Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Samples
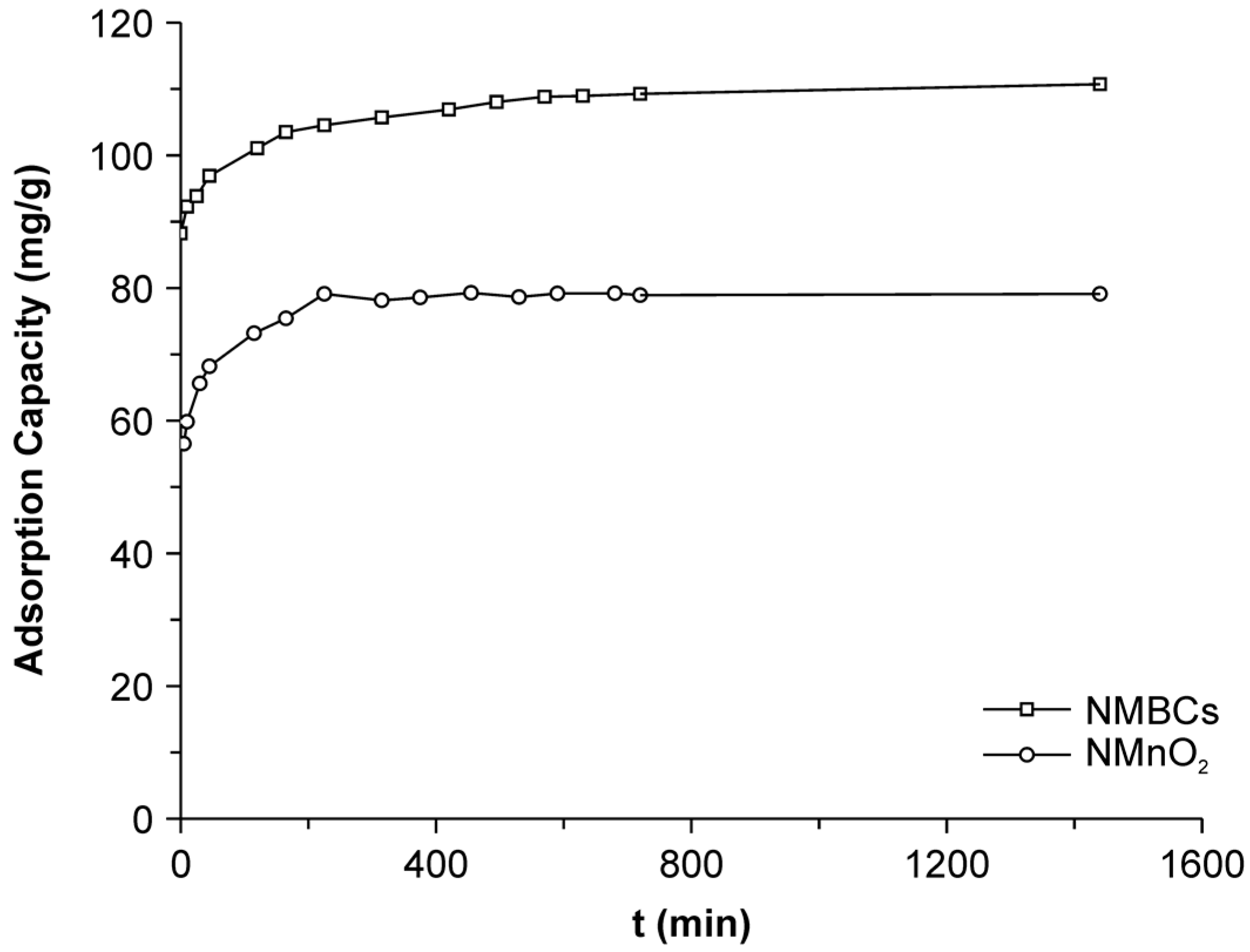
2.2. Adsorption Kinetics
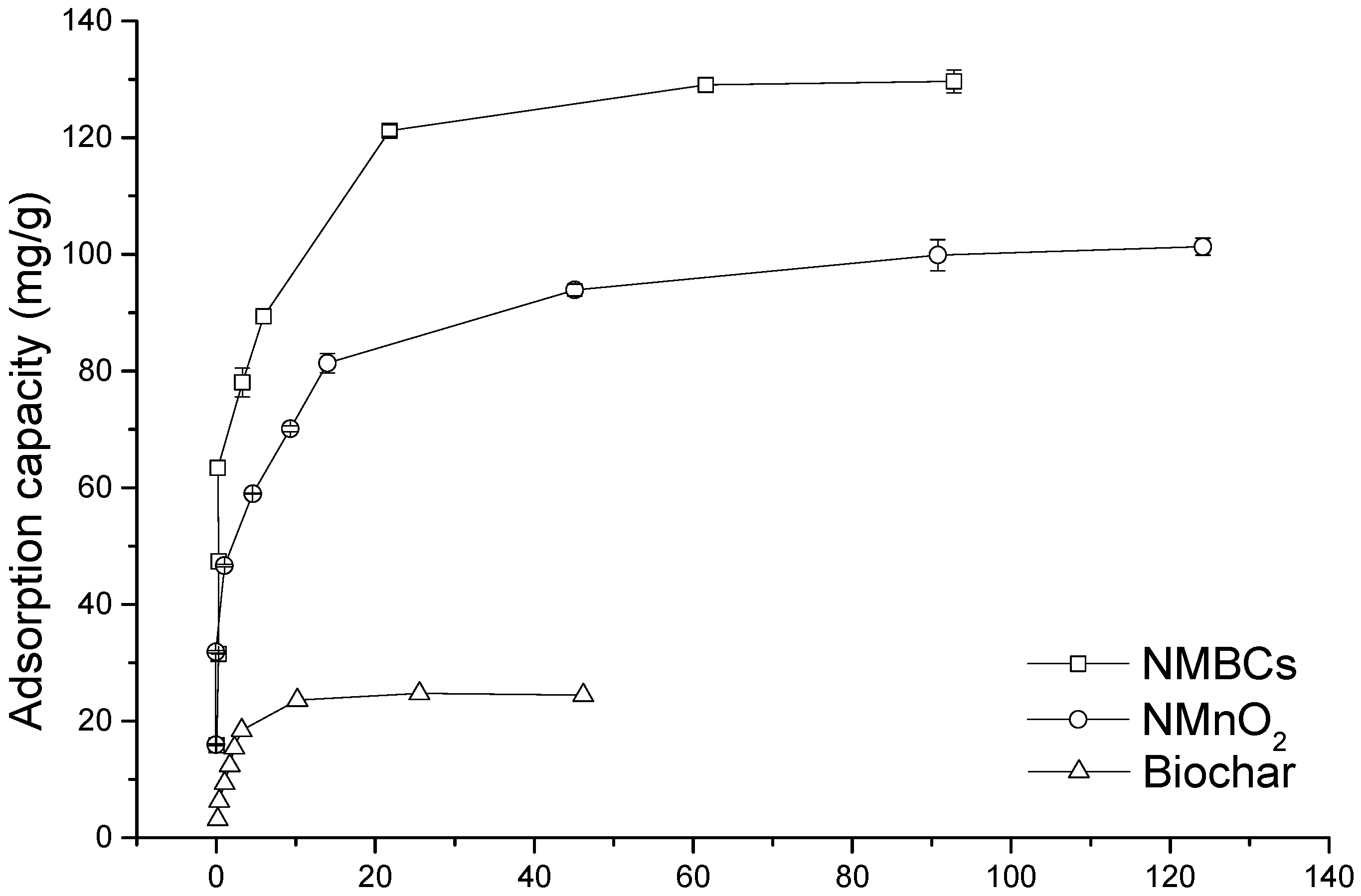
2.3. Adsorption Isotherms
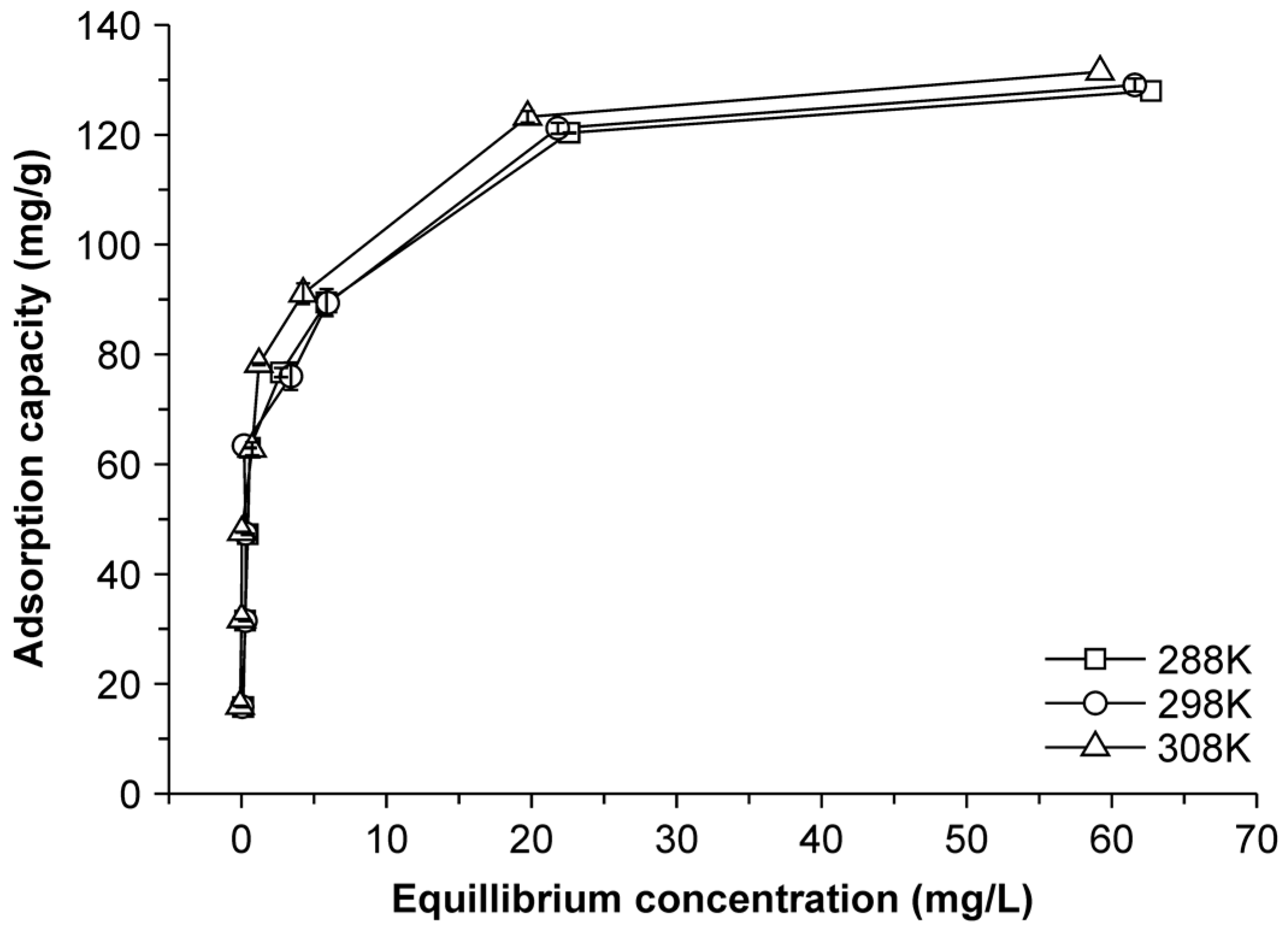
2.4. Adsorption Thermodynamics
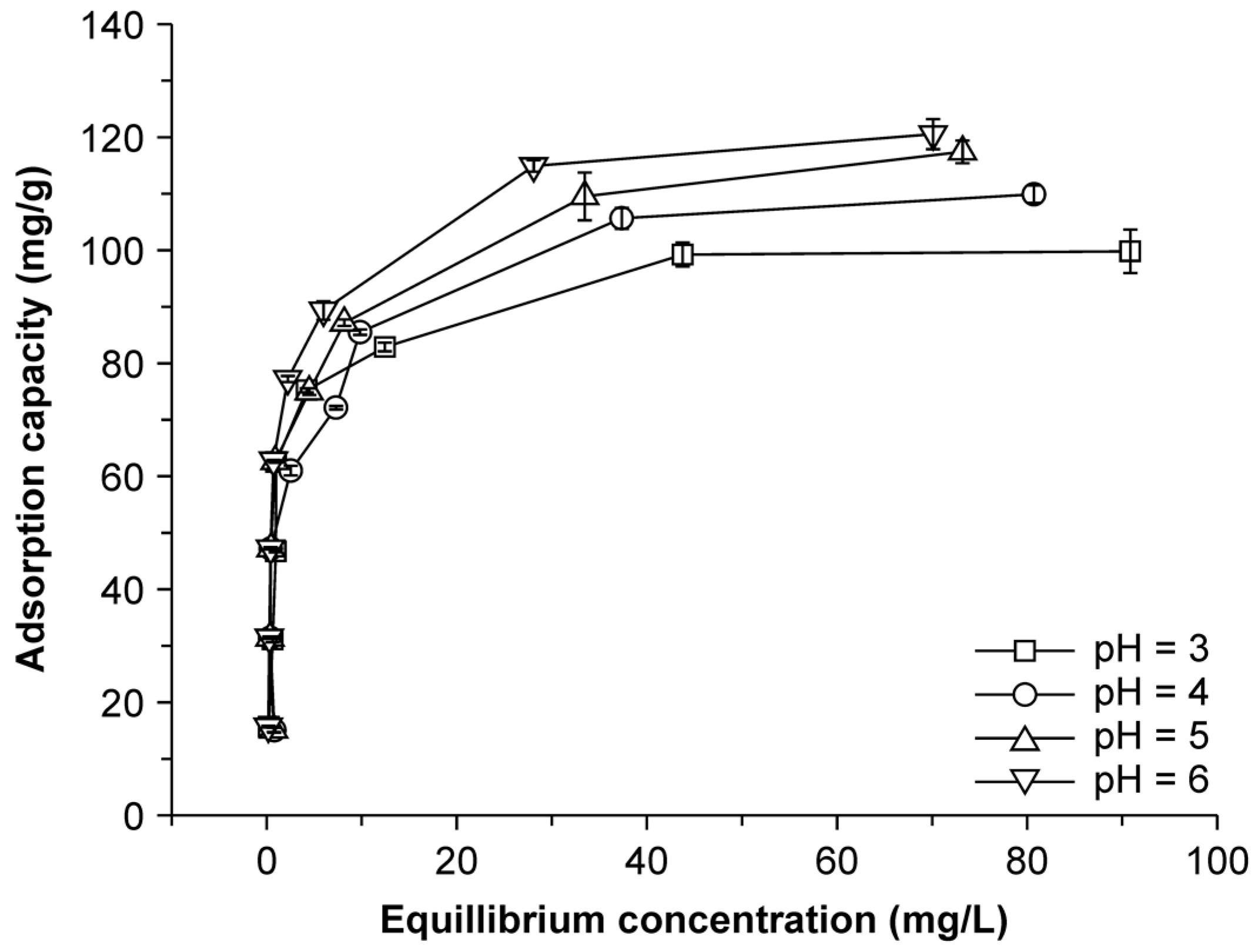
2.5. Effect of pH on Adsorption
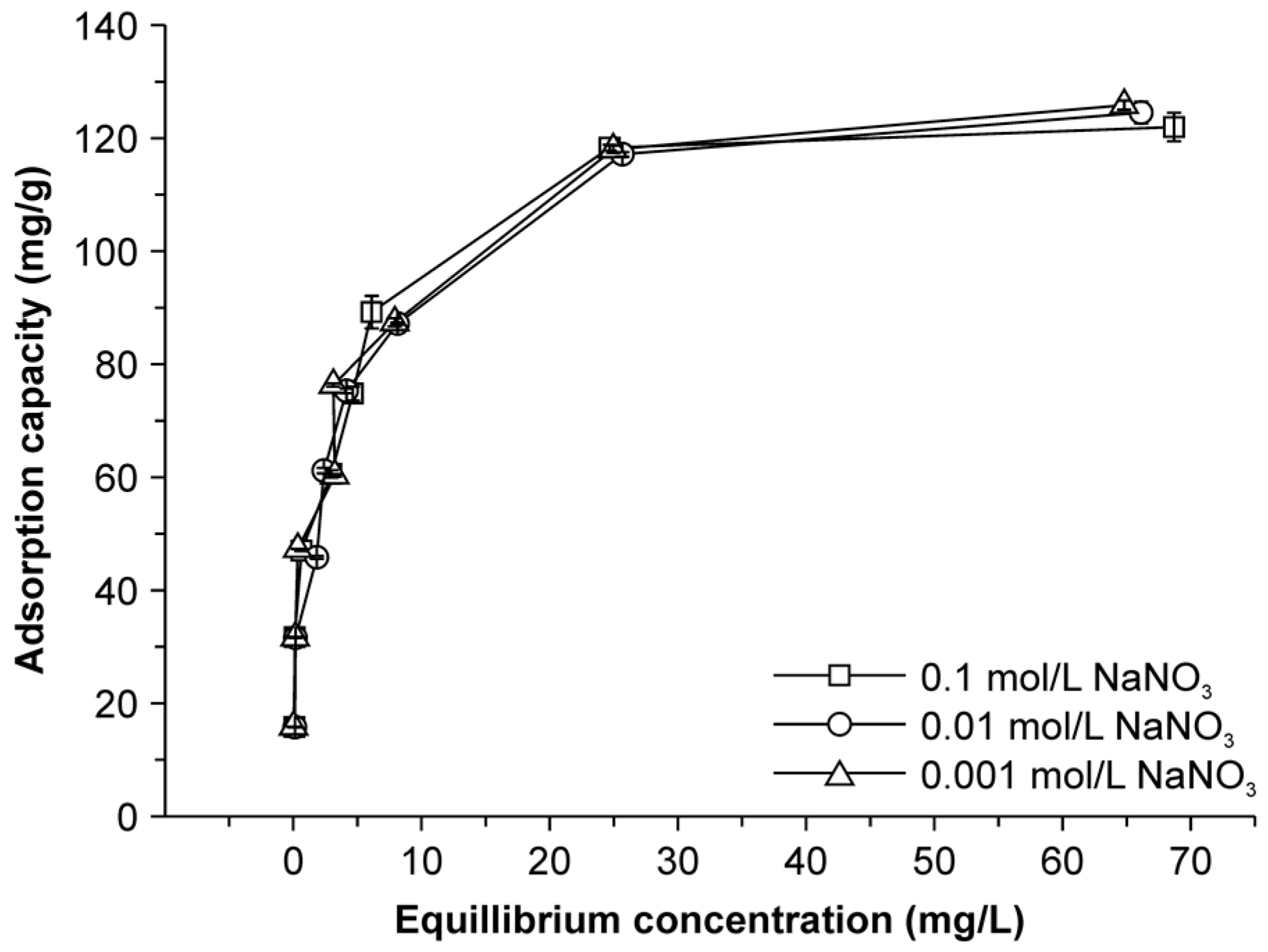
2.6. Effect of Ionic Strength on Adsorption
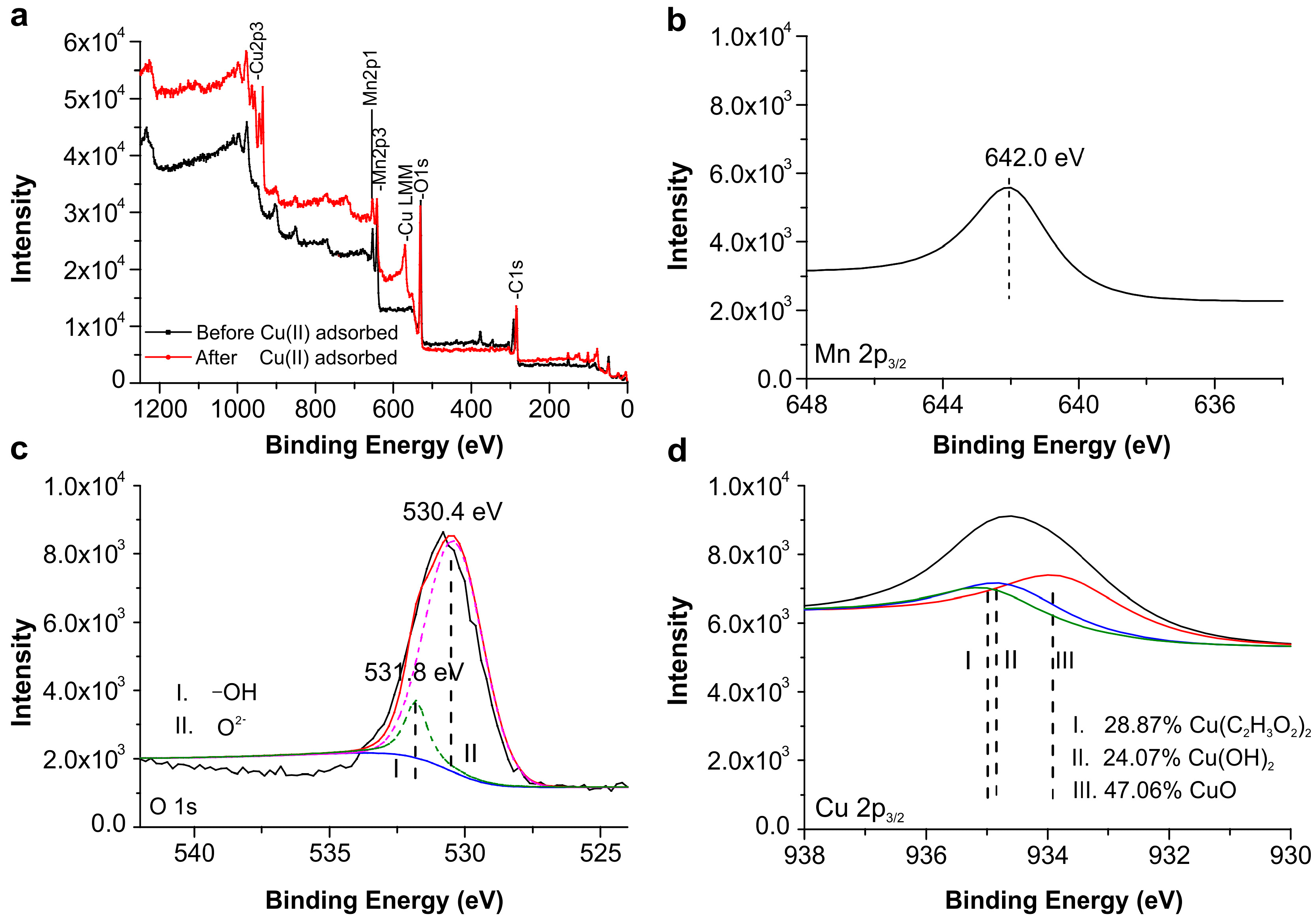
2.7. XPS Analysis
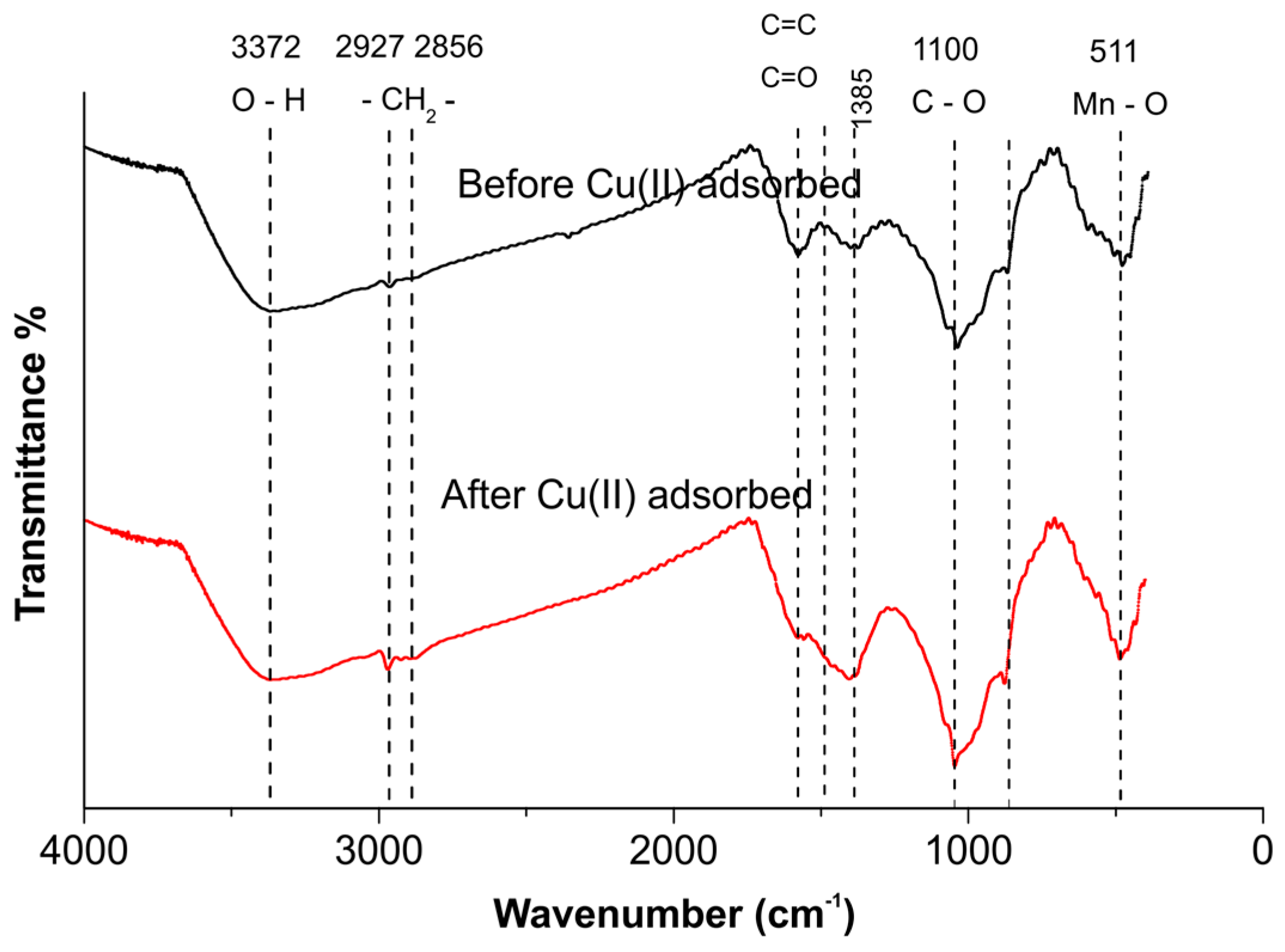
2.8. FTIR Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of NMBCs
3.2.2. Sample Characterization
3.2.3. Adsorption Experiments
3.2.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Aryal, M.; Ziagova, M.G.; Liakopoulou-Kyriakides, M. Cu(II) biosorption and competitive studies in multi-ions aqueous systems by Arthrobacter sp. Sphe3 and Bacillus sphaericus cells: Equillibrium and thermodynamic studies. Water Air Soil Pollut. 