One-Pot Multi-Enzymatic Synthesis of the Four Stereoisomers of 4-Methylheptan-3-ol
Abstract
:1. Introduction
2. Results
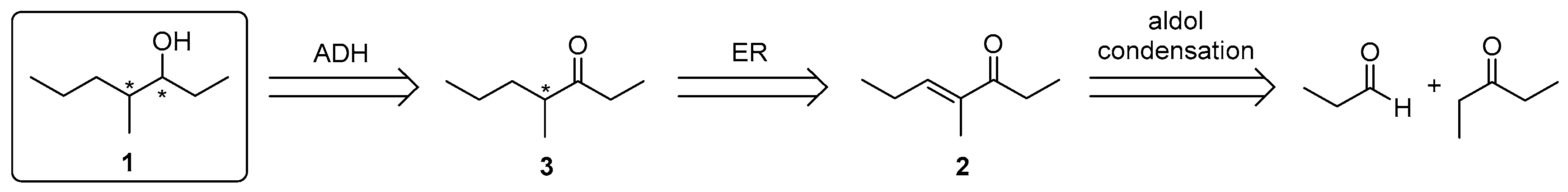
2.1. Known Synthetic Routes
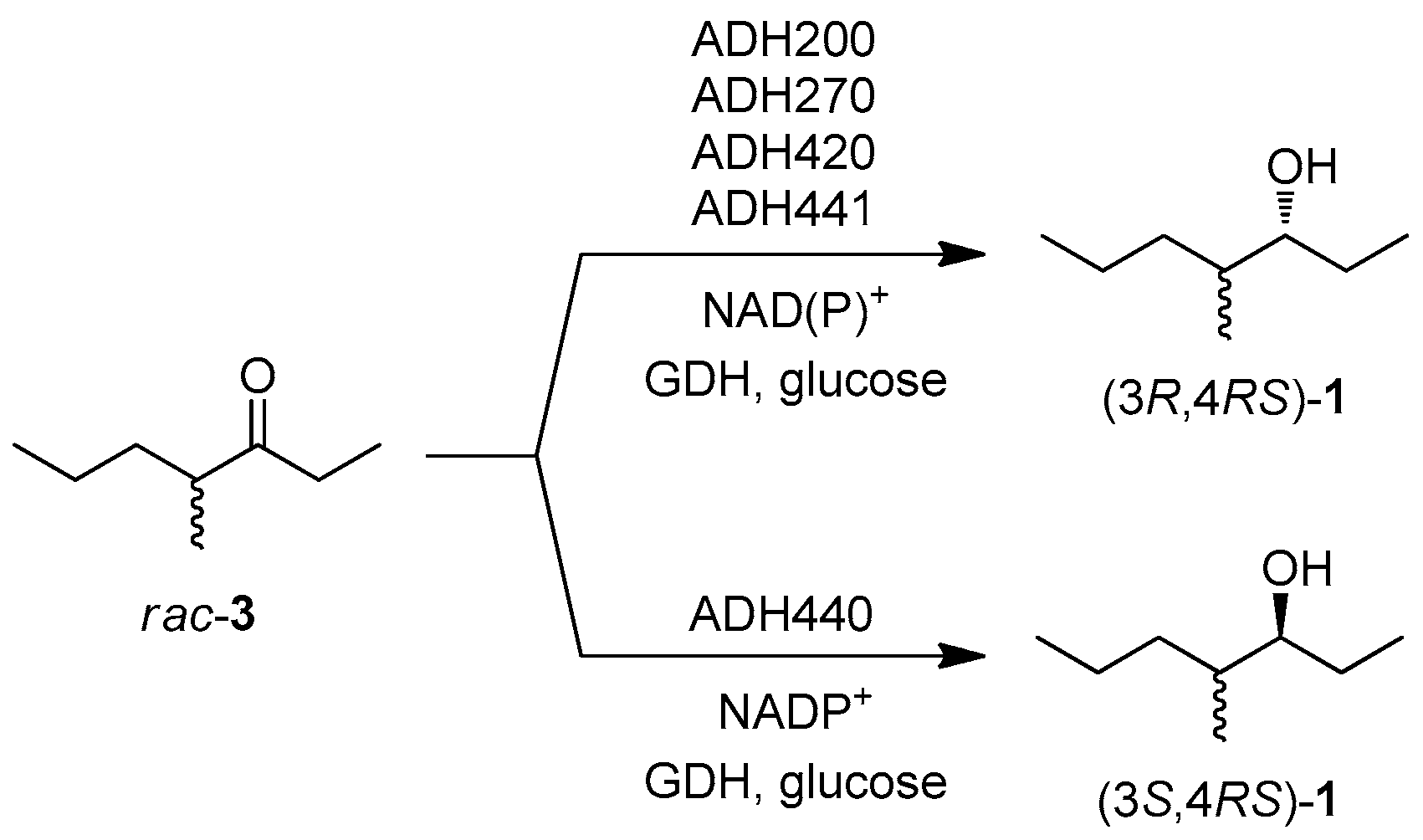
2.2. Selection of Ene-Reductases and Alcohol Dehydrogenases with the Desired Stereoselectivity
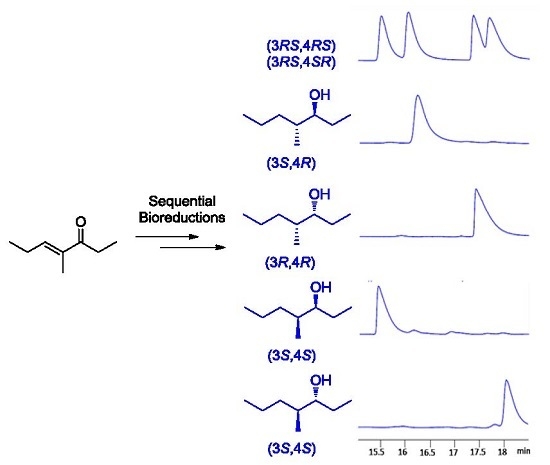
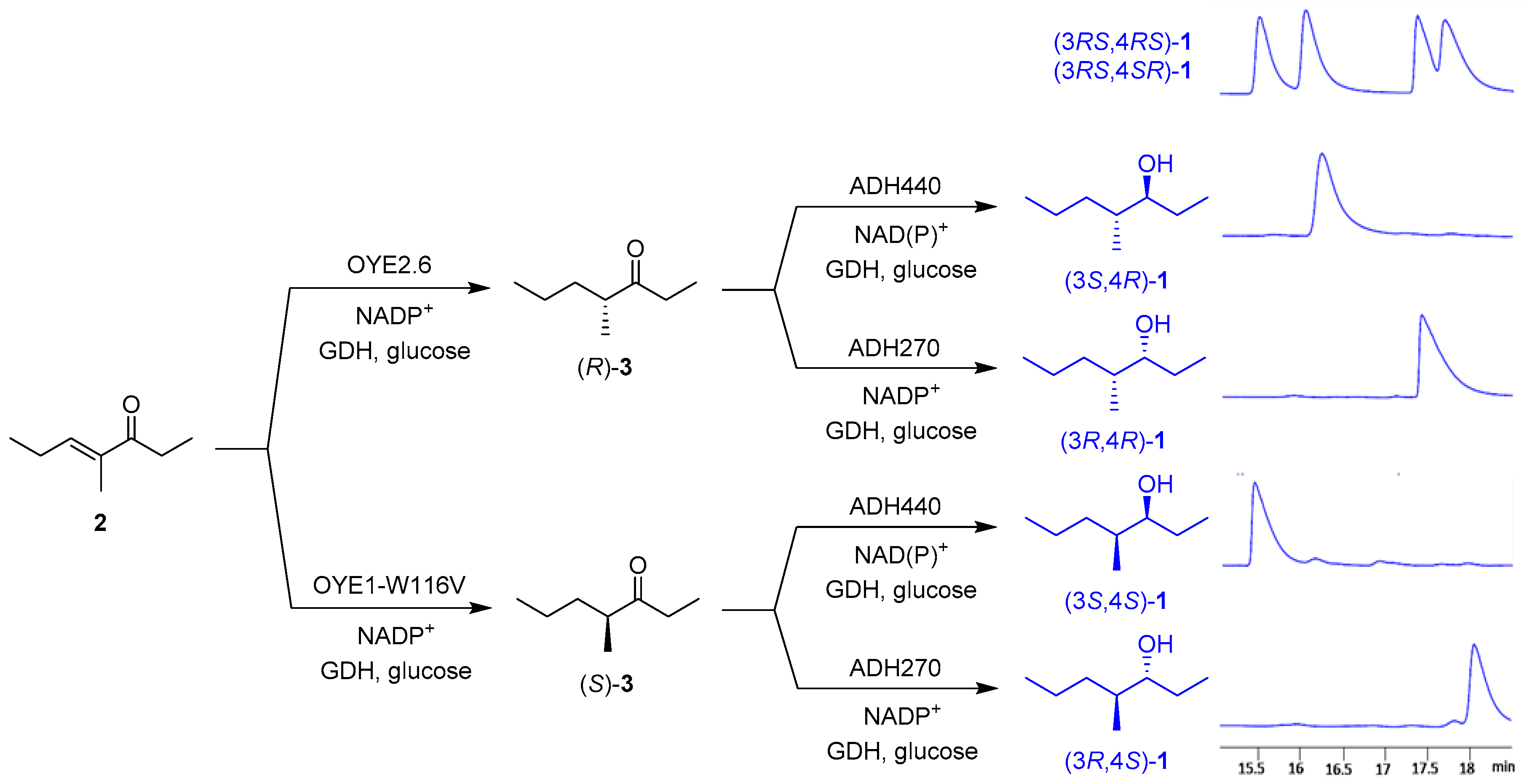
2.3. One-Pot Multi-Enzymatic Synthesis of the Four Stereoisomers of 4-Methylheptan-3-ol
3. Discussion
4. Materials and Methods
4.1. Strains and Enzymes
4.2. Overproduction of Enzymes in E. coli BL21(DE3)
4.3. One-Pot Conversion of 2 into the Four Stereoisomers of 1
4.3.1. (3R,4R)-4-Methylheptan-3-ol ((3R,4R)-1)
4.3.2. (3S,4R)-4-Methylheptan-3-ol ((3S,4R)-1)
4.3.3. (3R,4S)-4-Methylheptan-3-ol ((3R,4S)-1)
