Meadowsweet Teas as New Functional Beverages: Comparative Analysis of Nutrients, Phytochemicals and Biological Effects of Four Filipendula Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nutritional Profiles of Meadowsweet Teas
2.2. General Phytochemical Characteristics of Meadowsweet Teas
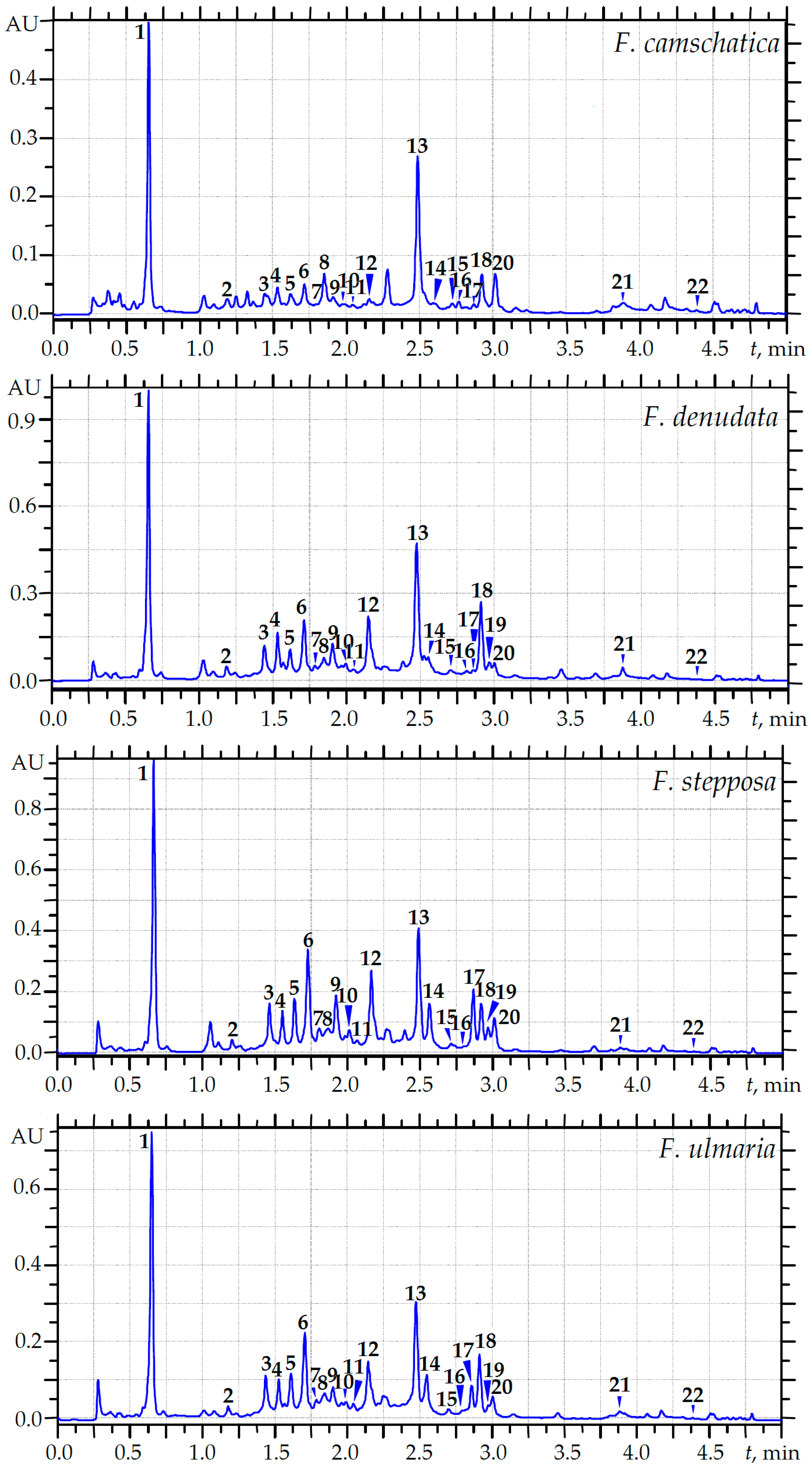
2.3. MC-RP-HPLC Quantification of the Main Phenolics of Meadowsweet Teas
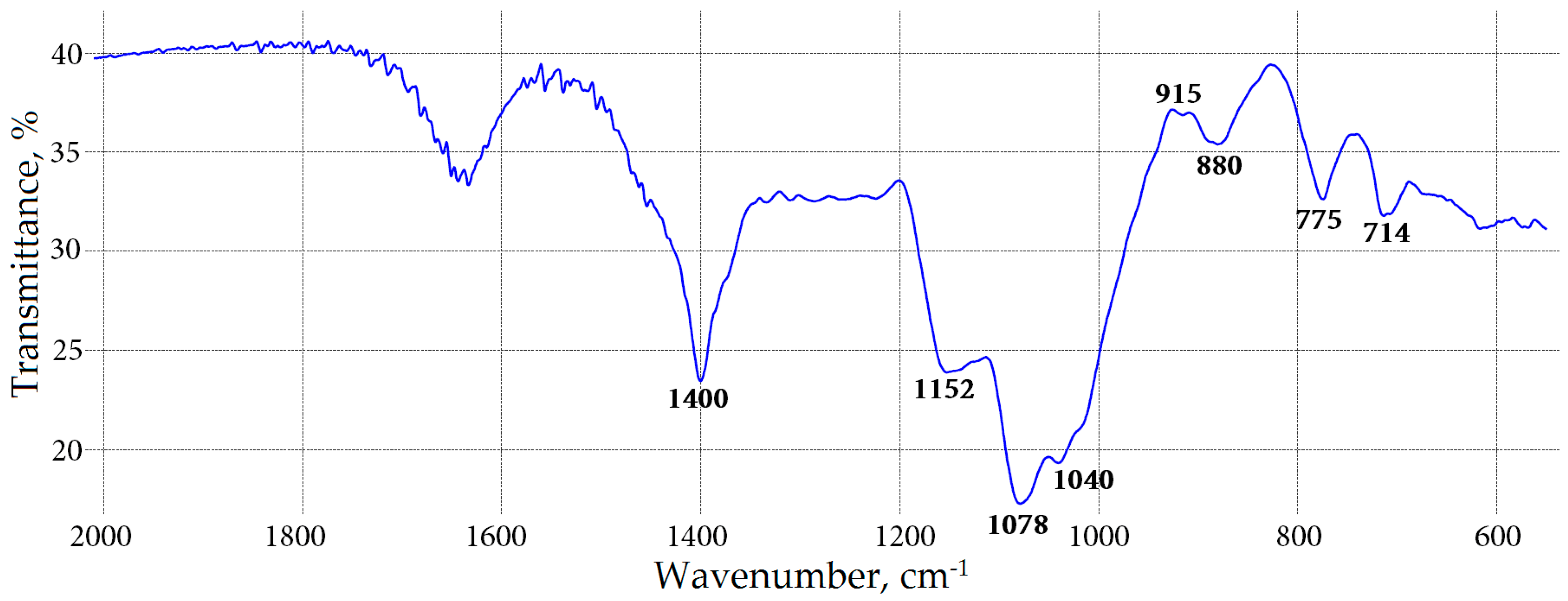
2.4. Essential Oil Compositions of Meadowsweet Flowers
2.5. Water-Soluble Polysaccharide Characterization of Meadowsweet Teas
2.6. Biological Activity of Meadowsweet Floral Teas Decoctions
2.6.1. Inhibitory Effect on Amylase, α-Glucosidase and AGEs Formation
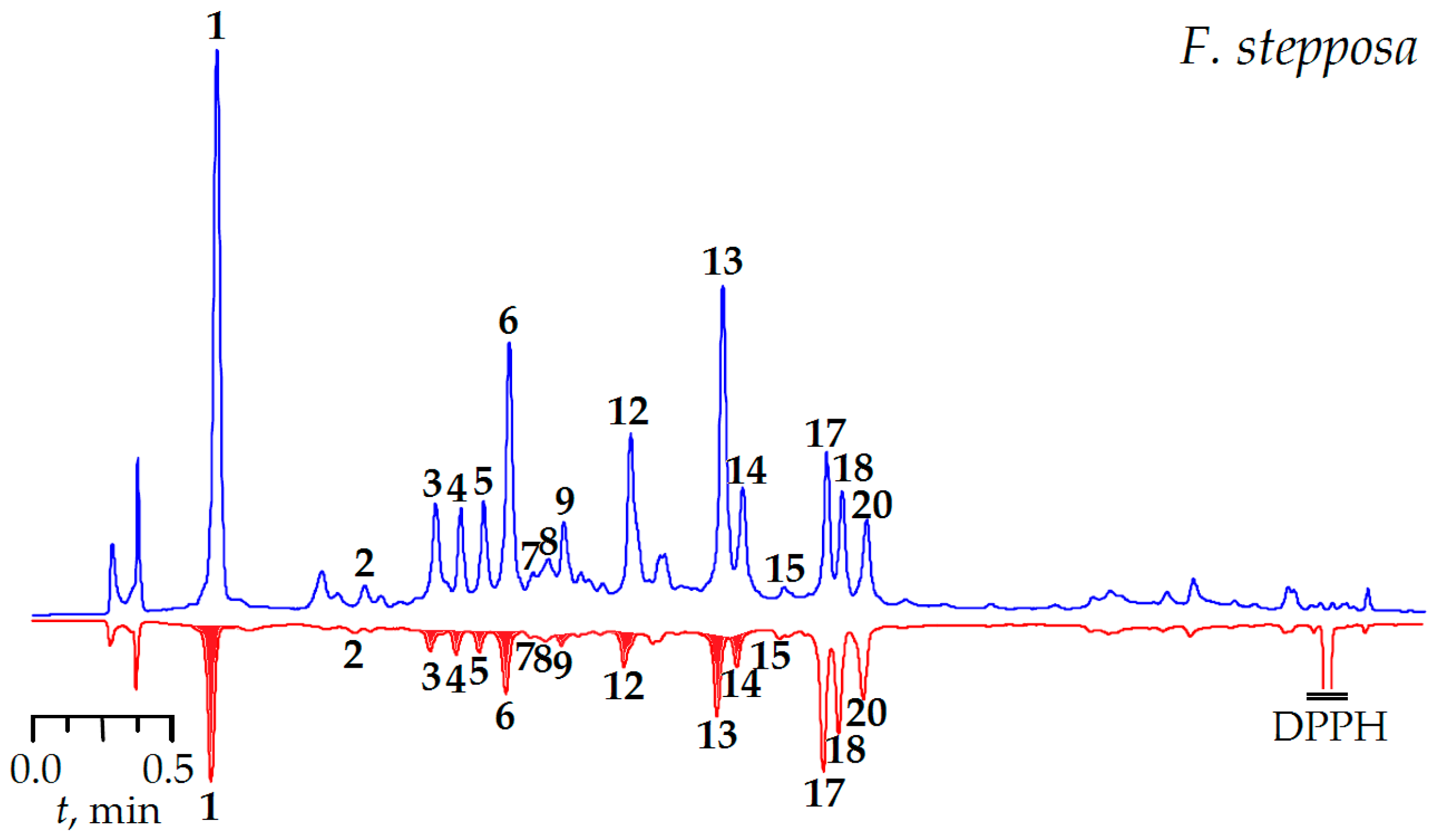
2.6.2. Antioxidant Activity
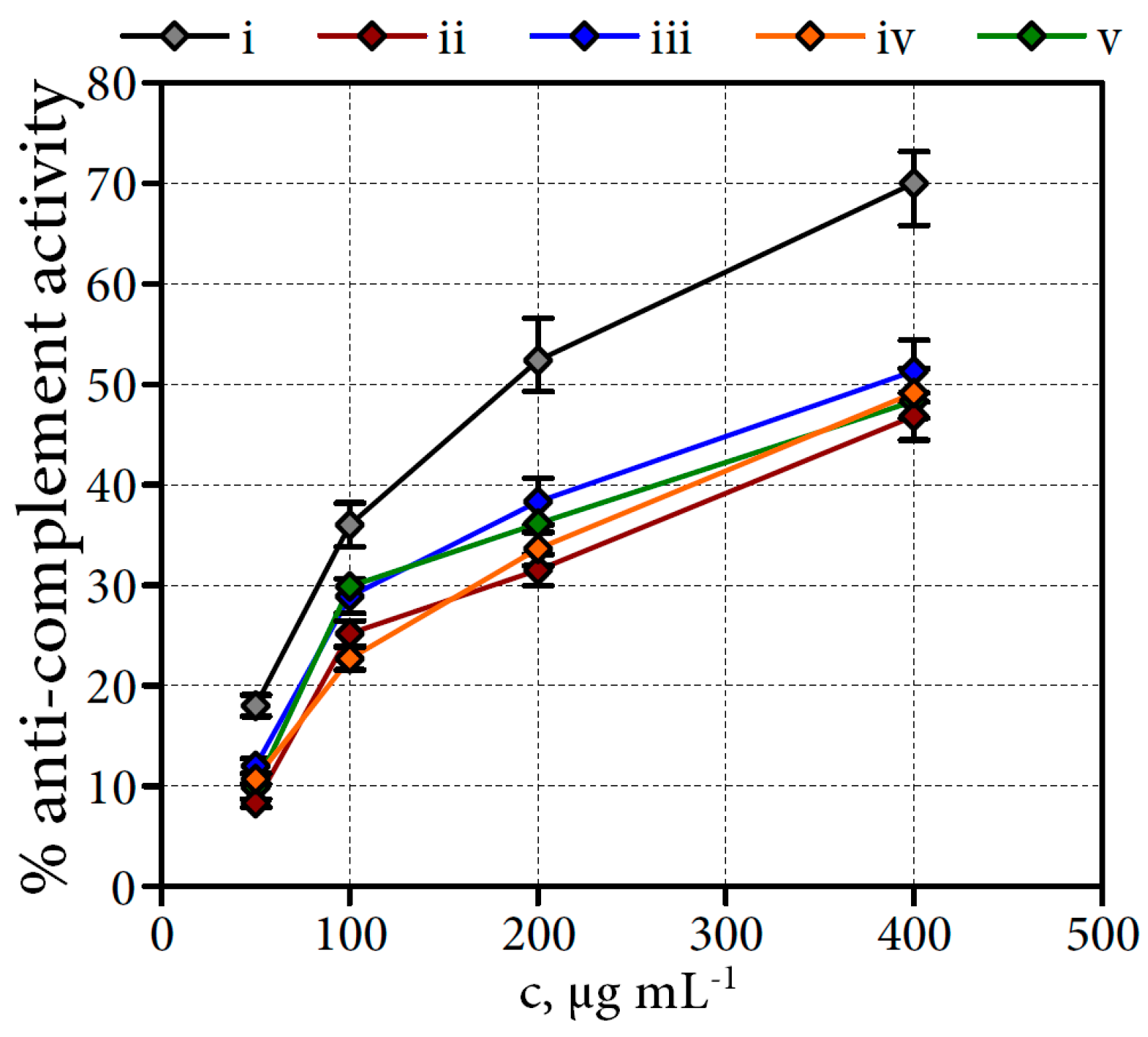
2.6.3. Anti-Complement Activity of Filipendula Flower Water-Soluble Polysaccharides
3. Materials and Methods
3.1. Plant Materials and Chemicals
3.2. Organoleptic and Nutritional Analysis
3.2.1. Decoction Preparation
3.2.2. Crude Composition
3.2.3. Free Sugars Composition
3.2.4. Organic Acids Composition
3.2.5. Amino Acids Composition
3.2.6. Minerals Composition
3.3. MC-RP-HPLC Quantification of Phytochemicals in Meadowsweet Teas
3.4. Essential Oil Analysis
3.5. Polysaccharide Analysis
3.6. Biological Activity
3.6.1. Amylase Inhibitory Activity
3.6.2. α-Glucosidase Inhibitory Activity
3.6.3. AGEs Formation Inhibitory Activity
3.6.4. DPPH-HPLC-UV Procedure
3.6.5. DPPH• Radical Scavenging Activity
3.6.6. ABTS•+ Radical Scavenging Activity
3.6.7. Br• Radical Scavenging Activity
3.6.8. β-Carotene Bleaching Assay
3.6.9. Complement Fixation Test
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zakaria, N.Z.I.; Masnan, M.J.; Zakaria, A.; Shakaff, A.Y.M. A bio-inspired herbal tea flavor assessment technique. Sensors 2014, 14, 12233–12255. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. Value chains of herbal medicines—Ethnopharmacological and analytical challenges in a globalizing world. In Evidence-Based Validation of Herbal Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 29–42. [Google Scholar]