2012, 223, 5119–5130. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Peternele, W.S.; Winkler-Hechenleitner, A.A.; Gómez Pineda, E.A. Adsorption of Cd(II) and Pb(II) onto functionalized formic lignin from sugar cane bagasse. Bioresour. Technol. 1999, 68, 95–100. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Rao, M.M.; Ramesh, A.; Rao, G.P.C.; Seshaiah, K. Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J. Hazard. Mater. 2006, 129, 123–129. [Google Scholar] [PubMed]
- Djeribi, R.; Hamdaoui, O. Sorption of copper(II) from aqueous solutions by cedar sawdust and crushed brick. Desalination 2008, 225, 95–112. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Aydın, H.; Bulut, Y.; Yerlikaya, Ç. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Mosa, A.; Zimmerman, A.R.; Ma, L.Q.; Harris, W.G.; Migliaccio, K.W. Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol. 2015, 181, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, S.E.; Hochella, M.F., Jr. Lead sorption efficiencies of natural and synthetic Mn and Fe-oxides. Geochim. Cosmochim. Acta 2003, 67, 4471–4487. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Kanungo, S.B. Adsorption of Co2+, Ni2+, Cu2+ and Zn2+ from 0.5 M NaCl and major ion sea water on a mixture of δ-MnO2 and amorphous FeOOH. J. Colloid Interface Sci. 2005, 284, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.G.; Lopez, M.L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Determination of arsenic(III) and arsenic(V) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents. Microchem. J. 2009, 91, 100–106. [Google Scholar] [CrossRef]
