3. Experimental Section
3.1. General Information
Melting points were recorded on a Thermocouple digital melting point apparatus (Stuart, Staffordshire, UK) and are uncorrected. IR spectra were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA) with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, kieselgel 60 (0.063–0.200 mm) (Merck KGaA, Frankfurt, Germany) was used as the stationary phase. NMR spectra were obtained as DMSO-
d6 solutions using Varian 300 MHz (Varian Inc., Palo Alto, CA, USA) or Agilent 500 MHz NMR (Agilent Technologies, Oxford, UK) spectrometers and the chemical shifts were quoted relative to the TMS peak. Low- and high-resolution mass spectra were recorded using a Waters Synapt G2 Quadrupole Time-of-flight mass spectrometer (Waters Corp., Milford, MA, USA) at the University of Stellenbosch Mass Spectrometry Unit. The synthesis and analytical data of compound
1 have been described previously [
13].
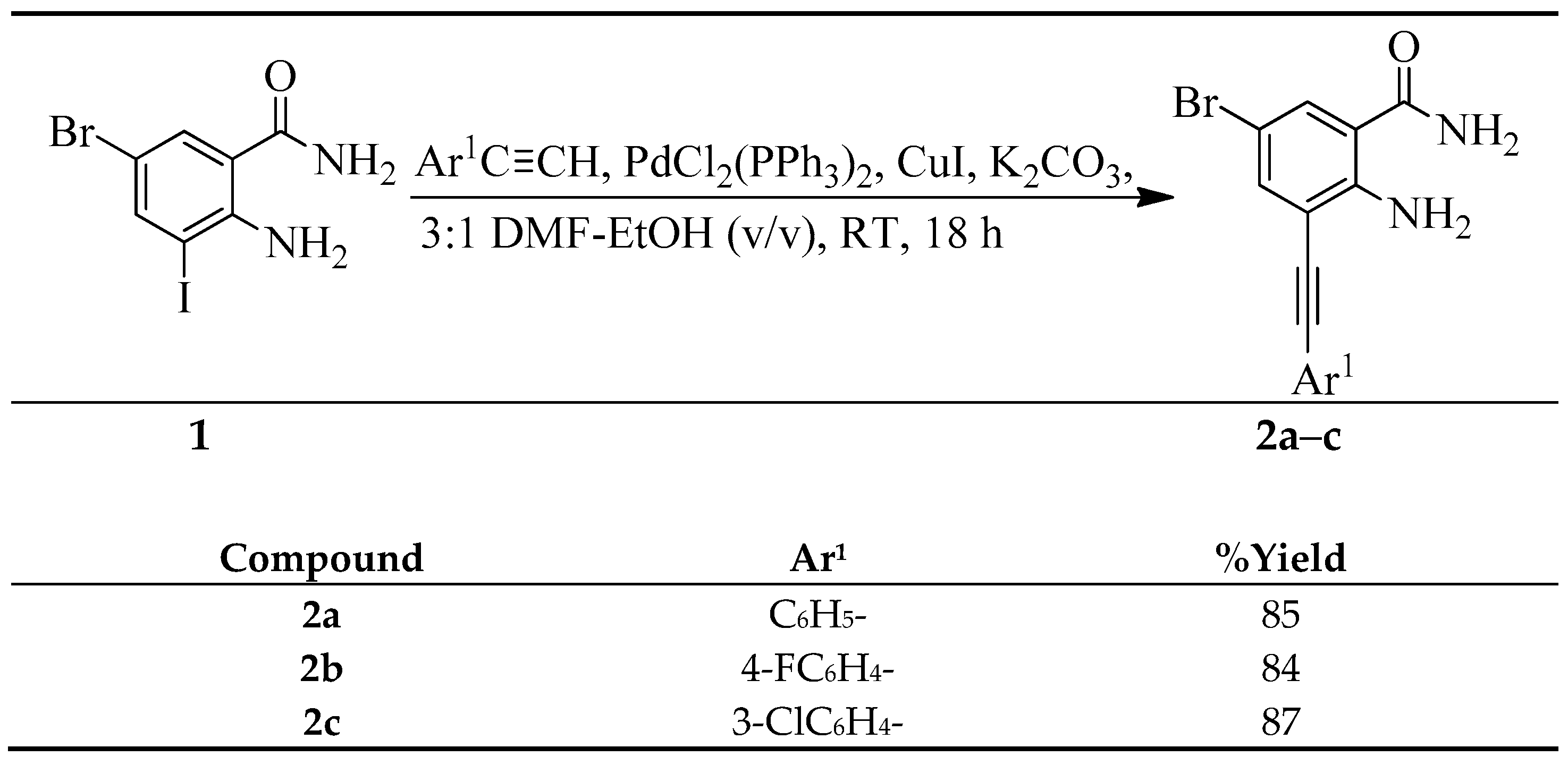
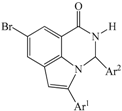
3.2. Typical Procedure for the Sonogashira Cross-Coupling of 1
A stirred mixture of 1 (1.00 g, 2.94 mmol), PdCl2(PPh3)2 (0.10 g, 0.15 mmol), CuI (0.06 g; 0.29 mmol) and K2CO3 (0.14 g, 1.66 mmol) in 3:1 DMF–EtOH (v/v, 20 mL) was purged with argon gas for 30 min. Phenyl acetylene (0.33 g, 3.22 mmol) was added via a syringe and the reaction mixture was stirred at RT for 18 h. The mixture was then quenched with ice-cold water and the product was extracted into chloroform. The combined organic layers were washed with water, dried over Na2SO4, filtered and then evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 2a. The following products were prepared in this fashion.
2-Amino-5-bromo-3-(phenylethynyl)benzamide (2a). Solid (1.00 g, 85%), Rf (7:3 hexane–EtOAc) 0.60, m.p. 181–182 °C; IR (ATR): 534, 632, 667, 758, 1239, 1399, 1545, 1594, 1647, 3171, 3368, 2477 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.68 (2H, br s, NH2), 6.46 (2H, s, -CONH2), 7.37–7.39 (3H, m, 3′,4′,5′-H), 7.47 (1H, d, J = 2.4 Hz, 5-H), 7.52–7.54 (2H, m, 2′,6′-H), 7.59 (1H, d, J = 2.4 Hz, 5-H); 13C-NMR (125 MHz, DMSO-d6) 83.6, 97.0, 105.9, 112.0, 114.8, 122.4, 128.4, 128.9, 130.7, 131.6, 137.8, 149.1, 169.8; HRMS (ES): MH+, found 314.0050. C15H11N2O79Br+ requires 314.0055.
2-Amino-5-bromo-3-(4-fluorophenylethynyl)benzamide (2b). Solid (0.81 g, 84%), Rf (7:3 hexane–EtOAc) 0.61, m.p. 187–188 °C; IR (ATR): 533, 835, 1155, 1232, 1451, 1506, 1599, 1648, 1664, 3197, 3299, 3347, 3429 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.68 (2H, br s, NH2), 6.44 (2H, s, CONH2), 7.06–7.09 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.47 (1H, d, J = 2.3 Hz, 5-H), 7.51 (2H, t, J = 8.7 Hz, 2′,6′-H), 7.57 (1H, d, J = 2.3 Hz, 5-H); 13C-NMR (125 MHz, DMSO-d6) 83.3, 95.8, 106.0, 111.8, 115.8 (d, 2JCF = 22.9 Hz), 118.5 (d, 4JCF = 3.7 Hz), 130.8, 133.5 (d, 2JCF = 8.4 Hz), 137.8, 149.1, 162.8 (d, 1JCF = 251.1 Hz), 169.8; HRMS (ES): MH+, found 331.9843. C15H10N2OF79Br+ requires 331.9882.
