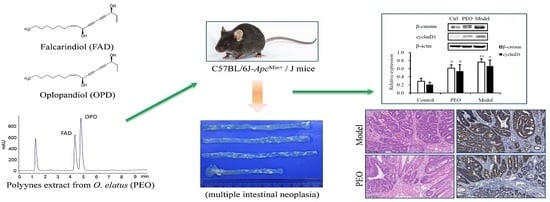
Polyyne-Enriched Extract from Oplopanax elatus Significantly Ameliorates the Progression of Colon Carcinogenesis in ApcMin/+ Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of the PEO in ApcMin/+ Mice
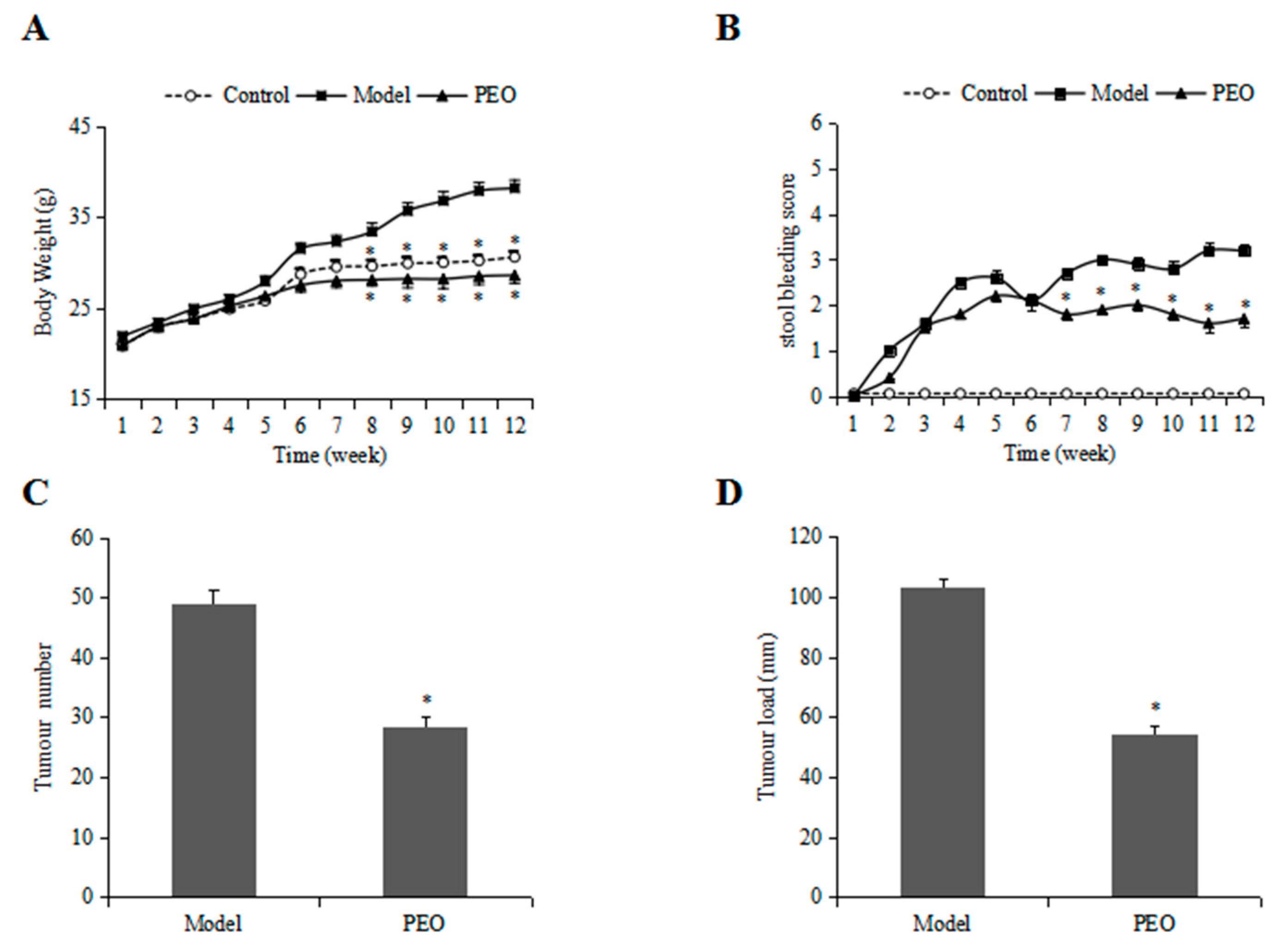
2.1.1. PEO Treatment Improved the Body Weight and Stool Bleeding in ApcMin/+ Mice
2.1.2. PEO Feeding Prevents Intestinal Tumorigenesis in ApcMin/+ Mice
2.1.3. PEO Decreased the Expression of β-Catenin and CyclinD1 in ApcMin/+ Mice
2.2. Effects of the PEO in HCT116 and SW480 Cells
2.2.1. Anti-Proliferative Effects of PEO in Colon Cancer Cells
2.2.2. PEO Decreased the Expression of β-Catenin, CyclinD1, C-myc and P-GSK-3β in HCT116 and SW480 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Material and Reagents
4.2. Preparation of PEO
4.3. Animal Studies
4.4. Western Blotting
4.5. Histology and Immunohistochemistry
4.6. Cell Culture and Treatment
4.7. MTT Assay
4.8. Q-PCR
4.9. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Dorudi, S.; Steele, R.J.; McArdle, C.S. Surgery for colorectal cancer. Br. Med. Bull. 2002, 64, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Choi, D.H. Radiation therapy for colorectal cancer. J. Korean Med. Assoc. 2010, 53, 592–602. [Google Scholar] [CrossRef]
- Moertel, C.G. Chemotherapy for colorectal cancer. N. Engl. J. Med. 1994, 330, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Koido, S.; Ohkusa, T.; Homma, S.; Namiki, Y.; Takakura, K.; Saito, K.; Ito, Z.; Kobayashi, H.; Kajihara, M.; Uchiyama, K.; et al. Immunotherapy for colorectal cancer. World J. Gastroenterol. 2013, 19, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Kopetz, S. Recent developments in the treatment of metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2017, 9, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.K.; Li, H.; Dong, C.L.; He, X.; Guo, C.R.; Zhang, C.F.; Yu, C.H.; Wang, C.Z.; Yuan, C.S. Palmatine from Mahonia bealei attenuates gut tumorigenesis in apcmin/+ mice via inhibition of inflammatory cytokines. Mol. Med. Rep. 2016, 14, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Hwang, H.S.; Lee, H.J.; Jung, S.Y.; Ji, S.J.; Oh, S.H.; Lee, Y.M. Distribution of vascular plants along the altitudinal gradient of Gyebangsan (mt.) in Korea. J. Asia Pac. Biodivers. 2014, 7, e40–e71. [Google Scholar] [CrossRef]
