Spectroscopic Studies on the Molecular Ageing of Serum Albumin
Abstract
:1. Introduction
2. Result and Discussion
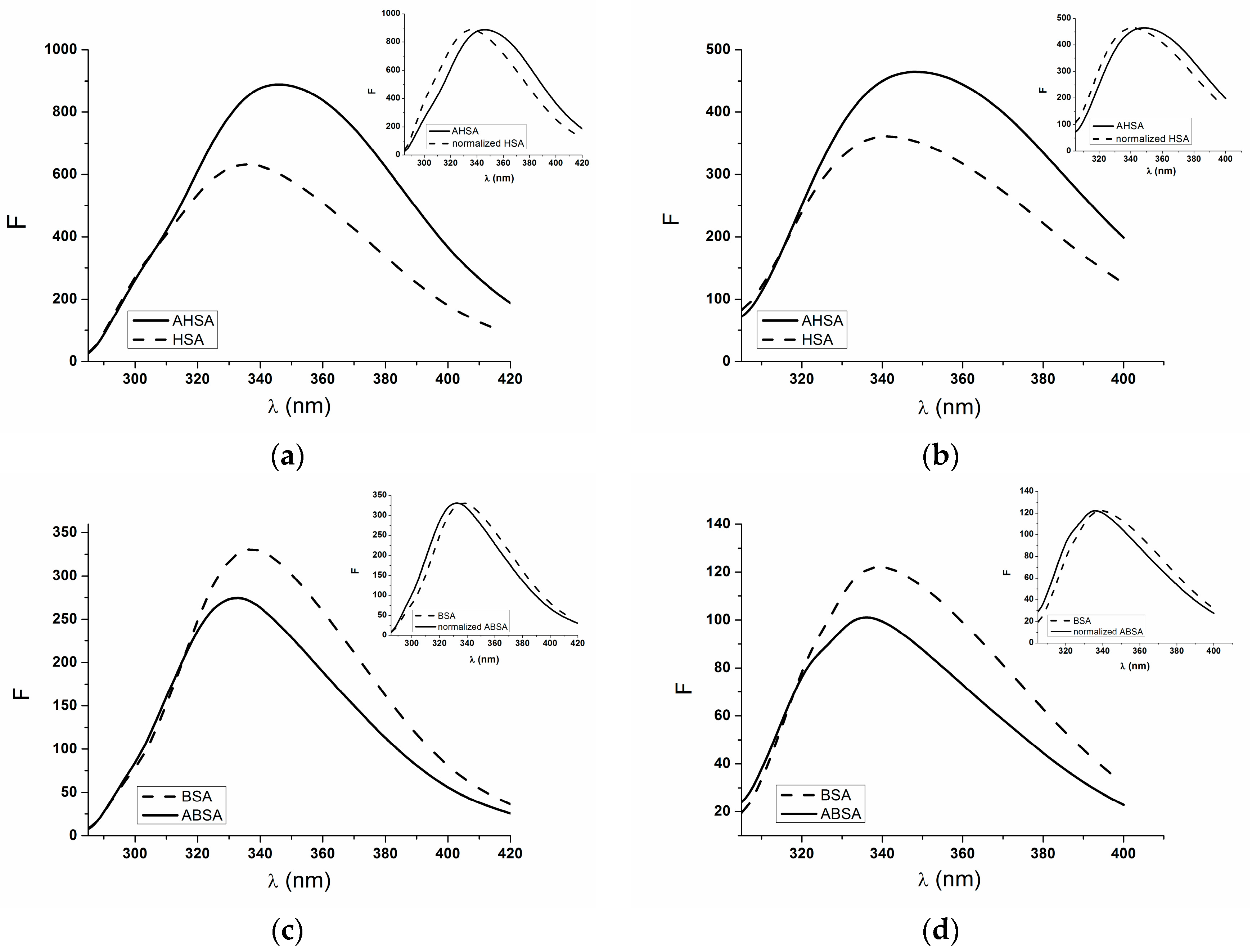
2.1. Absorption and Fluorescence Characteristic of Aged Serum Albumin—Second Derivative
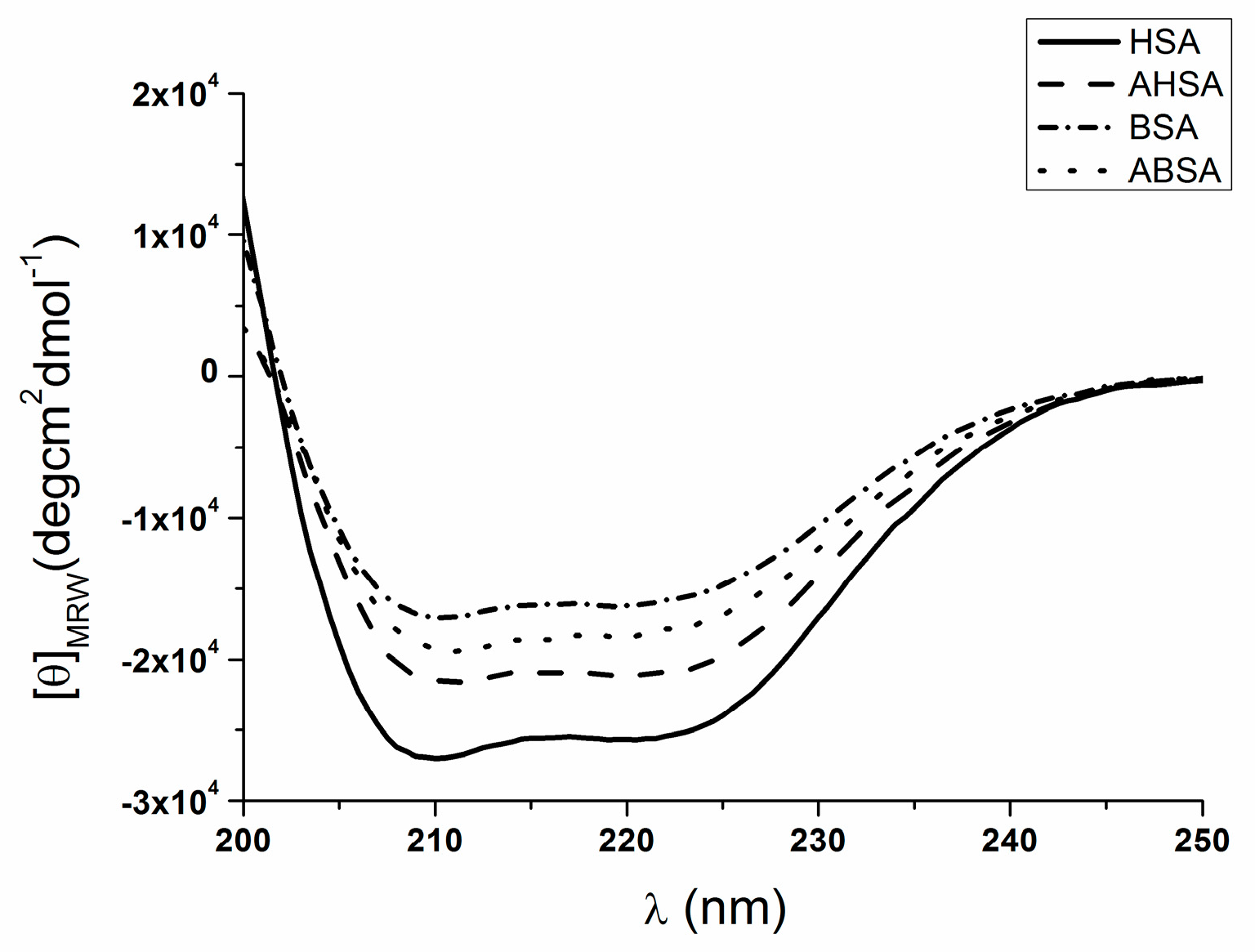
2.2. Circular Dichroism (CD) Characteristic of Aged Serum Albumin
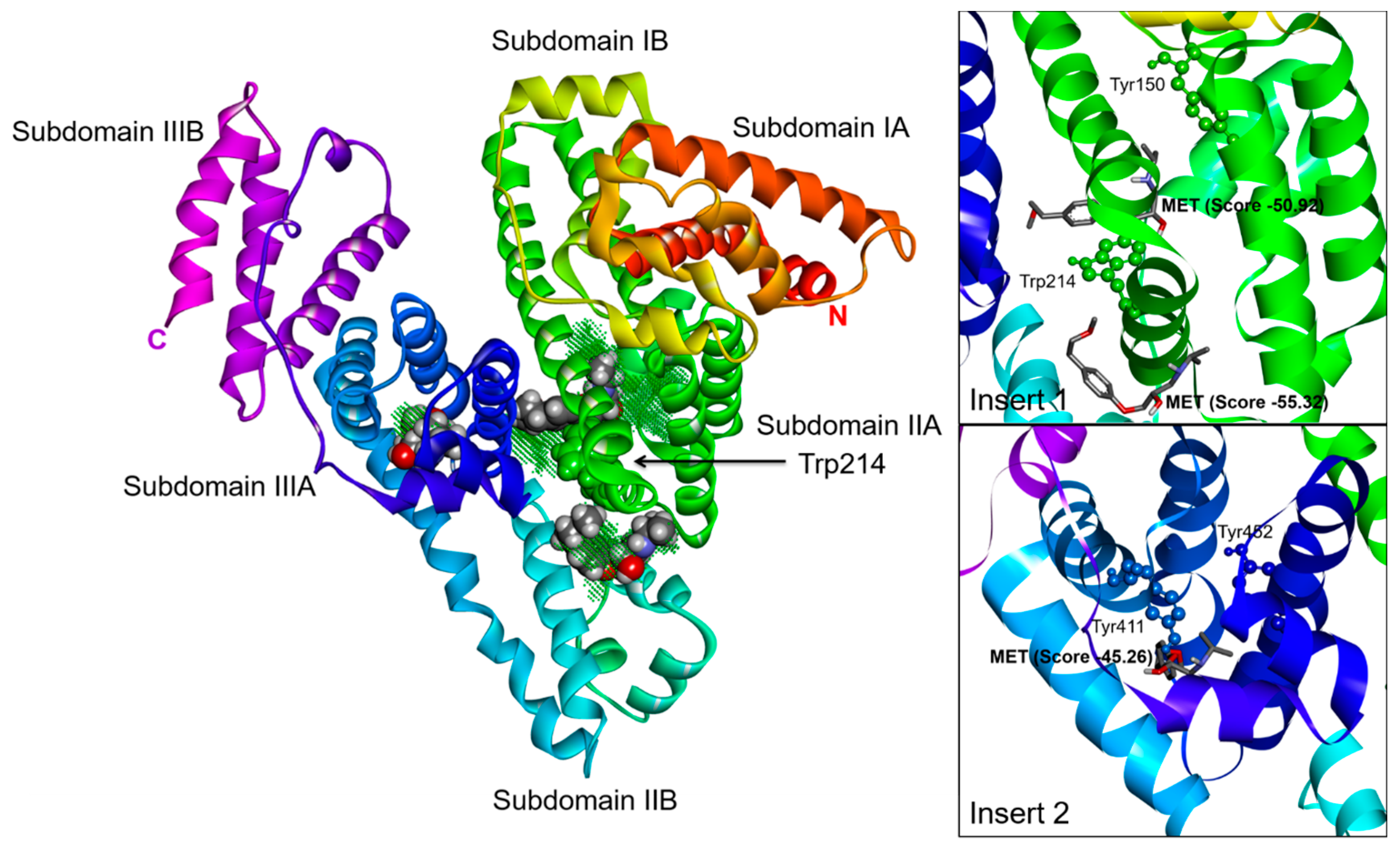
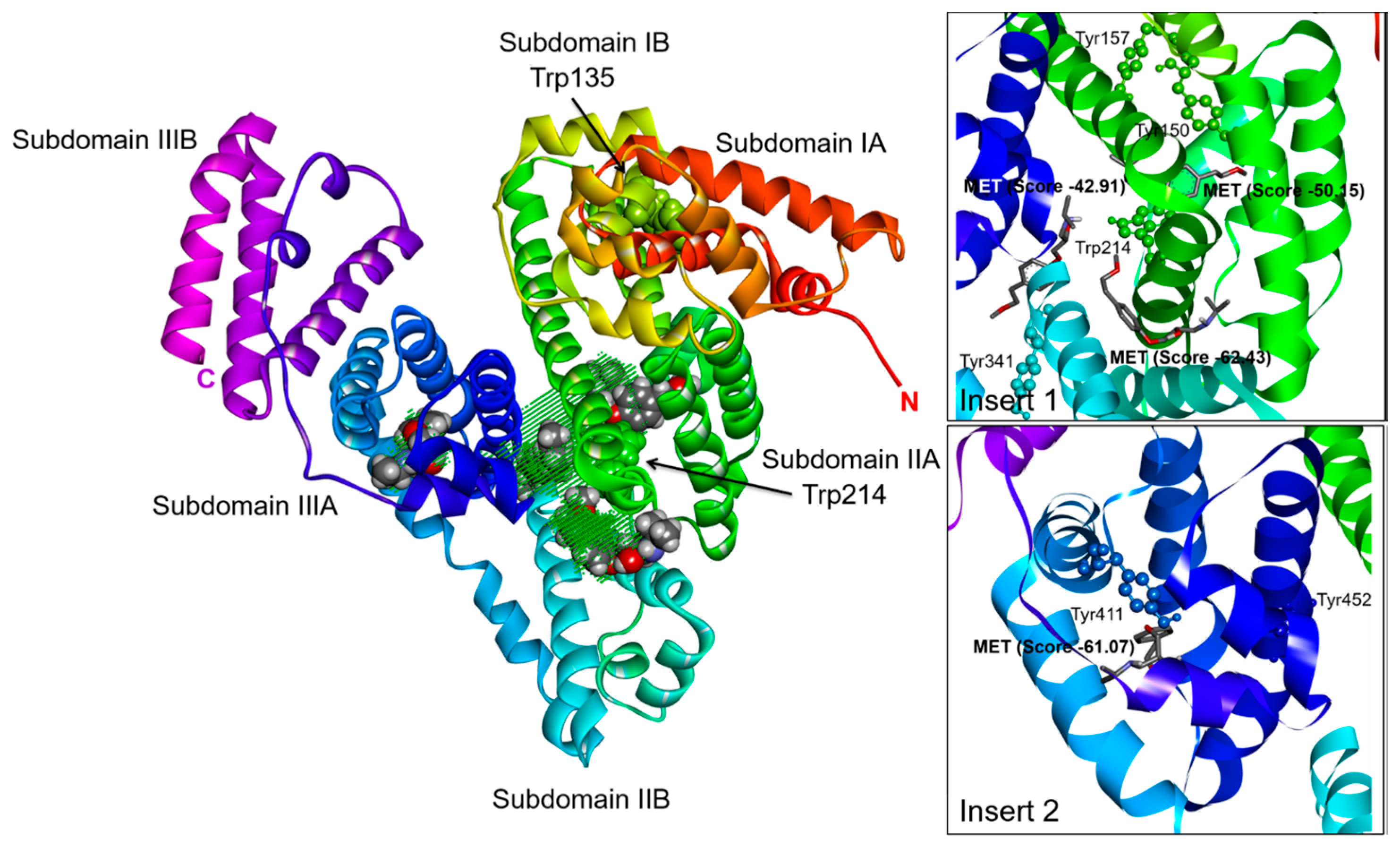
2.3. Effect of N-A Isomerization on Metoprolol (MET) Binding to Serum Albumin
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Aged serum Albumin in Vitro
3.3. Fluorescence and UV-Vis Spectra—Second Derivative
3.4. Circular Dichroism (CD) Spectra
3.5. Computational Simulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peters, T., Jr. All about Albumin. Biochemistry, Genetics and Medical Applications, 1st ed.; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Foster, J.F. Some aspects of the structure and conformational properties of serum albumin. In Albumin Structure, Functions and Uses; Rosenoer, V.M., Oratz, M., Rothschild, M.A., Eds.; Pergamon Press: Oxford, UK, 1977; pp. 53–84. [Google Scholar]
- Era, S.; Kuwata, K.; Imai, H.; Nakamura, K.; Hayashi, T.; Sogami, M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta 1995, 1247, 12–16. [Google Scholar] [CrossRef]