2-Amino-5-bromo-3-(3-chlorophenylethynyl)benzamide (2c). Solid (0.89 g, 87%), Rf (7:3 hexane–EtOAc) 0.63, m.p. 206–207 °C; IR (ATR): 554, 782, 876, 1092, 1243, 1402, 1558, 1601, 1648, 3298, 3347, 3428, 3452 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.92 (2H, s, -CONH2), 7.27 (1H, br s, NH), 7.31 (1H, d, J = 7.5 Hz, 4′-H), 7.32 (1H, dd, J = 1.5 and 7.5 Hz, 5′-H), 7.43 (1H, d, J = 2.5 Hz, 2′-H), 7.44 (1H, dd, J = 1.5 and 7.5 Hz, 6′-H), 7.64 (1H, d, J = 2.5 Hz, 6-H), 7.65 (1H, d, J = 2.5 Hz, 6-H), 7.88 (1H, br s, NH); 13C-NMR (125 MHz, DMSO-d6) 86.2, 94.6, 104.6, 109.8, 115.9, 124.5, 129.0, 130.3, 130.7, 131.2, 132.4, 133.4, 137.1, 149.8, 169.7; HRMS (ES): MH+, found 347.9652. C15H10N2O35Cl79Br+ requires 347.9665.
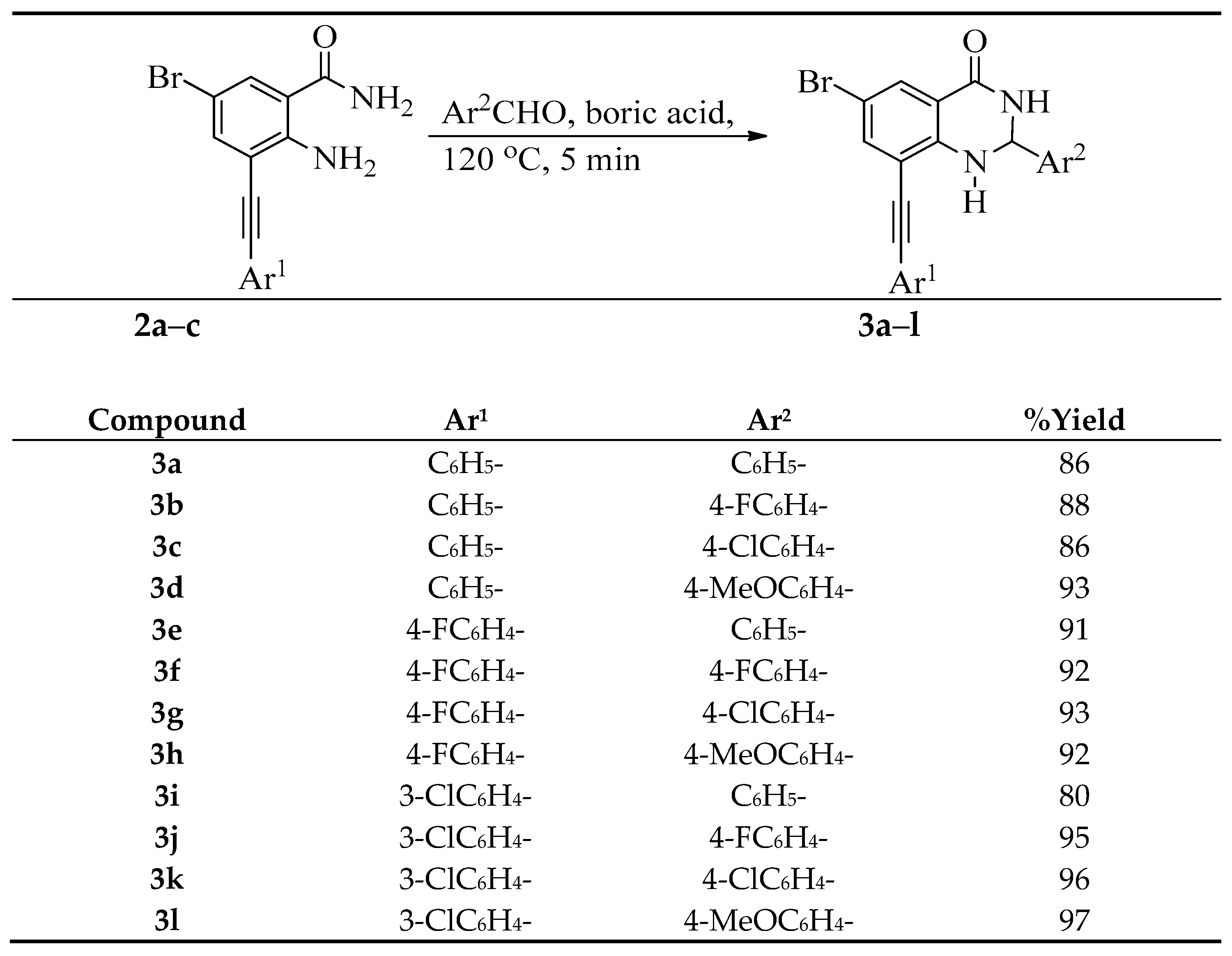
3.3. Typical Procedure for the Cyclocondensation of 2a–c with Benzaldehyde Derivatives
A mixture of 2 (0.25 g, 7.93 mmol), benzaldehyde (1.68 g, 15.86 mmol) and boric acid (0.01 g, 1.58 mmol) was finely grounded and transferred into a round bottomed flask and then heated at 120 °C for 5 min. The resultant precipitate was washed thoroughly with cold water and recrystallized from ethanol to afford 3 as a solid. The following products were prepared in this fashion.
6-Bromo-2-phenyl-8-(phenylethynyl)-2,3-dihydroquinazolin-4(1H)-one (3a). Solid (1.10 g, 86%), m.p. 226–227 °C (EtOH); IR (ATR) 491, 697, 785, 833, 1238, 1485, 1508, 1592, 1683, 3175, 3382 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.84 (1H, t, J = 3.0 Hz, 2-H), 7.36 (1H, d, J = 2.0 Hz, 1-NH), 7.42–7.46 (8H, m, ArH), 7.63–7.65 (2H, m, ArH), 7.66 (1H, d, J = 2.5 Hz, 7-H), 7.68 (1H, d, J = 2.5 Hz, 5-H), 8.84 (1H, d, J = 2.5 Hz, 2-NH); 13C-NMR (125 MHz, DMSO-d6) 65.0, 83.8, 96.7, 107.9, 110.2, 117.0, 122.4, 128.4, 129.0, 129.1, 129.6, 130.7, 132.2, 133.3, 138.4, 141.9, 146.6, 161.6; HRMS (ES): MH+, found 403.0457. C22H16N2O79Br+ requires 403.0446.