- Junior, E.L.C.; Morand, C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health—A review. J. Funct. Foods 2016, 21, 440–454. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Tankhaeva, L.M. Iridoids and flavonoids of four Siberian gentians: Chemical profile and gastric stimulatory effect. Molecules 2015, 20, 19172–19188. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Koryakina, L.P.; Vladimirov, L.N. Bitter gentian teas: Nutritional and phytochemical profiles, polysaccharide characterisation and bioactivity. Molecules 2015, 20, 20014–20030. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, K.; Goetz, P.; Jeune, R. Reine-des-prés (sommité fleurie de) Filipendula ulmariae (L.) Maxim. Phytothérapie 2011, 9, 318–322. [Google Scholar] [CrossRef]
- Lindeman, A.; Jounelaeriksson, P.; Lounasmaa, M. The aroma composition of the flower of meadowsweet (Filipendula ulmaria (L.) Maxim). Lebensm. Wiss. Technol. 1982, 15, 286–289. [Google Scholar]
- Toiu, A.; Vlase, L.; Oniga, I.; Benedec, D.; Tămaş, M. HPLC analysis of salicylic derivatives from natural products. Farmacia 2011, 59, 106–112. [Google Scholar]
- Blazics, B.; Papp, I.; Kery, A. LC-MS qualitative analysis and simultaneous determination of six Filipendula salicylates with two standards. Chromatographia 2010, 71, S61–S67. [Google Scholar] [CrossRef]
- Wilkes, S.; Glasl, H. Isolation, characterization, and systematic significance of 2-pyrone-4,6-dicarboxylic acid in Rosaceae. Phytochemistry 2001, 58, 441–449. [Google Scholar] [CrossRef]
- Bijttebier, S.; Van der, A.A.; Voorspoels, S.; Noten, B.; Hermans, N.; Pieters, L.; Apers, S. A first step in the quest for the active constituents in Filipendula ulmaria (meadowsweet): Comprehensive phytochemical identification by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. Planta Med. 2016, 82, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Bączek, K.; Cygan, M.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z. Seasonal variation of phenolics content in above- and underground organs of dropwort (Filipendula vulgaris Moench). Herba Pol. 2012, 58, 24–32. [Google Scholar]
- Pemp, E.; Reznicek, G.; Krenn, L. Fast quantification of flavonoids in Filipendulae ulmariae flos by HPLC/ESI-MS using a nonporous stationary phase. J. Anal. Chem. 2007, 62, 669–673. [Google Scholar] [CrossRef]