- Bajpai, S.; Chaudhuri, M. Removal of arsenic from ground water by manganese dioxide-coated sand. J. Environ. Eng. 1999, 125, 782–784. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, Z.; Ma, H.; Qiu, Y.; Zhao, J. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. J. Environ. Sci. 2010, 22, 225–229. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Lisha, K.P.; Pradeep, T. A novel cellulose-manganese oxide hybrid material by in situ soft chemical synthesis and its application for the removal of Pb(II) from water. J. Hazard. Mater. 2010, 181, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Bandosz, T.J.; Zhao, Z.; Han, M.; Qiu, J. Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes. J. Hazard. Mater. 2009, 167, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yan, N.; Feng, J.; Ma, J.; Wen, Q.; Li, N.; Dong, Q. Adsorption mechanism of copper and lead ions onto graphene nanosheet/δ-MnO2. Mater. Chem. Phys. 2012, 136, 538–544. [Google Scholar] [CrossRef]
- Sharma, Y.C. Thermodynamics of removal of cadmium by adsorption on an indigenous clay. Chem. Eng. J. 2008, 145, 64–68. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, D.; Zhu, S.; Hu, X. Adsorption of cadmium(II) on humic acid coated titanium dioxide. J. Colloid Interface Sci. 2012, 367, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-G.; Gong, W.-X.; Liu, X.-W.; Yao, Y.W.; Gao, B.-Y.; Yue, Q.-Y. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Wagner, C.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Data for Use in X-ray Photoelectron Spectroscopy; Physical Electronics Division, Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Hu, C.; Xing, S.; Qu, J.; He, H. Catalytic ozonation of herbicide 2,4-D over cobalt oxide supported on mesoporous zirconia. J. Phys. Chem. C 2008, 112, 5978–5983. