of novel 2-(5-(4-(allyloxy)-3-methoxyphenyl)-1H-pyrazol-3-yl)phenols analogues of 2-(4-(allyloxy)-3-methoxyphenyl)-4H-chromen-4-ones. Org. Commun. 2012, 5, 83–98. [Google Scholar]
- Gadhave, A.; Gaikar, R.; Kuchekar, S.; Karale, B. Synthesis and antimicrobial evaluations of some novel fluorinated chromones and pyrazoles. Indian J. Chem. 2015, 54B, 383–390. [Google Scholar]
- Chavhan, N.M.; Badadhe, P.V.; Bhagat, S.S.; Joshi, R.S.; Mandhane, P.G.; Gill, C.H. Synthesis of some biologically important pyrazoles and pyrazoline derivatives. J. Indian Chem. Soc. 2011, 88, 457–460. [Google Scholar]
- Pawar, S.B.; Dalvi, N.R.; Karale, B.K.; Gill, C.H. Synthesis of some 4-(2-hydroxy phenyl)-6-(1,3-diphenyl-1H-pyrazol-4-yl)pyrimidine-2(1H)-thiones and 2(5-(1,3-diphenyl-1H-pyrazol-4-yl)-1H-pyrazol-3-yl) phenols. Indian J. Heterocycl. Chem. 2005, 15, 197–198. [Google Scholar]
- More, M.S.; Shingare, M.S.; Kale, S.B.; Dalvi, N.R.; Karale, B.K. Synthesis and characterization of some 2-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)4H-chromon-4-ones, 4-(2-hydroxyphenyl)-6-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)pyrimidine-2-(1H)-thione, 2-(5-(1-phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-1H-pyrazol-3-yl)phenol and 2-(2,3-dihydro-2-(1phenyl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-benzo[b][1,4]thiazepin-4-yl)-phenol. Indian J. Chem. 2007, 46B, 360–365. [Google Scholar]
- Akolkar, H.N.; Jadhav, R.K.; Karale, B.K.; Randhavane, P.V. Synthesis and antimicrobial evaluation of some new theopiiene anchored chromones, aurones and pyrazoles. Indian J. Heterocycl. Chem. 2016, 25, 193–199. [Google Scholar]
- Karale, B.K.; Takate, S.J.; Salve, S.P.; Zaware, B.H.; Jadhav, S.S. Synthesis and biological screening of some novel thiazolyl chromones and pyrazoles. Indian J. Chem. 2015, 54B, 798–804. [Google Scholar]
- Badadhe, P.V.; Patil, L.R.; Bhagat, S.S.; Chate, A.V.; Shinde, D.W.; Nikam, M.D.; Gill, C.H. Synthesis and antimicrobial screening of some novel chromones and pyrazoles with incorporated isoxazole moieties. J. Heterocycl. Chem. 2013, 50, 999–1004. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Silva, V.L.M.; Elguero, J.; Silva, A.M.S. Synthesis of new pyrazole-1,2,3-triazole dyads. Tetrahedron Lett. 2013, 54, 5391–5394. [Google Scholar] [CrossRef]
- Safrygin, A.V.; Vetyugova, D.A.; Sosnovskikh, V.Y. Novel method for the synthesis of 3-[(3-arylpyrazol-5-yl)methylidene]-quinoxalin-2-ones on the basis of 2-methylchromones. Chem. Heterocycl. Compd. 2016, 52, 1035–1041. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdel-Hamed, M.A.M.; El-Gohary, N.M. A new approach for the synthesis of bioactive heteroaryl thiazolidine-2,4-diones. J. Braz. Chem. Soc. 2011, 22, 1130–1139. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Elguero, J. New bis(chalcones) and their transformation into bis(pyrazoline) and bis(pyrazole) derivatives. Eur. J. Org. Chem. 2003, 747–755. [Google Scholar] [CrossRef]