- Abe, I.; Kashiwagi, Y.; Noguchi, H.; Tanaka, T.; Ikeshiro, Y.; Kashiwada, Y. Ellagitannins and hexahydroxydiphenoyl esters as inhibitors of vertebrate squalene epoxidase. J. Nat. Prod. 2001, 64, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.; Mišić, M.; Aleksić, J.; Ðoković, D.; Palić, R.; Stojanović, G. Antimicrobial synergism and antagonism of salicylaldehyde in Filipendula vulgaris essential oil. Fitoterapia 2007, 78, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Petrovic, S.; Ristic, M.; Maksimovic, Z.; Kovacevic, N. Essential oil of Filipendula hexapetala. Chem. Nat. Comp. 2007, 43, 228–229. [Google Scholar] [CrossRef]
- Karimova, O.A.; Zhigunov, O.Y. Introduction of some varieties of Filipendula Mill. genus in Ufa Botanic Garden. Biol. Sci. 2016, 58, 146–148. (In Russian) [Google Scholar]
- Gudkova, N.Y. Perspectives of introduction of meadowsweet (Filipendula Mill.) as a source of medicinal raw material. Agric. Biol. 2012, 47, 73–79. (In Russian) [Google Scholar]
- Olennikov, D.N.; Kruglova, M.Y. A new quercetin glycoside and other phenolic compounds from the genus Filipendula. Chem. Nat. Comp. 2013, 49, 610–616. [Google Scholar] [CrossRef]
- Aseeva, T.A. Tibetan Medicine of Buryats; Publishing House of Russian Academy of Science: Novosibirsk, Russia, 2008. [Google Scholar]
- Makarov, A.A. Plant remedies of the Traditional Yakutian Medicine; Yakutian Scientific Center: Yakutsk, Russia, 1974. [Google Scholar]
- Anonymous. Buryats; Nauka: Moscow, Russia, 2004. [Google Scholar]
- Zykova, I.D.; Efremov, A.A.; Gerasimov, V.S.; Leshok, A.A. Features of the accumulation of macro- andmicronutrients in the aboveground parts of Filipendula ulmaria (L.) Maxim. in different phonological phases. Chem. Plant Raw Mater. 2013, 16, 189–193. [Google Scholar]
- Enel, M. Distribution of heavy metals in plants and their habitats in the outcrop area of Dictyonema shale. Oil Shale 2003, 20, 459–476. [Google Scholar]
- Szefer, P.; Nriagu, J.O. Mineral Components in Foods; CRC Press, Taylor Francis Group: London, UK; New York, NY, USA, 2007. [Google Scholar]