4.3.4. (3S,4S)-4-Methylheptan-3-ol ((3S,4S)-1)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.M.; Stringer, L.D.; Stephens, A.E.A.; Woods, B.; Williams, D.G.; Baker, G.; El-Sayed, A.M. From integrated pest management to integrated pest eradication: Technologies and future needs. Pest Manag. Sci. 2014, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Leskey, T.C.; Nielsen, A.L.; Rodriguez-Saona, C. Use of Pheromones in Insect Pest Management, with Special Attention to Weevil Pheromones. In Integrated Pest Management Current Concepts and Ecological Perspective, 1st ed.; Abrol, D.P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 141–168. [Google Scholar]
- Mori, K. Significance of chirality in pheromone science. Bioorg. Med. Chem. 2007, 15, 7505–7523. [Google Scholar] [CrossRef] [PubMed]
- Mori, K. Absolute configuration of (−)-4-methylheptan-3-ol, a pheromone of the smaller European elm bark beetle, as determined by the synthesis of its (3R,4R)-(+) and (3S,4R)-(+)-isomers. Tetrahedron 1977, 33, 289–294. [Google Scholar] [CrossRef]
- Blight, M.M.; Wadhams, L.J.; Wenham, M.J. The stereoisomeric composition of the 4-methyl-3-heptanol produced by Scolytus scolytus and the preparation and biological activity of the four synthetic stereoisomers. Insect Biochem. 1979, 9, 525–533. [Google Scholar] [CrossRef]
- Ben-Yehuda, S.; Tolasch, T.; Francke, W.; Gries, R.; Gries, G.; Dunkelblum, D.; Mendel, Z. Aggregation pheromone of the almond bark beetle Scolytus amygdali (Coleoptera: Scolytidae). IOBC WPRS Bull. 2002, 25. Available online: http://phero.net/iobc/samos/bulletin/ (accessed on 12 July 2017).