6-Bromo-2-(4-fluorophenyl)-8-(phenylethynyl)-2,3-dihydroquinazolin-4(1H)-one (3b). Solid (1.18 g, 88%), m.p. 235–237 °C (EtOH); IR (ATR): 557, 685, 753, 1230, 1488, 1589, 1681, 3372, 3477 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.85 (1H, t, J = 3.0 Hz, 2-H), 7.21 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.34 (1H, d, J = 2.0 Hz, 1-NH), 7.43–7.47 (5H, m, ArH), 7.64 (2H, t, J = 8.7 Hz, 2′,6′-H), 7.66 (1H, d, J = 2.5 Hz, 5-H), 7.69 (1H, d, J = 2.5 Hz, 5-H), 8.84 (1H, d, J = 2.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.0, 83.8, 96.7, 107.8, 110.2, 115.4 (d, 2JCF = 20.6 Hz), 117.1, 122.4, 128.5 (d, 3JCF = 8.6 Hz), 129.1, 129.6, 130.7, 132.2, 138.8 (d, 4JCF = 3.0 Hz), 139.1, 146.7, 161.6, 162.4 (d, 1JCF = 243.8 Hz); HRMS (ES): MH+, found 421.0342. C22H14N2OF79Br+ requires 421.0352.
6-Bromo-2-(4-chlorophenyl)-8-(phenylethynyl)-2,3-dihydroquinazolin-4(1H)-one (3c). Solid (1.20 g, 86%), m.p. 231–232 °C (EtOH); IR (ATR): 475, 532, 685, 752, 1279, 1382, 1486, 1587, 1682, 3176, 3380 cm−1; 13C-NMR (500 MHz, DMSO-d6) 5.84 (1H, t, J = 3.0 Hz, 2-H), 7.28 (1H, d, J = 2.0 Hz, 1-NH), 7.37 (2H, d, J = 7.5 Hz, 3′,5′-H), 7.38 (2H, d, J = 7.5 Hz, 2′,6′-H), 7.41–7.44 (3H, m, ArH), 7.64–7.65 (2H, m, ArH), 7.66 (1H, d, J = 2.5 Hz, 7-H), 7.69 (1H, d, J = 2.5 Hz, 5-H), 8.84 (1H, d, J = 3.0 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.5, 83.9, 96.7, 107.6, 110.1, 117.1, 122.4, 126.3, 128.6, 128.9, 129.1, 129.5, 130.7, 132.1, 138.3, 143.1, 146.7, 161.7; HRMS (ES): MH+, found 437.0053. C22H15N2O35Cl79Br+ requires 437.0053.
6-Bromo-2-(4-methoxyphenyl)-8-(phenylethynyl)-2,3-dihydroquinazolin-4(1H)-one (3d). Solid (1.31 g, 93%), m.p. 254–256 °C (EtOH); IR (ATR): 534, 686, 751, 1040, 1241, 1378, 1516, 1586, 1680, 3176, 3382 cm−1; 1H-NMR (500 MHz, DMSO-d6) 3.71 (3H, s, OCH3), 5.79 (1H, t, J = 3.5 Hz, 2-H), 6.91 (2H, d, J = 8.5 Hz, 3′,5′-H), 7.24 (1H, d, J = 2.0 Hz, 7-H), 7.24 (2H, d, J = 8.5 Hz, 2′,6′-H), 7.42–7.45 (3H, m, ArH), 7.63–7.65 (3H, m, 1-NH and ArH), 7.67 (1H, d, J = 2.0 Hz, 5-H), 8.76 (1H, d, J = 3.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 55.6, 65.2, 83.9, 96.6, 107.6, 110.1, 114.3, 117.2, 122.4, 127.6, 129.1, 129.5, 130.6, 132.1, 134.9, 138.3, 146.8, 159.6, 161.8; HRMS (ES): MH+, found 433.0553. C23H1879BrN2O2+ requires 433.0553.
6-Bromo-8-((4-fluorophenyl)ethynyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3e). Solid (1.15 g, 91%), m.p. 313 °C (EtOH); IR (ATR): 538, 686, 751, 1017, 1090, 1377, 1483, 1589, 1679, 3182, 3379 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.84 (1H, t, J = 3.5 Hz, 2-H), 7.30 (2H, t, J = Hz, 3′,5′-H), 7.31 (1H, d, J = 2.0 Hz, 1-NH), 7.35–7.38 (3H, m, ArH), 7.41 (2H, d, J = 7.0 Hz, ArH), 7.64 (1H, d, J = 2.5 Hz, 7-H), 7.68 (1H, d, J = 2.5 Hz, 5-H), 7.71 (2H, t, J = 8.5 Hz, 2′,6′-H), 8.84 (1H, d, J = 3.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.5, 83.6, 95.6, 107.6, 109.9, 115.3 (d, 2JCF = 21.7 Hz), 117.1, 118.9 (d, 4JCF = 3.3 Hz), 126.3, 128.6, 128.9, 130.7, 134.5 (d, 3JCF = 8.5 Hz), 138.3, 143.0, 146.8, 161.7, 162.7 (d, 1JCF = 246.0 Hz); HRMS (ES): MH+, found 421.0349. C22H15N2OF79Br+ requires 421.0352.
6-Bromo-2-(4-fluorophenyl)-8-((4-fluorophenyl)ethynyl)-2,3-dihydroquinazolin-4(1H)-one (3f). Solid (1.22 g, 92%), m.p. 286–287 °C (EtOH); IR (ATR): 491, 765, 783, 832, 1158, 1235, 1490, 1509, 1590, 1682, 3179, 3374 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.85 (1H, t, J = 3.5 Hz, 2-H), 7.31 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.39 (1H, d, J = 3.0 Hz, 1-NH), 7.44 (2H, t, J = 8.5 Hz, 2′,6′-H), 7.45 (2H, t, J = 8.5 Hz, 3″,5″-H), 7.66 (1H, d, J = 2.0 Hz, 7-H), 7.69 (1H, d, J = 2.0 Hz, 5-H), 7.71 (2H, t, J = 8.5 Hz, 2″,6″-H), 8.84 (1H, d, J = 3.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.0, 83,6, 95.6, 107.8, 110.1, 115.7 (d, 2JCF = 21.0 Hz), 116.3 (d, 2JCF = 22.7 Hz), 117.1, 118.9 (d, 4JCF = 3.8 Hz), 128.5 (d, 3JCF = 8.6 Hz), 130.7, 134.5 (d, 3JCF = 8.5 Hz), 138.4, 139.1 (d, 4JCF = 2.9 Hz), 146.7, 161.6, 162.4 (d, 1JCF = 244.5 Hz), 162.7 (d, 1JCF = 246.5 Hz); HRMS (ES): MH+, found 439.0241. C22H13N2OF279Br+ requires 439.0258.