- Era, S.; Sogami, M.; Kuwata, K. Comparative 1H NMR studies on the structural looseness of the aged (A) and non-aged (N) bovine mercaptalbumin in the alkaline region. Int. J. Biol. Macromol. 2009, 44, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Nikkel, H.J.; Foster, J.F. A reversible sulfhydryl-catalyzed structural alteraction of bovine mercaptalbumin. Biochemistry 1971, 10, 4479–4486. [Google Scholar] [CrossRef] [PubMed]
- Talsky, G. Derivative Spectrophotometry: Low and High Order; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1994. [Google Scholar]
- Kuś, S.; Marczenko, Z.; Obarski, N. Derivative UV-Vis Spectrophotometry in Analytical Chemistry. Chem. Anal. 1996, 41, 899–927. [Google Scholar]
- Patel1, D.D.; Patel, M.M. Simultaneous Estimation of Metoprolol Succinate and Olmesartan Medoxomil in Pharmaceutical Dosage Form by UV Spectroscopy. Int. J. Res. Pharm. Sci. 2012, 3, 935–939. [Google Scholar]
- Mihalyi, E. Numerical values of the absorbances of the aromatic amino acids in acid, neutral, and alkaline solutions. J. Chem. Eng. Data 1968, 13, 179–182. [Google Scholar] [CrossRef]
- Karpińska, J. Derivative spectrophotometry—recent applications and directions od developments. Talanta 2004, 64, 801–822. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, C.; Colonna, G.; Giovane, A.; Irace, G.; Servillo, L. Second—Derivative Spectroscopy of Proteins. Eur. J. Biochem. 