- Zada, A.; Ben-Yehuda, S.; Dunkelblum, E.; Harel, M.; Assael, F.; Mendel, Z. Synthesis and Biological Activity of the Four Stereoisomers of 4-Methyl-3-Heptanol: Main Component of the Aggregation Pheromone of Scolytus amygdali. J. Chem. Ecol. 2004, 30, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Attygale, A.B.; Vostrowsky, O.; Bestmann, H.J.; Steghaus-Kovac, S.; Maschwitz, U. (3S,4S)-4-Methyl-3-heptanol, the trail pheromone of the ant Leptogenys diminuta. Naturwissenschaften 1988, 75, 315–317. [Google Scholar] [CrossRef]
- Mori, K.; Iwasawa, H. Preparation of the both enantiomers of threo-2-amino-3-methylhexanoic acid by enzymatic. Resolution and their conversion to optically active forms of threo-4-methylheptan-3-ol, a pheromone component of the smaller european elm bark beetle. Tetrahedron 1980, 36, 2209–2213. [Google Scholar] [CrossRef]
- Matteson, D.S.; Sadhu, K.M. Boronic Ester Homologation with 99% Chiral Selectivity and Its Use in Syntheses of the Insect Pheromones (3S,4S)-4-Methyl-3-heptanol and exo-Brevicomin, J. Am. Chem. Soc. 1983, 105, 2077–2078. [Google Scholar] [CrossRef]
- Fujisawa, T.; Tajima, K.; Sato, T. Chirality transfer in the ester enolate Claisen rearrangement of (R)-1-methyl-(e)-2-butenyl hydroxyacetate and its application to the stereocontrolled pheromone synthesis. Chem. Lett. 1984, 1669–1672. [Google Scholar] [CrossRef]
- Sayo, N.; Azuma, K.-I.; Mikami, K.; Nakai, T. Acyclic stereocontrol via [2,3]-Wittig rearrangement with high enantio- and erythro-selectivity and its use in the chiral synthesis of insect pheromones. Tetrahedron Lett. 1984, 25, 565–568. [Google Scholar] [CrossRef]
- Fuganti, C.; Grasselli, P.; Servi, S.; Zirotti, C. Synthesis of the enantiomeric forms of cis and trans 1-benzyloxy-2,3-epoxy butane and of (3S,4S) 4-methyl-3-heptanol. Tetrahedron Lett. 1984, 23, 4269–4272. [Google Scholar] [CrossRef]
- Hoffmann, R.W.; Ladner, W.; Helbig, W. Stereoselective synthesis of alcohols, XVIII. Synthesis of (3S,4S)-4-methyl-3-heptanol and of (5S,6S)-anhydroserricornin. Liebigs Ann. Chem. 1984, 6, 1170–1179. [Google Scholar] [CrossRef]
- Oshima, M.; Yamazaki, H.; Shimizu, I.; Nisar, M.; Tsuji, J. Palladium-Catalyzed Selective Hydrogenolysis of Alkenyloxiranes with Formic Acid. Stereoselectivity and Synthetic Utility. J. Am. Chem. Soc. 1989, 111, 6280–6287. [Google Scholar] [CrossRef]
- Nakagawa, N.; Mori, K. Synthesis of (3S,4S)-4-methyl-3-heptanol and its (3S,4R)-isomer employing asymmetric epoxidation coupled with regioselective cleavage of epoxides with trimethylaluminum. Agric. Biol. Chem. 1984, 48, 2505–2510. [Google Scholar] [CrossRef]
- Tripathy, P.B.; Matteson, D.S. Asymmetric synthesis of the four stereoisomers of 4-methyl-3-heptanol via boronic esters: Sequential double stereodifferentiation leads to very high purity. Synthesis 1990, 200–206. [Google Scholar] [CrossRef]
- Unelius, C.R.; Sandell, J.; Orrenius, C. Enantioselective preparation of the stereoisomers of 4-methylheptan-3-ol using Candida antarctica lipase B. Collect. Czechoslov. Chem. Comun. 1998, 63, 525–533. [Google Scholar] [CrossRef]
- Růžička, J.; Koutek, B.; Streinz, L.; Šaman, D.; Lešetický, L. A new access to β-methyl substituted secondary alcohols. Application to the synthesis of 4-methylheptan-3-ol. Tetrahedron Asymmetry 1999, 10, 3521–3528. [Google Scholar] [CrossRef]