- Valle, M.G.; Nano, G.M.; Tira, S. The essential oil of Filipendula ulmaria. Planta Med. 1988, 54, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, X.; Liu, X.; Bao, Y.; Wang, S. Analysis of essential oils from flower-buds, leaves and stems of Filipendula palmata (Pall.) Maxim. Chem. Res. Chin. Univ. 2005, 21, 658–662. [Google Scholar]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Arifkhodzhaev, A.O. Galactans and galactan-containing polysaccharides of higher plants. Chem. Nat. Comp. 2000, 36, 229–244. [Google Scholar] [CrossRef]
- De Sales, P.M.; de Souza, P.M.; Simeoni, L.A.; de Olivera Magalhães, P.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from planr source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008, 10, 953–961. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tanaka, T.; Zhang, Y.J.; Yang, C.; Kouno, I. Rubusuaviins A-F, monomeric and oligomeric ellagitannins from Chinese sweet tea and their alpha-amylase inhibitory activity. Chem. Pharm. Bull. 2007, 55, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure-activity relationships of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glycosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Selenge, E.; Odontuya, G.; Murata, T.; Sasaki, K.; Kobayashi, K. Phytochemical constituents of Mongolian traditional medicinal plants, Chamaerhodos erecta and C. altaica, and its constituents prevents the extracellular matrix degradation factors. J. Nat. Med. 2013, 67, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Pukalskienė, M.; Venskutonis, P.R.; Pukalskas, A. Phytochemical characterization of Filipendula ulmaria by UPLC/Q-TOF-MS and evaluation of antioxidant activity. Rec. Nat. Prod. 2015, 9, 451–455. [Google Scholar]
- Katanić, J.; Boroja, T.; Stanković, N.; Mihailović, V.; Mladenović, M. Bioactivity, stability and phenolic characterization of Filipendula ulmaria (L.) Maxim. Food Funct. 2015, 6, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, Z.; Petrović, S.; Pavlović, M.; Kovačević, N.; Kukić, J. Antioxidant activity of Filipendula hexapetala flowers. Fitoterapia 2007, 78, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Katanić, J.; Mihailović, V.; Stanković, N.; Boroja, T.; Mladenović, M. Dropwort (Filipendula hexapetala Gilib.): Potential role as antioxidant and antimicrobial agent. EXCLI J. 2015, 14. [Google Scholar] [CrossRef]