1978, 90, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Federici, M.M. Quantitation of aromatic residues in proteins: Model compounds for second-derivative spectroscopy. Biochemistry 1982, 21, 2600–2606. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurin, V.A.; Borisenko, G.G.; Fabisiak, J.P.; Hubel, C.A.; Ness, R.B.; Gandley, R.; McLaughlin, M.K.; Roberts, J.M. Mishandling of cooper by albumin: Role in redox—cycling and oxydative stress in preeclampsia plasma. Hypertens. Pregnancy 2001, 20, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Yamasaki, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Effect of oxidative stress on the structure and function of human serum albumin. Pharm. Res. 2001, 18, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 2011, 20, 1298–1345. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.A.; Fahlman, R.P. The anti-apoptotic form of tyrosine kinase Lyn that is generated by proteolysis is degraded by the N-end rule pathway. Oncotarget 2014, 5, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.; Fahlman, R. The-N-End Rule: The Beginning Determines the End. Protein Pept. Lett. 2016, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.; Fahlman, R. Phosphorylation Impacts N-End Rule Degradation of the Proteolytically Activated Form of BMX Kinase. J. Biol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.K.; Kalonia, D.S. Second derivative tryptophan fluorescence spectroscopy as a tool to characterize partially unfolded intermediates of proteins. Int. J. Pharm. 