- Brenna, E.; Crotti, M.; Gatti, F.G.; Monti, D.; Parmeggiani, F.; Powell, R.W.; Santangelo, S.; Stewart, J.D. Opposite enantioselectivity in the bioreduction of (Z)-β-aryl-β-cyanoacrylates mediated by the tryptophan 116 mutants of Old Yellow Enzyme 1: Synthetic approach to (R)- and (S)-β-aryl-γ-lactams. Adv. Synth. Catal. 2015, 357, 1849–1860. [Google Scholar] [CrossRef]
- Brenna, E.; Gatti, F.G.; Manfredi, A.; Monti, D.; Parmeggiani, F. Old Yellow Enzyme-mediated reduction of β-cyano-α,β unsaturated esters for the synthesis of chiral building blocks: Stereochemical analysis of the reaction. Catal. Sci. Technol. 2013, 3, 1136–1146. [Google Scholar] [CrossRef]
- Powell, R.W.; Buteler, M.P.; Lenka, S.; Crotti, M.; Santangelo, S.; Burg, M.J.; Bruner, S.; Brenna, E.; Roitberg, A.E.; Stewart, J.D. Probing substrate interactions with Saccharomyces cerevisiae alkene reductase OYE3 using X-ray crystallography and computational methods. 2017; Submitted. [Google Scholar]
- Pompeu, Y.A.; Sullivan, B.; Stewart, J.D. X-ray Crystallography Reveals How Subtle Changes Control the Orientation of Substrate Binding in an Alkene Reductase. ACS Catal. 2013, 3, 2376–2390. [Google Scholar] [CrossRef]
- Riley, R.G.; Silverstein, R.M.; Moser, J.C. Biological Responses of Atta texana to Its Alarm Pheromone and the Enantiomer of the Pheromone. Science 1974, 183, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Williams, R.E.; Rathborne, D.A.; Scrutton, N.S.; Bruce, N.C. Biotransformation of Explosives by the Old Yellow Enzyme Family of Flavoproteins. Appl. Environ. Microbiol. 2004, 70, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Brenna, E.; Femmer, F.C.; Gatti, F.G.; Panke, S.; Parmeggiani, F.; Sacchetti, A. Biotechnological Development of a Practical Synthesis of Ethyl (S)-2-Ethoxy-3-(p-methoxyphenyl) propanoate (EEHP): Over 100-Fold Productivity Increase from Yeast Whole Cells to Recombinant Isolated Enzymes. Org. Process Res. Dev. 2012, 16, 269–276. [Google Scholar] [CrossRef]
- Turrini, N.G.; Cioc, R.C.; van der Niet, D.J.H.; Ruijter, E.; Orru, R.V.A.; Hall, M.; Faber, K. Biocatalytic access to nonracemic γ-oxo esters via stereoselective reduction using ene-reductases. Green Chem. 2017, 19, 511–518. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| ER | c (%) 2 | ee (%) 3 |
|---|---|---|
| OYE1 4 | 99 | 44, R |
| OYE3 4 | 99 | 78, S |
| OYE1-W116V 4 | 99 | 86, S |
| OYE2 | 99 | 40 R |
| OYE2.6 | 99 | 99, R |
| LeOPR1 | 99 | 70, R |
| YqjM | 99 | 60, S |
| PETN | 99 | 22, R |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenna, E.; Crotti, M.; Gatti, F.G.; Monti, D.; Parmeggiani, F.; Pugliese, A. One-Pot Multi-Enzymatic Synthesis of the Four Stereoisomers of 4-Methylheptan-3-ol. Molecules 2017, 22, 1591. https://doi.org/10.3390/molecules22101591
Brenna E, Crotti M, Gatti FG, Monti D, Parmeggiani F, Pugliese A. One-Pot Multi-Enzymatic Synthesis of the Four Stereoisomers of 4-Methylheptan-3-ol. Molecules. 2017; 22(10):1591. https://doi.org/10.3390/molecules22101591
Chicago/Turabian StyleBrenna, Elisabetta, Michele Crotti, Francesco G. Gatti, Daniela Monti, Fabio Parmeggiani, and Andrea Pugliese. 2017. "One-Pot Multi-Enzymatic Synthesis of the Four Stereoisomers of 4-Methylheptan-3-ol" Molecules 22, no. 10: 1591. https://doi.org/10.3390/molecules22101591