- Bondarenko, S.P.; Frasinyuk, M.S.; Vinogradova, V.I.; Khilya, V.P. Synthesis of 4-aryl-3-[2-hydroxy-4-(2-cytisin-12-ylethoxy)phenyl]pyrazoles. Chem. Nat. Compd. 2014, 50, 889–891. [Google Scholar] [CrossRef]
- Shokol, T.V.; Gorbulenko, N.V.; Frasinyuk, M.S.; Khilya, V.P. Synthesis of 6-(3-pyrazolyl)-4-methylumbelliferone derivatives substituted on the pyrazole ring. Chem. Nat. Compd. 2015, 51, 630–633. [Google Scholar] [CrossRef]
- Lévai, A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Alkorta, I.; Elguero, J.; Jekő, J. Synthesis of pyrazoles by treatment of 3-benzylchromones, 3-benzylflavones and their 4-thio analogues with hydrazine. Eur. J. Org. Chem. 2006, 2006, 2825–2832. [Google Scholar] [CrossRef]
- Lévai, A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Elguero, J.; Alkorta, I.; Jekő, J. Synthesis of 4-aryl-3(5)-(2-hydroxyphenyl)pyrazoles by reaction of isoflavones and their 4-thio analogues with hydrazine derivatives. Aust. J. Chem. 2007, 60, 905–914. [Google Scholar] [CrossRef]
- Usachev, B.I.; Shafeev, M.A.; Sosnovskikh, V.Y. Synthesis and reactivity of 2-polyfluoroalkylchromene-4(4H)-thiones. Russ. Chem. Bull. 2004, 53, 2285–2292. [Google Scholar] [CrossRef]
- Ali, T.E.; Abdel-Aziz, S.A.; El-Edfawy, S.M.; Mohamed, E.A.; Abdel-Kariem, S.M. Cleavage of diethyl chromonyl α-aminophosponate with nitrogen and carbon nucleophiles: A synthetic approach and biological evaluations of a series of novel azoles, azines, and azepines containing α-aminophosphonate and phosphonate groups. Synth. Commun. 2014, 44, 3610–3629. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Elguero, J. Novel (E)- and (Z)-3(5)-(2-hydroxyphenyl)-4-styrylpyrazoles from (E)- and (Z)-3-styrylchromones: The unexpected case of (E)-3(5)-(2-hydroxyphenyl)-4-(4-nitrostyryl)pyrazoles. Tetrahedron Lett. 2007, 48, 3859–3862. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Elguero, J. Synthesis of (E)- and (Z)-3(5)-(2-hydroxyphenyl)-4-styrylpyrazoles. Monatsh. Chem. 2009, 140, 87–95. [Google Scholar] [CrossRef]
- Gabbutt, C.D.; Hargrove, T.F.L.; Heron, B.M.; Jones, D.; Poyner, C.; Yildiz, E.; Horton, P.N.; Hursthouse, M.B. Oxidation and ring cleavage reactions of 3-benzhydrylchromones. Generation of triarylmethine cations from methylidenechroman-4-ones and benzopyrano[4,3-c]pyrazoles. Tetrahedron 2006, 62, 10945–10953. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Irgashev, R.A.; Levchenko, A.A. Uncatalyzed addition of indoles and N-methylpyrrole to 3-formylchromones: Synthesis and some reactions of (chromon-3-yl)bis(indol-3-yl)methanes and E-2-hydroxy-3-(1-methylpyrrol-2-ylmethylene)chroman-4-ones. Tetrahedron 2008, 64, 6607–6614. [Google Scholar] [CrossRef]
- Abass, M.; Abdel-Megid, M.; Hassan, M. Substituted quinolinones, part 12: Heterocyclization reactions of 3-(3-chromonyl)acryloylquinolinone with some bifunctional nucleophiles. Synth. Commun. 2007, 37, 329–352. [Google Scholar] [CrossRef]
- Lévai, A.; Silva, A.M.S.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Alkorta, I.; Elguero, J.; Jekő, J. Synthesis of pyrazolyl-2-pyrazolines by treatment of 3-(3-aryl-3-oxopropenyl)chromen-4-ones with hydrazine and their oxidation to bis(pyrazoles). Eur. J. Org. Chem. 2004, 2004, 4672–4679. [Google Scholar] [CrossRef]