2005, 294, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mozo-Vilları́as, A. Second derivative fluorescence spectroscopy of tryptophan in proteins. J. Biochem. Biophys. Methods 2002, 50, 163–178. [Google Scholar] [CrossRef]
- Kosa, T.; Maruyama, T.; Sakai, N.; Yonemura, N.; Yahara, S.; Otagiri, M. Species differences of serum albumins: III. Analysis of structural characteristics and ligand binding properties during N-B transitions. Pharm. Res. 1998, 15, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Oetl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influences its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Iwao, Y.; Anraku, M.; Yamasaki, K.; Kragh-Hansen, U.; Kawai, K.; Maruyama, T.; Otagiri, M. Oxidation of Arg-410 promotes the elimination of human serum albumin. Biochim. Biophys. Acta 2006, 1764, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end rule: Functions, mysteries, uses. Proc. Natl. Acad. Sci. USA 1996, 93, 12142–12149. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Suresh Kumar, G. Structural and thermodynamic studies on the interaction of iminium and alkanolamine forms of sanguinarine with hemoglobin. J. Phys. Chem. B. 2014, 118, 3771–3784. [Google Scholar] [CrossRef] [PubMed]
- Dockal, M.; Carter, D.C.; Rüker, F. Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J. Biol. Chem. 2000, 275, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Era, S.; Kuwata, K.; Sogami, M.; Kato, K.; Watari, H. Circular dichroic and 1H-NMR studies on the aged form of bovine plasma albumin. Int. J. Pept. Protein Res. 1991, 38, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein Oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Shringarpure, R.; Davies, K.J. Protein turnover by the proteasome in aging and disease. Free Radic. Biol. Med. 2002, 32, 1084–1089. [Google Scholar] [CrossRef]
- Grune, T.; Merker, K.; Sandig, G.; Davies, K.J. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem. Biophys. Res. Commun. 2003, 305, 709–718. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F. Fluorescence of dansyl amino acids in organic solvents and protein solutions. Arch. Biochem. Biophys. 1967, 120, 609–620. [Google Scholar] [CrossRef]
- Yamasaki, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Characterization of site I on human serum albumin: Concept about the structure of a drug binding site. Biochim. Biophys. Acta 1996, 1295, 147–157. [Google Scholar] [CrossRef]
- Chudzik, M.; Równicka-Zubik, J.; Pożycka, J.; Pawelczak, B.; Sulkowska, A. Analysis of Aged Human Serum Albumin Affinity for Doxazosin. Curr. Protein Pept. Sci. 2016, 17, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites of human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org (accessed on 7 October 2014).