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- López-Ramón, V.; Moreno-Castilla, C.; Rivera-Utrilla, J.; Radovic, L.R. Ionic strength effects in aqueous phase adsorption of metal ions on activated carbons. Carbon 2003, 41, 2020–2022. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Wei, B. Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudocapacitance properties in neutral electrolyte. Chem. Phys. Lett. 2008, 453, 242–249. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds can be obtained from the authors. Or not available.
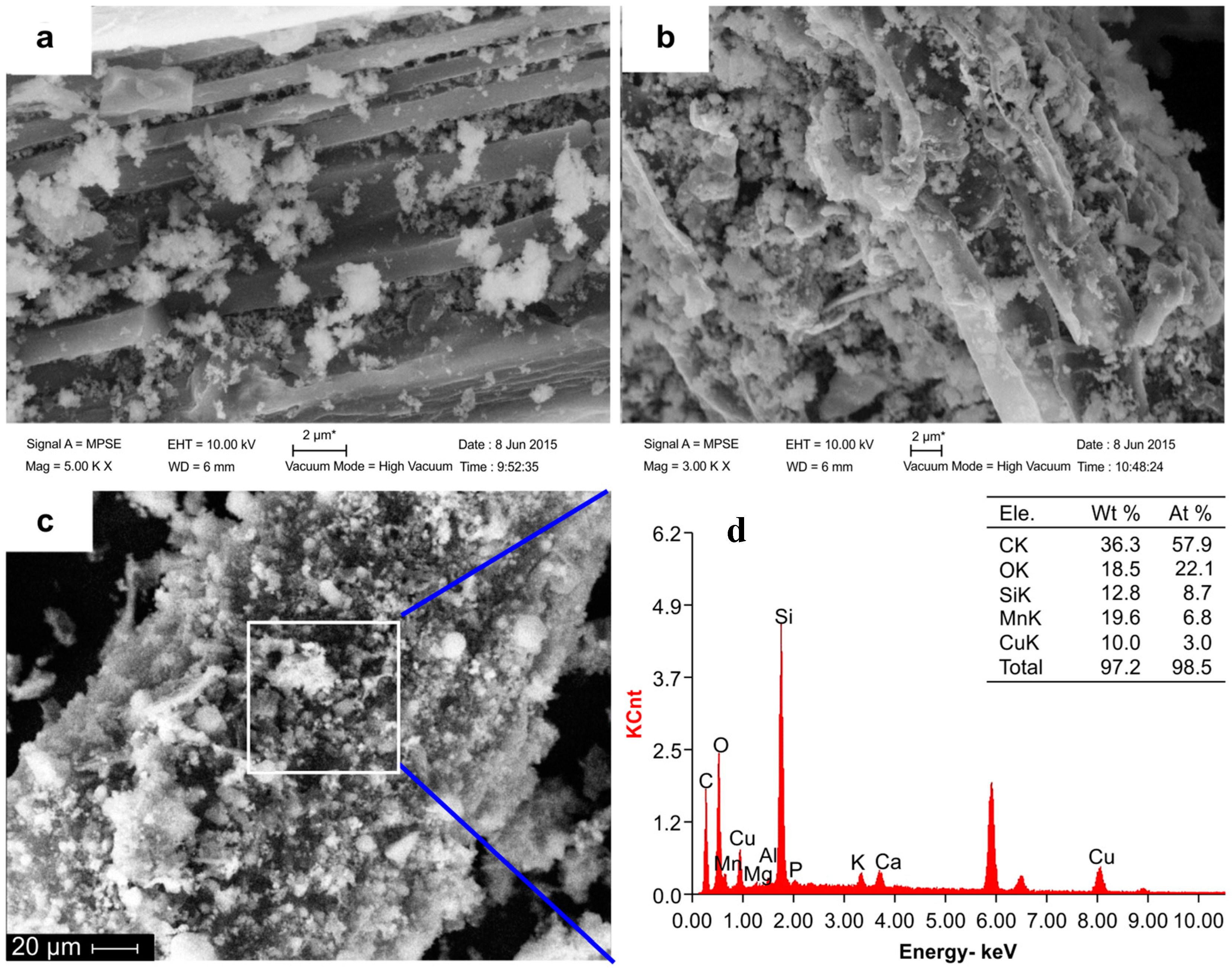
| Sample | Bulk Elemental Composition (%) | Surface Atomic Composition (%) | Ash Content (%) | SBET (m2/g) | Pore Width (nm) | Vtot (cm3/g) | pHZPC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | C | O | Mn | ||||||