- Moon, H.K.; Kim, J.A.; Park, S.Y.; Kim, Y.W.; Kang, H.D. Somatic embryogenesis and plantlet formation from a rare and endangered tree species, Oplopanax elatus. J. Plant Biol. 2006, 49, 320–325. [Google Scholar] [CrossRef]
- Calway, T.; Du, G.J.; Wang, C.Z.; Huang, W.H.; Zhao, J.; Li, S.P.; Yuan, C.S. Chemical and pharmacological studies of Oplopanax horridus, a north American botanical. J. Nat. Med. 2012, 66, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Yang, W.Z.; Guo, D.A. Oplopanaxelatus elatus (nakai) nakai: Chemistry, traditional use and pharmacology. Chin. J. Nat. Med. 2014, 12, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, J.K.; Kim, H.; Hyun, T.K. De novo transcriptomic analysis to reveal functional genes involved in triterpenoid saponin biosynthesis in Oplopanax elatus nakai. J. Appl. Bot. Food Qual. 2017, 90, 18–27. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G. Aralia elata var. Mandshurica (rupr. & maxim.) j.Wen: An overview of pharmacological studies. Phytomedicine 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Zhang, Q.W.; Yuan, C.S.; Wang, C.Z.; Li, S.P.; Zhou, H.H. Chemical constituents of the plants from the genus Oplopanax. Chem. Biodivers. 2014, 11, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhang, Z.; Huang, W.H.; Du, G.J.; Wen, X.D.; Calway, T.; Yu, C.; Nass, R.; Zhao, J.; Du, W.; et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine 2013, 20, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.Z.; Huang, W.H.; Li, S.P.; He, T.C.; Yuan, C.S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; He, Y.S.; Xu, L.H.; Zhang, B.Y.; Qi, L.W.; Yang, J.; Li, P.; Wen, X.D. Pharmacokinetic profiles of falcarindiol and oplopandiol in rats after oral administration of polyynes extract of Oplopanax elatus. Chin. J. Nat. Med. 2016, 14, 714–720. [Google Scholar] [CrossRef]
- Cao, H.; Song, S.; Zhang, H.; Zhang, Y.; Qu, R.; Yang, B.; Jing, Y.; Hu, T.; Yan, F.; Wang, B. Chemopreventive effects of berberine on intestinal tumor development in Apcmin/+ mice. BMC Gastroenterol. 2013, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chang, J.G.; Liu, C.Y.; Yang, S.F.; Ho, C.M.; Chen, W.T.L.; Chang, Y.S. Mutation analysis of 13 driver genes of colorectal cancer-related pathways in Taiwanese patients. World J. Gastroenterol. 2016, 22, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Wang, K.Z. The effect of Echinopanax elatum Nakai on experimental arthritis and the neuro-hypophyseal-adrenal system. Acta Pharm. Sin. 1980, 2, 81–85. [Google Scholar] [CrossRef]