6-Bromo-2-(4-chlorophenyl)-8-((4-fluorophenyl)ethynyl)-2,3-dihydroquinazolin-4(1H)-one (3g). Solid (1.28 g, 93%), m.p. 275–276 °C (EtOH); IR (ATR): 496, 329, 1091, 1228, 1379, 1483, 1507, 1588, 1681, 3172, 3379 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.85 (1H, t, J = 2.5 Hz, 2-H), 7.31 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.39 (1H, d, J = 2.0 Hz, 1-NH), 7.43 (2H, d, J = 8.5 Hz, 3″,5″-H), 7.45 (2H, d, J = 8.5 Hz, 2″,6″-H), 7.66 (1H, d, J = 2.5 Hz, 7-H), 7.69 (1H, d, J = 2.5 Hz, 5-H) 7.71 (2H, d, J = 8.5 Hz, 2′,6′-H), 8.84 (1H, d, J = 2.5 Hz, 3-NH); 13C NMR (125 MHz, DMSO-d6) 65.0, 83,6. 95.7, 107.8, 110.1, 116.3 (d, 2JCF = 21.9 Hz), 117.1, 118.9 (d, 4JCF = 3.5 Hz), 128.4, 129.0, 130.7, 133.3, 134.5 (d, 3JCF = 8.5 Hz), 138.4, 141.9, 146.6, 161.6, 162.7 (d, 1JCF = 248.6 Hz); HRMS (ES): MH+, found 455.9967. C22H14N2OF35Cl79Br+ requires 455.9962.
6-Bromo-8-((4-fluorophenyl)ethynyl)-2-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (3h). Solid (1.24 g, 92%), m.p. 215–216 °C (EtOH); νmax (ATR) 443, 648, 781, 883, 1041, 1178, 1489, 1586, 1684, 3179, 3390 cm−1; 1H-NMR (500 MHz, DMSO-d6) 3.69 (3H, s, OCH3), 5.77 (1H, t, J = 3.0 Hz, 2-H), 6.89 (2H, d, J = 8.5 Hz, 3″,5″-H), 7.27 (1H, d, J = 2.2 Hz, 1-NH), 7.28 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.61 (2H, d, J = 8.5 Hz, 2″,6″-H), 7.62 (1H, d, J = 3.0 Hz, 8-H), 7.66 (1H, d, J = 3.0 Hz, 5-H), 7.69 (2H, t, J = 8.7 Hz, 2′,6′-H), 8.75 (1H, d, J = 3.0 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 55.6, 65.1, 83.7, 95.5, 107.6, 109.9, 114.2, 116.3 (d, 2JCF = 21.8 Hz), 117.2, 118.9 (d, 4JCF = 3.6 Hz), 127.6, 130.7, 134. 4, 134.4 (d, 3JCF = 8.5 Hz), 134.9, 138.2, 146.8, 159.6, 161.7 (d, 1JCF = 246.6 Hz); HRMS (ES): MH+, found 451.0457. C23H17N2O2F79Br+ requires 451.0457.
6-Bromo-8-((3-chlorophenyl)ethynyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3i). Solid (1.00 g, 80%); m.p. 218–219 °C (EtOH); IR (ATR): 678, 780, 873, 1289, 1487, 1592, 1682, 3172, 3379 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.84 (1H, t, J = 2.5 Hz, 2-H), 7.20 (2H, t, J = 8.5 Hz, ArH), 7.42–7.50 (6H, m, ArH), 7.58 (1H, dd, J = 2.5 and 7.5 Hz, 5′-H), 7.67 (1H, d, J = 2.0 Hz, 7-H), 7.68 (1H, d, J = 2.0 Hz, 5-H), 7.73 (1H, s, 1-NH), 8.84 (1H, d, J = 2.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.5, 85.2, 95.0, 107.6, 109.5, 117.1, 124.5, 126.3, 128.7, 129.0, 129.5, 130.7, 130.9, 131.0, 131.5, 133.6, 138.5, 143.0, 146.9, 161.6; HRMS (ES): MH+, found 437.0049. C22H14N2O35Cl79Br+ requires 437.0057.
6-Bromo-8-((3-chlorophenyl)ethynyl)-2-(4-fluorophenyl)-2,3-dihydroquinazolin-4(1H)-one (3j). Solid (1.23 g, 95%), m.p. 211–212 °C (EtOH); IR (ATR): 529, 752, 1229, 1381, 1486, 1589, 1681, 3179, 3375 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.83 (1H, t, J = 2.5 Hz, 2-H), 7.20 (2H, t, J = 8.7 Hz, 3″,5″-H), (1H, d, J = 2.5 Hz, -NH), 7.41–7.44 (3H, m, ArH), 7.63 (2H, t, J = 8.7 Hz, 2″,6″-H), 7.66–7.68 (2H, m, ArH), 7.90 (1H, d, J = 2.0 Hz, 5-H), 8.81 (1H, d, J = 2.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 65.0, 83.8, 96.7, 107.8, 110.2, 115.6 (d, 2JCF = 21.9 Hz), 117.0, 122.4, 128.4, 128.6 (d, 3JCF = 8.5 Hz), 129.1, 129.4, 129.6, 130.7, 132.1, 138.4, 139.1 (d, 4JCF = 2.9 Hz), 146.6, 161.6, 162.3 (d, 1JCF = 242.8 Hz); HRMS (ES): MH+, found 454.9970. C22H13N2OF35Cl79Br+ requires 454.9962.
6-Bromo-2-(4-chlorophenyl)-8-((3-chlorophenyl)ethynyl)-2,3-dihydroquinazolin-4(1H)-one (3k). Solid (1.30 g, 96%), m.p. 214–215 °C (EtOH); IR (ATR): 540, 676, 777, 1089, 1379, 1484, 1589, 1684, 3175, 3373 cm−1; 1H-NMR (500 MHz, DMSO-d6) 5.84 (1H, t, J = 2.5 Hz, 2-H), 7.40–7.50 (7H, m, ArH), 7.58 (1H, dd, J = 2.5 and 7.5 Hz, 5′-H), 7.67 (1H, d, J = 2.5 Hz, 7-H), 7.68 (1H, d, J = 2.5 Hz, 5-H), 7.77 (1H, s, 1-NH), 8.86 (1H, d, J = 2.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 64.9, 85.1, 95.1, 107.8, 109.6, 117.1, 124.4, 128.3, 129.0, 129.6, 130.7, 131.0, 131.1, 131.5, 133.3, 133.6, 138.6, 141.8, 146.7, 161.5; HRMS (ES): MH+, found 470.9651. C22H14N2O35Cl279Br+ requires 470.9667.
6-Bromo-8-((3-chlorophenyl)ethynyl)-2-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (3l). Solid (1.30 g, 97%), m.p. 200–201 °C (EtOH); IR (ATR): 499, 826, 1037, 1230, 1253, 1384, 1484, 1508, 1588, 1685, 3177, 3379 cm−1; 1H-NMR (500 MHz, DMSO-d6) 3.70 (3H, s, OCH3), 5.78 (1H, t, J = 3.0 Hz, 2-H), 6.91 (2H, d, J = 8.5 Hz, 3″,5″-H), 7.31 (2H, d, J = 8.5 Hz, 2″,6″-H), 7.35 (1H, d, J = 2.0 Hz, 1-NH), 7.45 (1H, d, J = 7.5 Hz, 4′-H), 7.48 (1H, d, J = 6.5 Hz, 2′-H), 7.81 (1H, dd, J = 2.0 and 7.5 Hz, 5′-H), 7.58 (1H, d, J = 7.5 Hz, 6′-H), 7.65 (1H, d, J = 2.5 Hz, 7-H), 7.78 (1H, d, J = 2.5 Hz, 5-H), 8.79 (1H, d, J = 3.0 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 55.6, 65.2, 85.3, 95.0, 107.5, 109.4, 114.3, 117.2, 124.5, 127.6, 129.5, 130.7, 130.9, 131.0, 131.5, 133.6, 134.9, 138.5, 146.9, 159.6, 161.6; HRMS (ES): MH+, found 467.0160. C23H16N2O235Cl79Br+ requires 467.0162.