- Lévai, A. Synthesis of 2-pyrazolines by the reactions of α,β-unsaturated aldehydes, ketones, and esters with diazoalkanes, nitrile imines, and hydrazines. J. Heterocycl. Chem. 2002, 39, 1–13. [Google Scholar] [CrossRef]
- Shokol, T.V.; Lozinskii, O.A.; Tkachuk, T.M.; Khilya, V. 7-Hydroxy-3-phenoxy-8-formylchromones, analogs of natural flavonoids. Chem. Nat. Compd. 2009, 45, 350–355. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S.; Almeida, L.M.P.M.; Cavaleiro, J.A.S.; Elguero, J. 3-Aroyl-5-hydroxyflavones: Synthesis and transformation into aroylpyrazoles. Eur. J. Org. Chem. 2002, 2002, 3807–3815. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Irgashev, R.A.; Moshkin, V.S.; Kodess, M.I. Reaction of 3-(polyfluoroacyl)chromones with hydrazines: New regioselective synthesis of RF-containing pyrazoles. Russ. Chem. Bull. 2008, 57, 2146–2155. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S. Synthesis of 3-(2-benzyloxy-6-hydroxyphenyl)-1-methylpyrazoles by the reaction of chromones with methylhydrazine. J. Heterocycl. Chem. 2000, 37, 1629–1634. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Moshkin, V.S.; Kodess, M.I. On the reaction of 3-cyanochromones with phenyl- and methylhydrazines: Structural revision and a simple synthesis of chromeno[4,3-c]pyrazol-4-ones. J. Heterocycl. Chem. 2010, 47, 629–633. [Google Scholar] [CrossRef]
- Budzisz, E.; Malecka, M.; Nawrot, B. Synthesis and structure of highly substituted pyrazole ligands and their complexes with platinum(II) and palladium(II) metal ions. Tetrahedron 2004, 60, 1749–1759. [Google Scholar] [CrossRef]
- Małecka, M.; Massa, W.; Harms, K.; Budzisz, E. Molecular structure of 4-methoxy-2,3-dimethyl-2,4-dihydro[1,2]benzoxaphosphinino-[4,3-c]pyrazole-4-oxide and its carboxylic analogues. J. Mol. Struct. 2005, 737, 259–265. [Google Scholar] [CrossRef]
- Hassaine, R.; Talhi, O.; Taibi, N.; Paz, F.A.A.; Bensaid, O.; Bachari, K.; Silva, A.M.S. Actions of bisnucleophiles on (E)-3-[3-(2-hydroxyaryl)-3-oxoprop-1-en-1-yl]chromones: Versatile transformations into oxygen- and nitrogen-containing heterocycles. Synlett 2016, 27, 465–470. [Google Scholar] [CrossRef]
- Maurya, M.R.; Parhate, V.V.; Rajput, P.R. Synthesis and antibacterial activity of some new chlorosubstituted pyrazoles. Asian J. Chem. 2003, 15, 1843–1844. [Google Scholar]
- Mahale, J.D.; Manoja, S.C.; Belsare, N.G.; Rajput, P.R. Synthesis and study of chlorosubstituted 4-aroyl and 4-alkoyl-pyrazolines, pyrazoles and their effect on some flowering plants. Indian J. Chem. 2010, 49B, 505–511. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S.; Jekő, J.; Lévai, A. Synthesis and structure elucidation of novel pyrazolyl-2-pyrazolines obtained by the reaction of 3-(3-aryl-3-oxopropenyl)chromen-4-ones with phenylhydrazine. Arkivoc 2012, v, 265–281. [Google Scholar] [CrossRef]