- Kown, H.S.; Kim, D.H.; Shin, H.K.; Yu, C.Y.; Kim, M.J.; Lim, J.D.; Park, J.K.; Kim, J.K. Fourteen-day repeated-dose oral toxicity study of the ethanol extracts isolated from Oplopanax elatus in Sprague-Dawley rat. Korean J. Food Sci. Technol. 2007, 39, 470–475. [Google Scholar]
- Rao, T.P.; Kuhl, M. An updated overview on Wnt signaling pathways: A prelude for more. Circ. Res. 2010, 106, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T. The Wnt/β-catenin pathway. Sci. Signal. 2003, 2003, 271. [Google Scholar] [CrossRef]
- Fodde, R. The Apc gene in colorectal cancer. Eur. J. Cancer 2002, 38, 867–871. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchezde, D.C.; Pradilla, D.A.; Cerrada, E.; Rodriguez, Y.M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of Falcarinol and related aliphatic C(17)-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Nie, M.K.; Chen, M.Y.; Wang, J.; Wang, C.Z.; Huang, W.H.; Yuan, C.S.; Zhou, H.H. Screening and identifying antioxidants from Oplopanax elatus using 2,2′-diphenyl-1-picrylhydrazyl with off-line two-dimensional HPLC coupled with diode array detection and tandem time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 4269–4280. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.D.; Wang, C.Z.; Yu, C.; Zhao, L.; Zhang, Z.; Matin, A.; Wang, Y.; Li, P.; Xiao, S.Y.; Du, W.; et al. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytother. Res. 2014, 28, 892–898. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound PEO is available from the authors. |




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; Sun, W.; Wang, C.; Zhang, L.; Li, P.; Wen, X.; Yang, J.; Yuan, C. Polyyne-Enriched Extract from Oplopanax elatus Significantly Ameliorates the Progression of Colon Carcinogenesis in ApcMin/+ Mice. Molecules 2017, 22, 1593. https://doi.org/10.3390/molecules22101593
Qiao X, Sun W, Wang C, Zhang L, Li P, Wen X, Yang J, Yuan C. Polyyne-Enriched Extract from Oplopanax elatus Significantly Ameliorates the Progression of Colon Carcinogenesis in ApcMin/+ Mice. Molecules. 2017; 22(10):1593. https://doi.org/10.3390/molecules22101593
Chicago/Turabian StyleQiao, Xin, Wei Sun, Chongzhi Wang, Li Zhang, Ping Li, Xiaodong Wen, Jie Yang, and Chunsu Yuan. 2017. "Polyyne-Enriched Extract from Oplopanax elatus Significantly Ameliorates the Progression of Colon Carcinogenesis in ApcMin/+ Mice" Molecules 22, no. 10: 1593. https://doi.org/10.3390/molecules22101593