- Pukalskiene, M.; Venskutonis, P.R.; Pukalaskas, A. Phytochemical composition and antioxidant properties of Filipendula vulgaris as a source of healthy functional ingredients. J. Funct. Foods. 2015, 15, 233–242. [Google Scholar] [CrossRef]
- Samuelsen, A.B.; Lund, I.; Djahromi, J.M.; Paulsen, B.S.; Wold, J.K. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr. Polym. 1999, 38, 133–143. [Google Scholar] [CrossRef]
- Kiyohara, H.; Yamada, H. Structure of an anti-complementary arabinogalactan from the root of Angelica acutiloba Kitagawa. Carbohydr. Res. 1989, 193, 173–192. [Google Scholar] [CrossRef]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Knutsen, S.H.; Yamada, H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydr. Polym. 1998, 35, 145–153. [Google Scholar] [CrossRef]
- Varljen, J.; Lipták, A.; Wagner, H. Structural analysis of a rhamnoarabinogalactan and arabinogalactans with immuno-stimulating activity from Calendula officinalis. Phytochemistry 1989, 28, 2379–2383. [Google Scholar] [CrossRef]
- Anonymous. Organoleptic Analysis of Herbal Ingredients; American Herbal Products Association: Silver Spring, MD, USA, 2013. [Google Scholar]
- Anonymous. Quality Control Methods for Herbal Materials; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 76, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Scholtze, H. Determination of phenylthiocarbamyl amino acids by reversed-phase high-performance liquide chromatography. J. Chromatogr. 1985, 350, 453–460. [Google Scholar] [CrossRef]
- Wolf, R.E.; Adams, M. Multi-Elemental Analysis of Aqueous Geochemical Samples by Quadrupole Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). U.S. Geological Survey Open-File Report 2015-1010. 2015. Available online: http://dx.doi.org/10.3133/ofr20151010 (accessed on 5 June 2015).
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major using the Dreywood method. Chem. Nat. Comp. 2006, 42, 265–268. [Google Scholar] [CrossRef]