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.J.; et al. General Atomic and Molecular Electronic Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced Protein-Ligand docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- CLC Drug Discovery Workbench version 1.5; CLC Bio, a QIAGEN Company: Aarhus, Denmark, 2014.
- Dessault Systemes BIOVIA. Discovery Studio Modeling Environment; Release 2017; Dessault Systemes: San Diego, CA, USA, 2016. [Google Scholar]
- Sample Availability: Samples of the compounds MET and MET_HSA complexes (in Mol Files) are available from the authors.
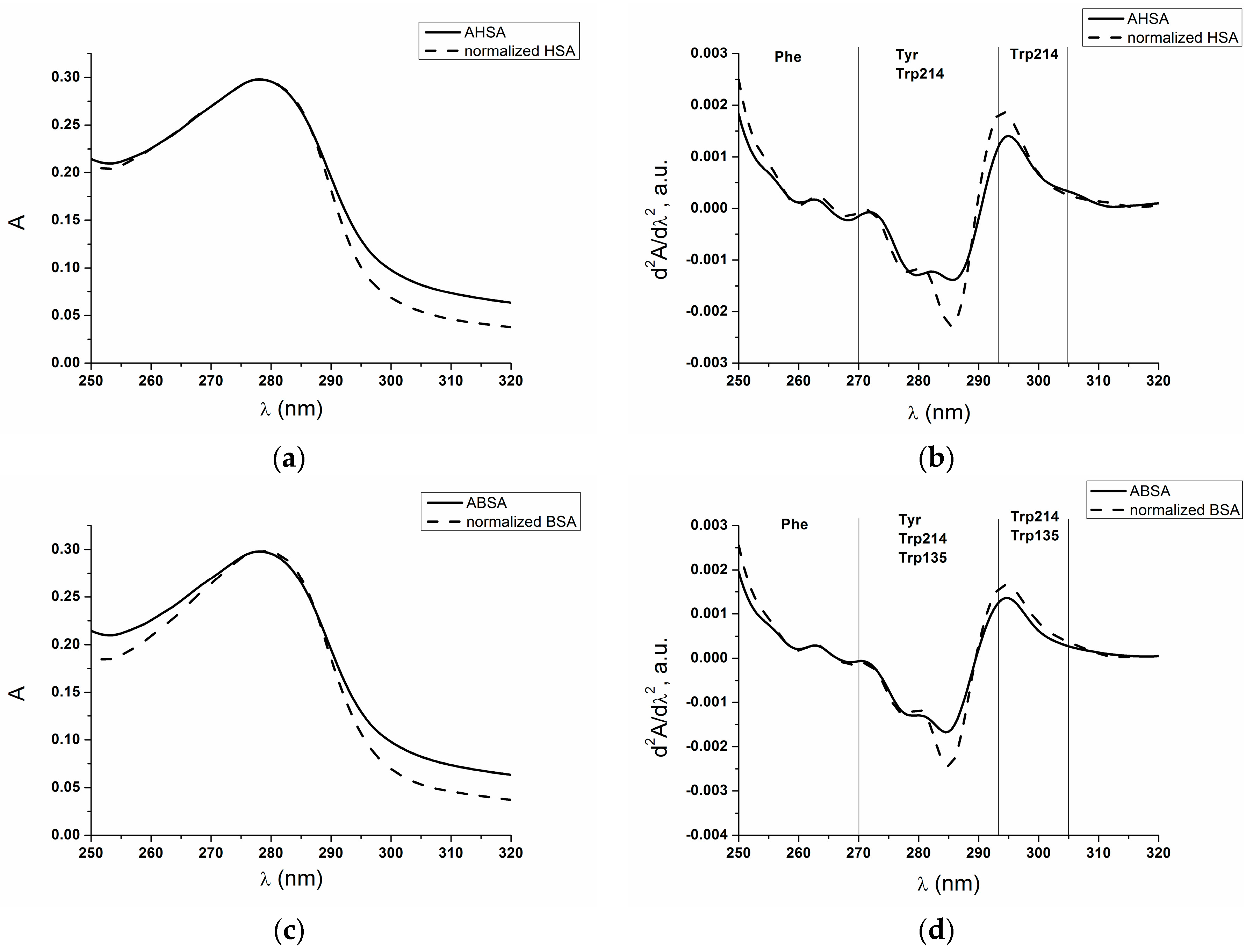


| λ (nm) | 295 | 283 | 287 | 260 | 252 |
|---|---|---|---|---|---|
| AHSA | 0.0014 | −0.0012 | −0.0013 | 0.0001 | 0.0011 |
| normalized HSA | 0.0018 | −0.0017 | −0.0019 | 0.0001 | 0.0015 |
| ABSA | 0.0013 | −0.0015 | −0.0012 | 0.0002 | 0.0012 |
| normalized BSA | 0.0016 | −0.0020 | −0.0018 | 0.0002 | 0.0016 |
| H275nm | λmin (nm) | λmax (nm) | H295nm | λmin (nm) | λmax (nm) | |
|---|---|---|---|---|---|---|
| normalized HSA | 0.017 | 311 | 289 | 0.550 | 385 | 395 |
| AHSA | 0.46 | 312 | 289 | 0.065 | 385 | 395 |
| BSA | 0.71 | 289 | 304 | 0.800 | 385 | 395 |
| normalized ABSA | 0.850 | 303 | 289 | 0.990 | 385 | 393 |