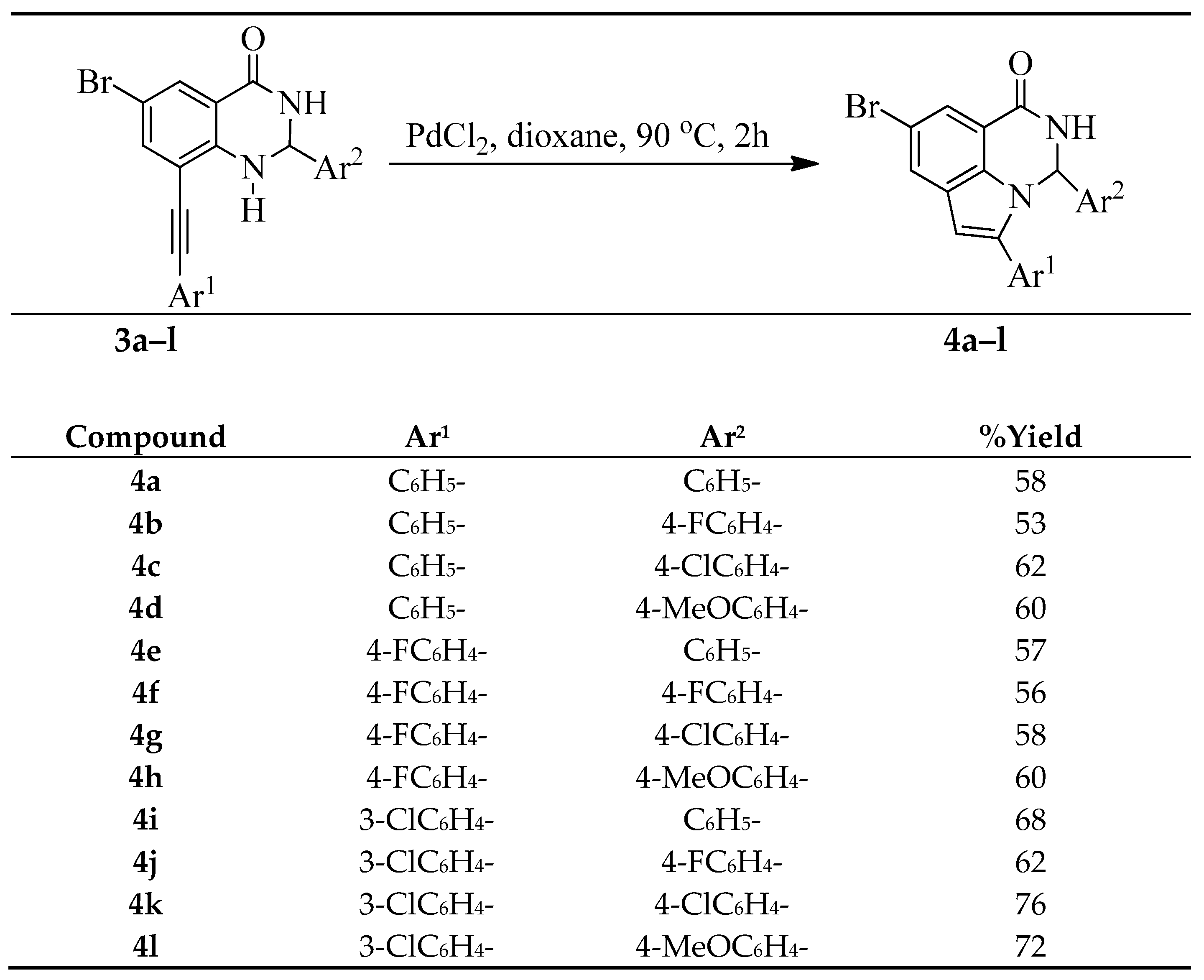
3.4. Typical Procedure for the PdCl2-Mediated Heteroannulation of 3a–l to Afford 4a–l
A stirred mixture of 3a (0.50 g, 1.24 mmol) and PdCl2 (0.03 g, 0.25 mmol) in dioxane (20 mL) was heated at 90 °C for 2 h. The mixture was allowed to cool to room temperature and then quenched with an ice-cold water. The precipitate was filtered and dissolved in chloroform. The organic layer was washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 4a. The following products were prepared in this fashion:
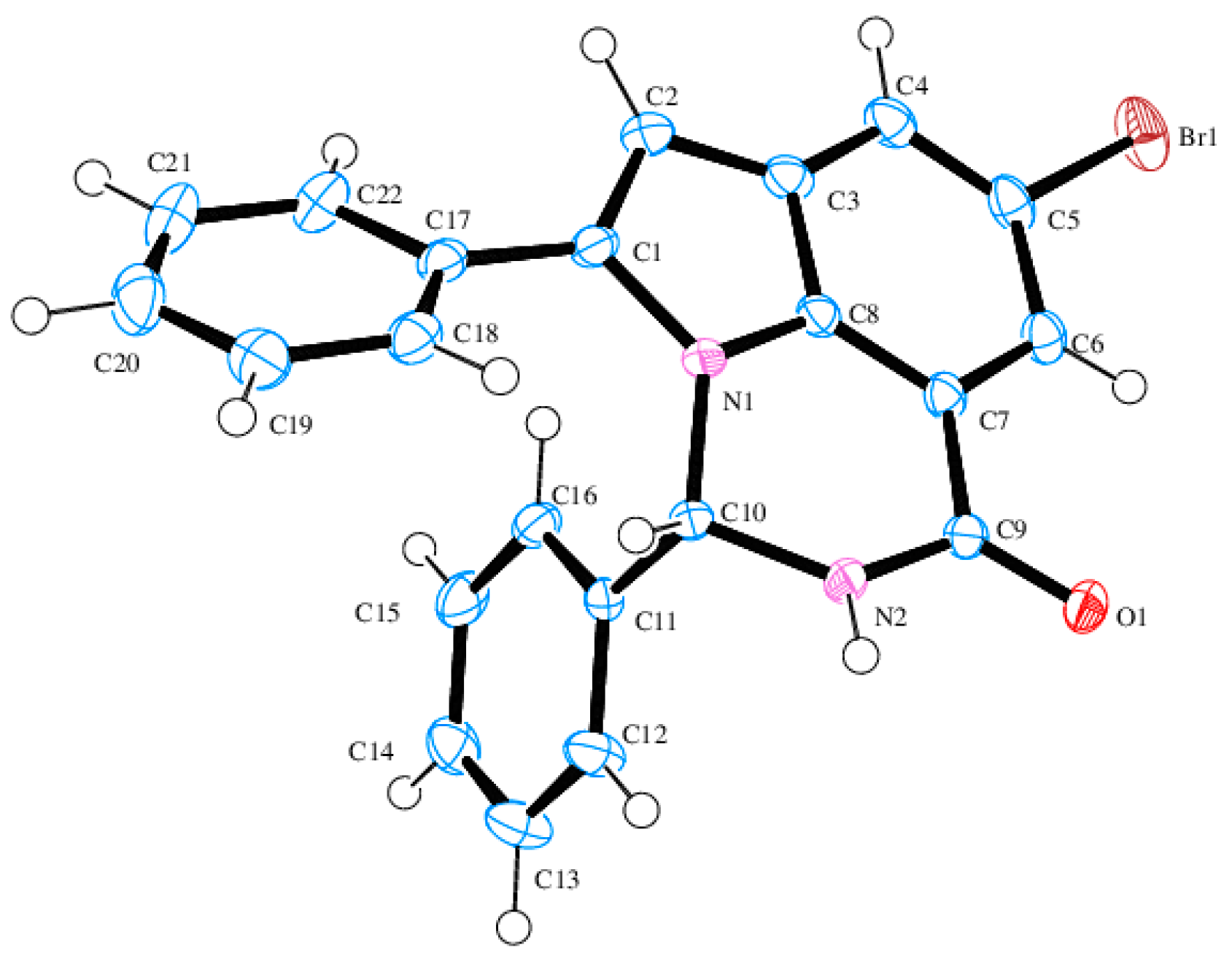
8-Bromo-3,5-diphenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4a). Solid (0.29 g, 58%), Rf (8:2 hexane–EtOAc) 0.21, m.p. 186–187 °C; IR (ATR): 527, 694, 755.0, 1276, 1456, 1647, 3074, 3185 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.53 (2H, d, J = 7.0 Hz, ArH), 6.77 (1H, s, 6-H), 7.05 (1H, d, J = 3.5 Hz, 3-H), 7.06–7.20 (3H, m, ArH), 7.34–7.42 (3H, m, ArH), 7.56 (2H, d, J = 7.0 Hz, ArH), 7.60 (1H, d, J = 1.5 Hz, 7-H), 8.00 (1H, d, J = 1.5 Hz, 9-H), 9.16 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 70.2, 104.1, 113.7, 115.6, 121.5, 125.2, 127.2, 128.4, 128.5, 129.1, 129.2, 129.3, 129.4, 131.1, 137.3, 141.3, 142.6, 160.3; HRMS (ES): MH+, found 403.0457. C22H1679BrN2O+ requires 403.0446.
8-Bromo-3-(4-fluorophenyl)-5-phenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4b). Solid (0.25 g, 53%), Rf (8:2 hexane–EtOAc) 0.21, m.p. 185–186 °C; IR (ATR): 524, 701, 765, 842, 1224, 1474, 1675, 3076, 3189 cm−1; 1H-NMR (300 MHz, DMSO-d6) 6.56 (2H, d, J = 8.5 Hz, 3′,5′-H), 6.92 (1H, s, 6-H), 7.08 (1H, d, J = 3.5 Hz, 3-H), 7.35–7.43 (3H, m, ArH), 7.56 (2H, dd, J = 1.8 and 8.1 Hz, ArH), 7.61 (1H, d, J = 1.5 Hz, 7-H), 8.01 (1H, d, J = 1.5 Hz, 9-H), 9.16 (1H, d, J = 3.0 Hz, NH); 13C-NMR (75 MHz, DMSO-d6) 69.6, 104.2, 113.7, 115.3, 116.0 (d, 2JCF = 21.9 Hz), 121.6, 127.4, 127.5 (d, 3JCF = 8.5 Hz), 128.4, 128.5, 129.3, 129.5, 130.9, 137.1, 137.6 (d, 4JCF = 2.9 Hz), 142.5, 160.3, 162.3 (d, 1JCF = 246.3 Hz); HRMS (ES): MH+, found 421.0342. C22H15N2OF79Br+ requires 421.0352.