- Usov, A.T.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulitara P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 35, 43–51. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Stolbikova, A.V.; Rokhin, A.V.; Khobrakova, V.B.; Tankhaeva, L.M. Polysaccharides from Fabaceae. V. α-Glucan from Sophora flavescens roots. Chem. Nat. Comp. 2011, 47, 1–6. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. A quantitative assay for total fructans in burdock (Arctium spp.) roots. Russ. J. Bioorg. Chem. 2011, 37, 893–898. [Google Scholar] [CrossRef]
- Togola, A.; Inngjerdingen, M.; Diallo, D.; Barsett, H.; Rolstad, B. Polysaccharides with complement fixing and macrophage stimulation activity from Opilia celtidifolia, isolation and partial characterization. J. Ethnopharmacol. 2007, 115, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Rokhin, A.V. Water-soluble glucans from true cardamom (Elettaria cardamomum White at Maton) seeds. Appl. Biochem. Microbiol. 2013, 49, 182–187. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef] [PubMed]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. BioMed Res. Int. 2012, 2012, 281078. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Aradate, T.; Sasaki, C.; Kojima, H.; Ohara, M. Screening sysytem for the Maillard reaction inhibitor from natural product extract. J. Health Sci. 2002, 48, 520–526. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.M.S.; Shawky, B.T. Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010, 123, 315–320. [Google Scholar] [CrossRef]
- Ding, H.; Chou, T.; Liang, C. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010, 123, 254–262. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014, 150, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Michaelsen, T.E.; Gilje, A.; Samuelsen, A.B.; Hogasen, K.; Paulsen, B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol. 2000, 52, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Tankhaeva, L.M. Lamiaceae carbohydrates. 1. Pectinic substances and hemicelluloses from Mentha × piperita. Chem. Nat. Comp. 2007, 43, 501–507. [Google Scholar] [CrossRef]
- Sample Availability: Samples of Filipendula camschatica, F. denudata, F. stepposa and F. ulmaria plants and extracts are available from the authors.