| θMRE at 222 nm * | % α-Helix | % β-Sheet | % Turn | % Other | |
|---|---|---|---|---|---|
| HSA | −25436.1 | 58.9 | 23.1 | 2.0 | 16.2 |
| AHSA | −20892.2 | 49.2 | 13.8 | 4.8 | 32.2 |
| BSA | −15826.2 | 40.9 | 27.9 | 6.5 | 24.8 |
| ABSA | −17859.2 | 39.1 | 22.7 | 4.8 | 33.5 |
| Molar Ratio Serum Albumin:Probe | % of Displacement | |||
|---|---|---|---|---|
| HSA | AHSA | BSA | ABSA | |
| SA:DG 1:0.5 | 47.12 | 23.60 | 10.10 | 11.81 |
| SA:DG 1:1 | 27.98 | 59.85 | 12.06 | 21.05 |
| SA:DP 1:0.5 | 39.16 | 33.28 | 41.20 | 26.27 |
| SA:DP 1:1 | 33.29 | 47.59 | 46.05 | 25.94 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudzik, M.; Maciążek-Jurczyk, M.; Pawełczak, B.; Sułkowska, A. Spectroscopic Studies on the Molecular Ageing of Serum Albumin. Molecules 2017, 22, 34. https://doi.org/10.3390/molecules22010034
Chudzik M, Maciążek-Jurczyk M, Pawełczak B, Sułkowska A. Spectroscopic Studies on the Molecular Ageing of Serum Albumin. Molecules. 2017; 22(1):34. https://doi.org/10.3390/molecules22010034
Chicago/Turabian StyleChudzik, Mariola, Małgorzata Maciążek-Jurczyk, Bartosz Pawełczak, and Anna Sułkowska. 2017. "Spectroscopic Studies on the Molecular Ageing of Serum Albumin" Molecules 22, no. 1: 34. https://doi.org/10.3390/molecules22010034