8-Bromo-3-(4-chlorophenyl)-5-phenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4c). Solid (0.31 g, 62%), Rf (8:2 hexane-EtOAc) 0.23, m.p. 208–209 °C; IR (ATR): 455, 517, 697, 757, 851, 1110, 1323, 1469, 1676, 3072, 3182 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.54 (2H, d, J = 8.5 Hz, 3′,5′-H), 6.78 (1H, s, 6-H), 7.08 (1H, d, J = 3.5 Hz, 3-H), 7.16 (2H, d, J = 8.5 Hz, 2′,6′-H), 7.37–7.43 (3H, m, ArH), 7.57 (2H, d, J = 7.0 Hz, ArH), 7.61 (1H, d, J = 1.5 Hz, 7-H), 8.02 (1H, d, J = 1.5 Hz, 9-H), 9.16 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 69.6, 104.2, 113.8, 115.4, 121.6, 127.2, 127.3, 128.4, 128.5, 129.1, 129.3, 129.5, 130.9, 133.7, 137.1, 140.2, 142.5, 160.2; HRMS (ES): MH+, found 437.0053. C22H15N2O35Cl79Br+ requires 437.0056.
8-Bromo-3-(4-methoxyphenyl)-5-phenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4d). Solid (0.30 g, 60%), Rf (8:2 hexane–EtOAc) 0.19, m.p. 174–175 °C; IR (ATR): 526, 573, 687, 759, 1032, 1174, 1246, 1465, 1512, 1614, 1669, 3074, 3182 cm−1; 1H-NMR (300 MHz, DMSO-d6) 3.57 (3H, s, OCH3), 6.45 (2H, d, J = 8.7 Hz, 3′,5′-H), 6.62 (2H, d, J = 8.7 Hz, 2′,6′-H), 6.70 (1H, s, 6-H), 7.00 (1H, d, J = 3.5 Hz, 3-H), 7.33–7.45 (3H, m, ArH), 7.58 (2H, d, J = 7.0 Hz, ArH), 7.99 (1H, d, J = 1.5 Hz, 9-H), 9.11 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 5.4, 69.9, 104.1, 113.6, 114.3, 121.4, 126.5, 127.2, 127.8, 128.4, 128.5, 129.2, 129.5, 131.2, 133.6, 137.2, 142.6, 159.7, 160.4; HRMS (ES): MH+, found 433.0553. C23H18N2O279Br+ requires 433.0552.
8-Bromo-5-(4-fluorophenyl)-3-phenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4e). Solid (0.27 g, 57%), Rf (8:2 hexane–EtOAc) 0.26, m.p. 220–222 °C; IR (ATR): 518, 563, 694, 775, 845, 1226, 1319, 1492, 1607, 1672, 3065, 3185 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.55 (2H, d, J = 7.0 Hz, ArH), 6.74 (1H, s, 6-H), 7.03 (1H, d, J = 3.0 Hz, 3-H), 7.07–7.14 (3H, m, ArH), 7.22 (2H, t, J = 8.7 Hz, 3′,5′-H),7.60 (2H, t, J = 8.7 Hz, 2′,6′-H), 7.61 (1H, d, J = 1.5 Hz, 6-H), 8.00 (1H, d, J = 1.5 Hz, 9-H), 9.16 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 64.9, 85,2, 95.0, 107.7, 109.6, 115.7 (d, 2JCF = 21.8 Hz), 117.1, 124.4, 128.5 (d, 3JCF = 8.5 Hz), 129.5, 130.7, 130.9, 131.1, 131.5, 133.6, 138.6, 139.1 (d, 4JCF = 2.9 Hz), 146.8, 161.5, 162.4 (d, 1JCF = 242.8 Hz); HRMS (ES): MH+, found 421.0341. C22H14N2OF79Br+ requires 421.0352.