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Extractives b | 310 ± 12 | 390 ± 15 | 394 ± 15 | 374 ± 14 |
| Organoleptic characteristics | ||||
| Color | Yellow | Dark yellow | Yellow | Pale yellow |
| Odor | Specific, sharp, methyl salicylate like | Specific, sweet | Specific, sweet, honey like | Specific, sweet |
| Taste | Bitterish, astringent | Bitterish, astringent | Bitterish, astringent | Bitterish, astringent |
| Macronutrients | ||||
| Carbohydrates b | 173.81 ± 5.04 | 132.05 ± 3.82 | 163.25 ± 4.87 | 147.11 ± 3.97 |
| Protein b | 10.30 ± 0.35 | 8.57 ± 0.31 | 12.49 ± 0.40 | 9.73 ± 0.32 |
| Lipids b | <1.00 | <1.00 | <1.00 | <1.00 |
| Ash b | 54.59 ± 1.91 | 40.98 ± 1.59 | 34.40 ± 1.16 | 45.63 ± 1.59 |
| Energy c | 0.75 | 0.57 | 0.71 | 0.64 |
| Free sugars | ||||
| Fructose b | tr. | tr. | tr. | tr. |
| Galactose b | 11.22 ± 0.30 | 8.07 ± 0.21 | 9.02 ± 0.25 | 9.74 ± 0.30 |
| Glucose b | 66.31 ± 1.85 | 54.39 ± 1.63 | 67.31 ± 2.01 | 65.27 ± 2.02 |
| Sucrose b | 15.61 ± 0.43 | 11.86 ± 0.35 | 12.08 ± 0.34 | 10.18 ± 0.32 |
| Total free sugars b | 93.14 | 74.32 | 88.41 | 85.19 |
| Organic acids | ||||
| Citric acid b | 5.84 ± 0.15 | 10.84 ± 0.25 | 8.24 ± 0.19 | 7.11 ± 0.17 |
| Malic acid b | 6.52 ± 0.15 | 11.67 ± 0.29 | 10.30 ± 0.26 | 9.58 ± 0.24 |
| Oxalic acid b | 9.62 ± 0.24 | 6.04 ± 0.14 | 4.93 ± 0.12 | 5.74 ± 0.12 |
| Quinic acid b | 0.11 ± 0.00 | tr. | tr. | tr. |
| Succinic acid b | tr. | 0.10 ± 0.00 | tr. | tr. |
| Tartaric acid b | tr. | 0.35 ± 0.01 | 0.19 ± 0.00 | 0.27 ± 0.00 |
| Total organic acids b | 22.09 | 29.00 | 23.66 | 22.70 |
| Amino acids | ||||
| Alanine b | 0.89 ± 0.02 | 0.26 ± 0.00 | 0.62 ± 0.02 | 0.75 ± 0.02 |
| Arginine b | 1.28 ± 0.04 | 0.32 ± 0.01 | 0.98 ± 0.03 | 0.73 ± 0.02 |
| Asparagine b | 0.56 ± 0.01 | 0.39 ± 0.01 | 0.58 ± 0.02 | 0.26 ± 0.00 |
| Aspartic acid b | 3.35 ± 0.07 | 0.90 ± 0.02 | 3.71 ± 0.08 | 2.03 ± 0.05 |
| Cisteine b | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| Glutamine b | 0.02 ± 0.00 | 0.10 ± 0.00 | 0.14 ± 0.00 | 0.08 ± 0.00 |
| Glutamic acid b | 3.65 ± 0.09 | 0.68 ± 0.02 | 2.59 ± 0.06 | 1.48 ± 0.03 |
| Glycine b | 1.26 ± 0.04 | 0.29 ± 0.00 | 0.84 ± 0.02 | 0.67 ± 0.02 |
| Histidine b | 0.81 ± 0.02 | 0.08 ± 0.00 | 0.37 ± 0.01 | 0.35 ± 0.00 |
| Isoleicine b | 0.97 ± 0.03 | 0.17 ± 0.00 | 0.65 ± 0.01 | 0.59 ± 0.02 |
| Leucine b | 1.55 ± 0.04 | 0.72 ± 0.02 | 1.45 ± 0.03 | 1.04 ± 0.03 |
| Lysine b | 1.44 ± 0.04 | 0.28 ± 0.00 | 0.77 ± 0.02 | 0.86 ± 0.02 |