| BC | 85.3 | 5.2 | 5.16 | 0.81 | 75.0 | 15.3 | - | 10.2 | 61.0 | 23 | 0.036 | 10.0 |
| NMnO2 | - | - | - | - | 17.48 | 45.38 | 31.91 | - | 161 | 2.54 | 0.020 | 7.80 |
| NMBCs | 73.4 | 2.1 | 17.2 | 0.68 | 35.6 | 41.2 | 19.68 | 12.6 | 80.3 | 3.86 | 0.013 | 11.0 |
| Samples | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe | K1 | R2 | qe | K2 | R2 | |
| NMBCs | 105.01 | 0.2 | 0.52 | 110.86 | 12.26 | 0.99 |
| NMnO2 | 76.10 | 0.22 | 0.55 | 75.99 | 0.025 | 0.99 |
| Samples | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF (mg1−n·Ln/g) | n | R2 | qm (mg/g) | b (L/mg) | R2 | |
| BC | 23.77 (0.35) | 3.40 (0.06) | 0.923 | 26.88 (0.23) | 0.57 (0.07) | 0.984 |
| NMnO2 | 298.7 (0.27) | 26.96 (0.11) | 0.971 | 93.91 (0.22) | 0.57 (0.05) | 0.954 |
| NMBCs | 8316.6 (0.36) | 632.91 (0.02) | 0.974 | 142.02 (0.31) | 0.81 (0.02) | 0.978 |
| T (K) | qe (mg/g) | ln Ke | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/K·mol) | R |
|---|---|---|---|---|---|---|
| 288 | 139.95 | 4.35 | −10.41 | 0.45 | 0.038 | 0.980 |
| 298 | 142.02 | 4.32 | −10.71 | 0.45 | 0.037 | 0.985 |
| 308 | 144.45 | 4.36 | −11.16 | 0.45 | 0.038 | 0.983 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Huang, Y.; Qiu, W.; Sun, Z.; Liu, Z.; Song, Z. Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution. Molecules 2017, 22, 173. https://doi.org/10.3390/molecules22010173
Zhou L, Huang Y, Qiu W, Sun Z, Liu Z, Song Z. Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution. Molecules. 2017; 22(1):173. https://doi.org/10.3390/molecules22010173
Chicago/Turabian StyleZhou, Li, Yifan Huang, Weiwen Qiu, Zhanxiang Sun, Zhongqi Liu, and Zhengguo Song. 2017. "Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution" Molecules 22, no. 1: 173. https://doi.org/10.3390/molecules22010173