8-Bromo-3,5-bis(4-fluorophenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4f). Solid (0.28 g, 56%), Rf (8:2 hexane–EtOAc) 0.26, m.p. 239–240 °C; IR (ATR): 510, 566, 772, 835, 1157, 1225, 1320, 1494, 1509, 1620, 1671, 3073, 3187 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.54 (1H, s, 6-H), 6.59 (2H, d, J = 8.5 Hz, 3′,5′-H), 6.74 (2H, d, J = 8.5 Hz, 3″,5″-H), 6.85 (1H, d, J = 3.5 Hz, 3-H), 7.08 (2H, d, J = 8.5 Hz, 2′,6′-H), 7.29 (2H, d, J = 8.5 Hz, 2″,6″-H), 7.86 (1H, d, J = 1.5 Hz, 7-H), 7.80 (1H, d, J = 1.5 Hz, 9-H), 7.95 (1H, d, J = 2.0 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 70.3, 104.2, 114.4, 115.9 (d, 2JCF = 21.9 Hz), 116.1 (d, 2JCF = 20.9 Hz), 122.9, 127.0 (d, 3JCF = 8.5 Hz), 127.1 (d, 4JCF = 3.8 Hz), 127.8 (2C), 128.1, 130.3 (d, 3JCF = 8.5 Hz), 135.9 (d, 4JCF = 3.8 Hz), 136.8, 140.8, 162.0, 162.8 (d, 1JCF = 248.4 Hz), 163.0 (d, 1JCF = 249.3 Hz); HRMS (ES): MH+, found 439.0241. C22H13N2OF279Br+ requires 439.0258.
8-Bromo-3-(4-chlorophenyl)-5-(4-fluorophenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4g). Solid (0.32 g, 58%), Rf (8:2 hexane–EtOAc) 0.27, m.p. 216–217 °C; IR (ATR): 510.0, 563, 812, 824, 1088, 1160, 1225, 1491, 1620, 1672, 3074, 3185 cm−1. 1H-NMR (500 MHz, DMSO-d6) 6.56 (2H, d, J = 7.5 Hz, 3″,5″-H), 7.76 (1H, s, 6-H), 7.05 (1H, d, J = 2.5 Hz, 3-H), 7.18 (2H, d, J = 7.5 Hz, 2″,6"-H), 7.24 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.61 (2H, t, J = 8.5 Hz, 2′,6′-H), 7.62 (1H, d, J = 1.5 Hz, 7-H), 8.01 (1H, d, J = 1.5 Hz, 9-H), 9.17 (1H, d, J = 2.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 70.3, 104.1, 113.7, 115.5, (116.4 (d, 2JCF = 21.9Hz), 121.5, 127.2, 127.6 (d, 3JCF = 3.8 Hz), 128.4, 129.1 (2C), 129.2, 130.8 (d, 3JCF = 8.5 Hz), 137.1, 141.2, 141.5, 160.2, 162.7 (d, 1JCF = 245.5 Hz); HRMS (ES): MH+, found 455.9970. C22H13N2OF35Cl79Br+ requires 454.9962.
8-Bromo-5-(4-fluorophenyl)-3-(4-methoxyphenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4h). Solid (0.30 g, 60%), Rf (8:2 hexane–EtOAc) 0.24, m.p. 200–201 °C; IR (ATR): 511, 590, 837, 1174, 1246, 1466, 1493, 1620, 1670, 3071, 3184 cm−1; 1H-NMR (500 MHz, DMSO-d6) 3.58 (3H, s, OCH3), 6.46 (2H, d, J = 8.5 Hz, 3",5"-H), 6.64 (2H, d, J = 8.0 Hz, 2″,6″-H), 6.74 (1H, s, 6-H), 6.97 (1H, d, J = 1.5 Hz, 3-H), 7.24 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.59 (1H, d, J = 1.5 Hz, 9-H), 7.61 (2H, t, J = 8.7 Hz, 2′,6′-H), 7.98 (1H, d, J = 1.5 Hz, 5-H), 9.10 (1H, d, J = 3.5 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 55.5, 69.9, 104.1, 113.6, 114.3, 115.5, 116.4 (d, 2JCF = 21.8 Hz), 121.4, 126.6, 127.2, 127.7 (d, 4JCF = 2.9 Hz), 128.3, 130.8 (d, 3JCF = 8.8 Hz), 133.5, 137.1, 141.5, 159.7, 160.2, 162.6 (d, JCF = 250.5 Hz); HRMS (ES): MH+, found 451.0457. C23H1779BrFN2O2+ requires 451.0457.
8-Bromo-5-(3-chlorophenyl)-3-phenyl-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4i). Solid (0.34 g, 68%), Rf (8:2 hexane–EtOAc) 0.24, m.p. 234–235 °C; IR (ATR) 517, 534, 692, 773, 1269, 1318, 1456, 1621, 1677, 3062, 3176 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.56 (2H, d, J = 7.0 Hz, ArH), 6.84 (1H, s, 6-H), 7.08 (1H, d, J = 1.5 Hz, 3-H), 7.10–7.16 (4H, m, ArH), 7.41–7.43 (2H, m, 4′-H and 6′-H), 7.54 (1H, dd, J = 2.0 and 3.5 Hz, 5′-H), 7.63 (1H, d, J = 1.5 Hz, 7-H), 8.02 (1H, d, J = 1.5 Hz, 9-H), 9.15 (1H, d, J = 3.5 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 70.3, 104.9, 113.8, 115.6, 121.9, 125.3, 127.2, 127.4, 128.0, 128.2, 129.1, 129.2, 129.3, 131.2, 133.1, 134.0, 137.3, 140.8, 141.2, 160.0; m/z 437 (100, MH+); HRMS (ES): MH+, found 437.0049. C22H15N2O35Cl79Br+ requires 437.0056.
8-Bromo-5-(3-chlorophenyl)-3-(4-fluorophenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4j). Solid (0.34 g, 62%), Rf (8:2 hexane-EtOAc) 0.27, m.p. 227–228 °C; IR (ATR): 529, 566, 692.1, 772, 788, 1148, 1173, 1232, 1319, 1461, 1508, 1599, 1671, 3073, 3187 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.60 (2H, t, J = 8.5 Hz, 3″,5″-H), 6.84 (1H, s, 6-H), 6.95 (2H, t, J = 8.5 Hz, 2″,6″-H), 7.11 (1H, d, J = 3.5 Hz, 3-H), 7.41–7.43 (2H, m, 4′-H and 6′-H), 7.34 (1H, dd, J = 1.5 and 3.5 Hz, 5′-H), 7.62 (1H, d, J = 1.5 Hz, 7-H), 7.72 (1H, s, 2′-H), 8.02 (1H, d, J = 1.5 Hz, 9-H), 9.14 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 69.7, 105.0, 113.8, 115.4, 116.0 (d, 2JCF = 21.8 Hz), 121.9, 127.2, 127.5, 127.6 (d, 3JCF = 85 Hz), 128.0, 128.2, 129.1, 131.2, 133.0, 134.0, 137.1, 137.5 (d, 3JCF = 2.9 Hz), 140.6, 159.9, 162.3 (d, 1JCF = 243.6 Hz); HRMS (ES): MH+, found 454.9970. C22H14N2OF35Cl79Br + requires 454.9962.