- Jamode, V.S.; Kale, A.S. Synthesis and characterization of 1-isonicotinoyl/1-carboxamido/1-thiocarboxamido-3-(2-hydroxy-5-chlorophenyl)-4-aroyl-3-methyl pyrazoles. Asian J. Chem. 2006, 18, 3197–3200. [Google Scholar]
- Chate, A.V.; Joshi, R.S.; Mandhane, P.G.; Mohekar, S.R.; Gill, C.H. Synthesis and antimicrobial evaluation of new 5-(2-hydroxyphenyl)-3-(4-(phenylthio)phenyl)-1H-pyrazole-2(5H)-carbothioamide analogues of 2-(4-(phenylthio)phenyl)-4H-chromen-4-one. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 327–335. [Google Scholar] [CrossRef]
- Plaskon, A.S.; Grygorenko, O.O.; Ryabukhin, S.V. Recyclizations of 3-formylchromones with binucleophiles. Tetrahedron 2012, 68, 2743–2757. [Google Scholar] [CrossRef]
- Ghosh, C.K.; Patra, A. Chemistry and application of 4-oxo-4H-1-benzopyran-3-carboxaldehyde. J. Heterocycl. Chem. 2008, 45, 1529–1547. [Google Scholar] [CrossRef]
- Ulven, T.; Receveur, J.-M.; Grimstrup, M.; Rist, O.; Frimurer, T.M.; Gerlach, L.-O.; Mathiesen, J.M.; Kostenis, E.; Uller, L.; Hoegberg, T. Novel selective orally active CRTH2 antagonists for allergic inflammation developed from in silico derived hits. J. Med. Chem. 2006, 49, 6638–6641. [Google Scholar] [CrossRef] [PubMed]
- Kornev, M.Y.; Moshkin, V.S.; Eltsov, O.S.; Sosnovskikh, V.Y. Reactions of chromone-3-carboxylic acid and chromone-3-carboxamides with cyanoacetic acid hydrazide. Mendeleev Commun. 2015, 26, 72–74. [Google Scholar] [CrossRef]
- Gadakh, A.V.; Rindhe, S.S.; Pandit, C.; Karale, B.K. Synthesis and antimicrobial activity of novel fluorine containing 4-(substituted-2-hydroxybenzoyl)-1H-pyrazoles and pyrazolyl benzo[d]oxazoles. Bioorg. Med. Chem. Lett. 2010, 20, 5572–5576. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, E.; Urdaneta, N.; Gutierrez, K.J.; Herrera, J.C. In vitro cytotoxicity of hydrazones, pyrazoles, pyrazolo-pyrimidines, and pyrazolo-pyridine synthesized from 6-substituted 3-formylchromones. Z. Naturforsch. 2015, 70, 305–310. [Google Scholar] [CrossRef]
- Figarella, K.; Marsiccobetre, S.; Galindo-Castro, I.; Urdaneta, N.; Herrera, J.C.; Canudas, N.; Galarraga, E. Antileishmanial and antitrypanosomal activity of synthesized hydrazones, pyrazoles, pyrazolo[1,5-a]-pyrimidines and pyrazolo[3,4-b]-pyridine. Curr. Bioact. Compd. 2017, 13. [Google Scholar] [CrossRef]
- Akolkar, H.N.; Karale, B.K. Synthesis and characterization of novel thiazole anchored pyrazolyl benzoxazoles. Orient. J. Chem. 2015, 31, 431–433. [Google Scholar] [CrossRef]
- Ghosh, C.K.; Ghosh, C.; Karak, S.K.; Chakravarty, A.K. Benzopyrans. Part 45(1). Reactions of monobrominated 3-acetyl-2-methyl-1-benzopyran-4-one with some binucleophiles. J. Chem. Res. 2004, 2004, 84–86. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Silva, V.L.M.; Elguero, J.; Silva, A.M.S. (E)-3-Halo-2-styryl-4H-chromen-4-ones: Synthesis and transformation to novel pyrazoles. Tetrahedron 2013, 69, 9701–9709. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.T.; Dai, L.Y.; Du, Y.L.; Xue, D. Synthesis of novel disubstituted pyrazolo[1,5-a]pyrimidines, imidazo[1,2-a]pyrimidines, and pyrimido[1,2-a]benzimidazoles containing thioether and aryl moieties. Helv. Chim. Acta 2012, 95, 989–997. [Google Scholar] [CrossRef]