8-Bromo-5-(3-chlorophenyl)-3-(4-chlorophenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4k). Solid (0.38 g, 76%), Rf (8:2 hexane–EtOAc) 0.29, m.p. 238–239 °C; IR (ATR): 523, 773, 1089, 1318, 1464, 1599, 1677, 3069, 3178 cm−1; 1H-NMR (500 MHz, DMSO-d6) 6.57 (2H, d, J = 8.5 Hz, 3″,5″-H), 6.86 (1H, s, 6-H), 7.11 (1H, d, J = 3.5 Hz, 3-H), 7.20 (2H, d, J = 8.5 Hz, 2″,6″-H), 7.41–7.43 (2H, m, 4′-H and 6′-H), 7.54 (1H, dd, J = 1.5 and 3.5 Hz, 5′-H), 7.62 (1H, d, J = 1.5 Hz, 7-H), 7.65 (1H, s, 2′-H), 8.03 (1H, d, J = 1.5 Hz, 9-H), 9.16 (1H, d, J = 3.0 Hz, NH); 13C-NMR (125 MHz, DMSO-d6) 69.7, 105.1, 113.9, 115.4, 122.0, 127.2, 127.3, 127.6, 128.0, 128.2, 129.2, 129.3, 131.3, 133.0, 133.8, 134.0, 137.1, 140.1, 140.6, 159.9; HRMS (ES): MH+, found 470.9651. C22H13N2O35Cl279Br+ requires 470.9667.
8-Bromo-5-(3-chlorophenyl)-3-(4-methoxyphenyl)-2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinazolin-1-one (4l). Solid (0.36 g, 72%), Rf (8:2 hexane–EtOAc) 0.22, m.p. 177–178 °C; IR (ATR): 514, 572, 772, 1033, 1175, 1247, 1456, 1512, 1598, 1671, 3068, 3187 cm−1; 1H-NMR (500 MHz, DMSO-d6) 3.58 (3H, s, OCH3), 6.47 (2H, d, J = 8.5 Hz, 3″,5″-H), 6.64 (2H, d, J = 8.5 Hz, 3″,5″-H), 6.84 (1H, s, 6-H), 7.02 (1H, d, J = 3.5 Hz, 6′-H), 7.43 (1H, d, J = 7.5 Hz, 5′-H), 7.40–7.55 (2H, m, ArH), 7.61 (1H, d, J = 1.5 Hz, 7-H), 7.65 (1H, s, 2′-H), 8.00 (1H, d, J = 1.5 Hz, 9-H), 9.10 (1H, d, J = 3.0 Hz, 3-NH); 13C-NMR (125 MHz, DMSO-d6) 55.5, 69.9, 104.1, 113.6, 114.3, 115.5, 116.4 (d, 2JCF = 21.9 Hz), 121.4, 126.6, 127.1, 127.6 (d, 3JCF = 3.8 Hz), 128.4, 130.8 (d, 3JCF = 8.5 Hz), 133.5, 137.1, 141.5, 159.7, 160.2, 162,7 (d, 1JCF = 246.0 Hz); HRMS (ES): MH+, found 467.0139. C23H17N2O235Cl79Br+ requires 467.0162.
3.5. Materials and Methods for Bioassays
3.5.1. Materials and Methods for In Vitro Cytotoxicity Assay
Cell Culture
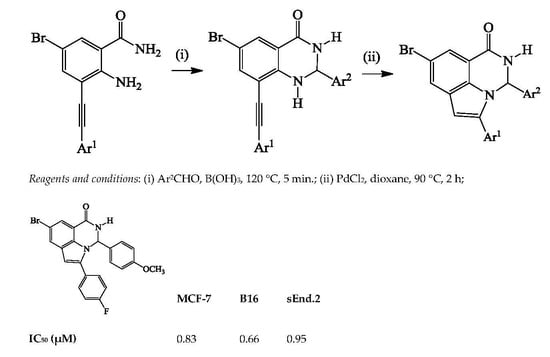
Human breast cancer (MCF-7) cells, mouse endothelial (End.2) cells derived from benign vascular tumors, and mouse melanoma (B16) cells were cultured in Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% penicillin-streptomycin and 20 mM glutamine. The cells were maintained in a 37 °C incubator in a humidified atmosphere containing 5% CO2.
Cell Viability Assay
Cell viability was assessed using the crystal violet nuclear staining assay. Cells were seeded in 96-well culture plates at a density of 5000 cells/well for 24 h, and then treated with test compounds (0–50 μM) or 0.05% dimethylsulfoxide (DMSO) for 48 h. The time and dose range were chosen following initial screening undertaken over a 72 h period. Following 48 h of treatment, the cells were fixed with 1% glutaraldehyde in phosphate buffered saline (PBS) for 15 min, and stained with a 0.1% crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA). After 30 min the cells were incubated in 0.1% Triton X-100 (Sigma-Aldrich) for 90 min. The absorbance was read at 570 nm on an ELx 800 Universal Microplate Reader (Bio-Tek Instruments Inc., Analytical Diagnostic Products, Weltevreden, South Africa. Three wells were analysed for each concentration. The percentage of viable cells was calculated as follows: viability (%) = [A570 (treated) − A570 (blank)]/[A570 (control) − A570 (blank)] × 100 [
22].
Statistics
The results are expressed as mean ± SD of at least three separate experiments. Data was analysed with GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). One way analysis of variance (ANOVA) and post-hoc Tukeys test were used. Values of p < 0.05 were considered to be statistically significant.
3.5.2. In Vitro pLDH Assay
Three-fold serial dilutions of the test compounds 4a–l were incubated in triplicate with 3D7 strain P. falciparum parasites in a transparent 96-well flat bottom plate (Nest Biotechnology Co., Ltd., Wuxi, Jiangsu, China). DMSO and chloroquine were used as negative and positive controls, respectively. The plate was put in an airtight box, gassed and incubated with complete RPMI 1640 medium for 48 h. At the end of incubation, Malstat reagent was added to the 96-well plate followed by developing with NBT/PES (nitro blue tetrazolium + phenazine ethosulphate) reagent. Parasite growth was determined spectrophotometrically at 620 nm, by measuring the activity of the pLDH in control and drug-treated cultures using an Infinite F500 multiwell plate reader (Tecan Group Ltd., Männedorf, Switzerland). The OD values from control wells devoid of drug were referred to as having 100% pLDH activity. The IC50 are expressed as the % parasite survival relative to the control, calculated from fitted sigmoidal dose response curves. The dose response curves were obtained by plotting percentage parasite survival against the logarithm of the concentration using the GraphPad Prism software package. IC50 values were calculated graphically by interpolation from these curves. A prerequisite for all experiments was to have a Z′-factor > 0.5 as a measure of the quality of the screening assay.