- Bharate, S.B.; Mahajan, T.R.; Gole, Y.R.; Nambiar, M.; Matan, T.T.; Kulkarni-Almeida, A.; Balachandran, S.; Junjappa, H.; Balakrishnan, A.; Vishwakarma, R.A. Synthesis and evaluation of pyrazolo[3,4-b]pyridines and its structural analogues as TNF-α and IL-6 inhibitors. Bioorg. Med. Chem. 2008, 16, 7167–7176. [Google Scholar] [CrossRef] [PubMed]
- Lácová, M.; Puchała, A.; Solčanyov, E.; Lac, J.; Koiš, P.; Chovancová, J.; Rasala, D. 3-Formylchromones IV. The rearrangement of 3-formylchromone enamines as a simple, facile route to novel pyrazolo[3,4-b]pyridines and the synthetic utility of the latter. Molecules 2005, 10, 809–821. [Google Scholar] [CrossRef]
- Stankovičová, H.; Gáplovský, A.; Lácová, M.; Chovancová, J.; Puchała, A. Transformation of 4-oxo-4H-[1]-benzopyran-3-carboxaldehydes into pyrazolo[3,4-b]pyridines. J. Heterocycl. Chem. 2006, 43, 843–848. [Google Scholar] [CrossRef]
- Quiroga, J.; Mejía, D.; Insuasty, B.; Abonía, R.; Nogueras, M.; Sánchez, A.; Cobo, J.; Low, J.N. Synthesis of 6-(2-hydroxybenzoyl)pyrazolo[1,5-a]pyrimidines by reaction of 5-amino-1H-pyrazoles and 3-formylchromone. J. Heterocycl. Chem., 2002, 39, 51–54. [Google Scholar] [CrossRef]
- Zimmerman, J.R.; Myers, B.J.; Bouhall, S.; McCarthy, A.; Johntony, O.; Manpadi, M. A two-step, single pot procedure for the synthesis of substituted dihydropyrazolo-pyrimidines. Tetrahedron Lett. 2014, 55, 936–940. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Ma, Y.-Q.; Liang, Y.; Xue, D.; He, Q. An efficient one-pot synthesis of diarylpyrazolo[1,5-a]pyrimidine from isoflavones. J. Heterocycl. Chem. 2011, 48, 279–285. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Zhang, Z.-T.; He, Q.; Xue, D.; Zhang, P.-F. Simple and efficient method for synthesis of 3-cyano-6,7-diarylpyrazolo[1,5-a]pyrimidine from isoflavones. Synth. Commun. 2012, 42, 933–941. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; He, Q. Facile synthesis of diarylpyrazolo[1,5-a]pyrimidine from isoflavones. J. Heterocycl. Chem. 2014, 51, 24–29. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, J.F.; Wang, T.; Wang, P.; Zhang, Z.T. Synthesis, crystal structure, characterization and antifungal activity of pyrazolo[1,5-a]pyrimidines derivatives. J. Mol. Struct. 2016, 1120, 228–233. [Google Scholar] [CrossRef]
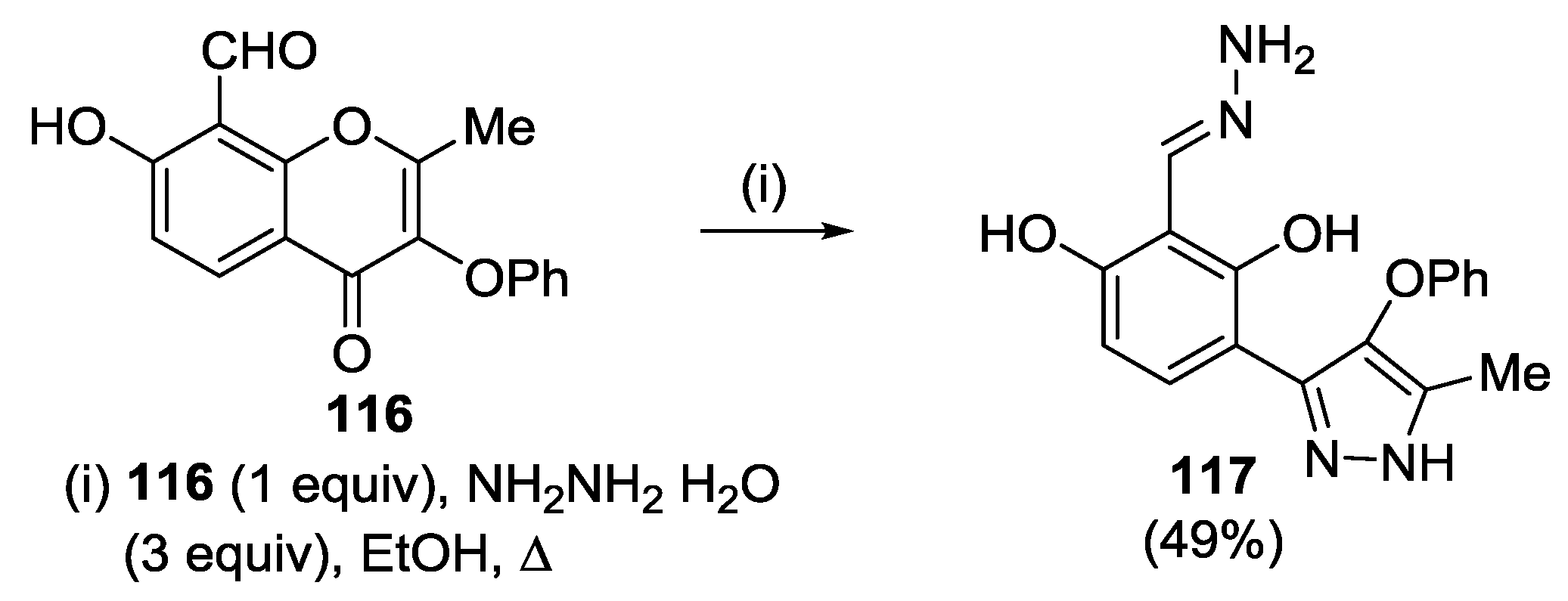



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.M.M.; Silva, V.L.M.; Silva, A.M.S. Synthesis of Chromone-Related Pyrazole Compounds. Molecules 2017, 22, 1665. https://doi.org/10.3390/molecules22101665
Santos CMM, Silva VLM, Silva AMS. Synthesis of Chromone-Related Pyrazole Compounds. Molecules. 2017; 22(10):1665. https://doi.org/10.3390/molecules22101665
Chicago/Turabian StyleSantos, Clementina M. M., Vera L. M. Silva, and Artur M. S. Silva. 2017. "Synthesis of Chromone-Related Pyrazole Compounds" Molecules 22, no. 10: 1665. https://doi.org/10.3390/molecules22101665