| Methionine b | 1.01 ± 0.02 | 0.12 ± 0.00 | 0.40 ± 0.01 | 0.14 ± 0.00 |
| Phenyalanine b | 0.89 ± 0.03 | 0.23 ± 0.00 | 0.57 ± 0.01 | 0.73 ± 0.01 |
| Proline b | 0.77 ± 0.02 | 0.18 ± 0.00 | 0.85 ± 0.02 | 0.72 ± 0.01 |
| Serine b | 1.43 ± 0.04 | 0.31 ± 0.01 | 0.78 ± 0.02 | 0.69 ± 0.01 |
| Treonine b | 0.75 ± 0.01 | 0.14 ± 0.00 | 0.38 ± 0.01 | 0.58 ± 0.01 |
| Tyrosine b | 0.65 ± 0.01 | 0.11 ± 0.00 | 0.02 ± 0.00 | 0.42 ± 0.01 |
| Valine b | 1.25 ± 0.03 | 0.32 ± 0.01 | 0.71 ± 0.01 | 0.76 ± 0.02 |
| Total amino acids b | 22.58 | 5.61 | 16.44 | 12.89 |
| Minerals | ||||
| Calcium d | 396.29 ± 28.93 | 295.84 ± 23.08 | 239.42 ± 22.51 | 273.72 ± 24.09 |
| Chromium d | 0.16 ± 0.01 | 0.23 ± 0.02 | 0.29 ± 0.02 | 0.20 ± 0.02 |
| Cobalt d | 0.11 ± 0.01 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 |
| Copper d | 4.83 ± 0.44 | 5.31 ± 0.45 | 4.38 ± 0.40 | 4.74 ± 0.42 |
| Iron d | 4.84 ± 0.42 | 4.70 ± 0.40 | 5.06 ± 0.41 | 11.12 ± 1.01 |
| Magnesium d | 4923.48 ± 428.34 | 3266.55 ± 264.59 | 2726.8 ± 245.41 | 2730.73 ± 251.23 |
| Manganese d | 2.78 ± 0.22 | 2.69 ± 0.22 | 1.21 ± 0.11 | 6.30 ± 0.60 |
| Molybdenum d | 0.03 ± 0.00 | 0.08 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.00 |
| Nickel d | 0.33 ± 0.02 | 1.24 ± 0.10 | 1.43 ± 0.12 | 1.28 ± 0.11 |
| Selenium d | 0.34 ± 0.03 | 0.01 ± 0.00 | 0.16 ± 0.01 | 0.26 ± 0.02 |
| Zinc d | 14.51 ± 1.03 | 16.14 ± 1.48 | 11.88 ± 1.01 | 12.55 ± 0.99 |
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Flavonoids | 18.34 ± 0.42 | 50.92 ± 1.22 | 52.25 ± 1.35 | 55.31 ± 1.43 |
| Tannins | 51.83 ± 1.50 | 114.81 ± 3.55 | 120.18 ± 3.72 | 100.88 ± 3.21 |
| Catechins | 9.57 ± 0.39 | 1.27 ± 0.05 | tr. | 5.69 ± 0.24 |
| Proanthocyanidins | 4.64 ± 0.15 | 2.15 ± 0.06 | 5.14 ± 0.16 | 3.83 ± 0.12 |
| WS-Polysaccharides | 21.39 ± 0.56 | 24.51 ± 0.61 | 30.47 ± 0.94 | 23.81 ± 0.59 |
| Compound | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Flavonoids | ||||
| Kaempferol-3-O-α-l-rhamnoside | tr. | 2.27 ± 0.07 | 2.78 ± 0.07 | 0.87 ± 0.02 |
| Kaempferol-4′-O-β-d-glucoside | 5.43 ± 0.14 | 4.70 ± 0.14 | 6.50 ± 0.19 | 5.71 ± 0.12 |
| Quercetin | 0.40 ± 0.01 | 1.71 ± 0.04 | 0.72 ± 0.02 | 1.20 ± 0.03 |
| Quercetin-3-O-β-d-glucoside | 0.74 ± 0.02 | 4.95 ± 0.14 | 10.18 ± 0.30 | 6.92 ± 0.16 |
| Quercetin-3-O-α-l-arabinoside | 0.76 ± 0.02 | 5.96 ± 0.17 | 4.21 ± 0.13 | 2.60 ± 0.05 |
| Quercetin-3-O-α-l-rhamnoside | 0.29 ± 0.01 | 1.18 ± 0.03 | 9.50 ± 0.27 | 3.92 ± 0.09 |
| Quercetin-4′-O-β-d-glucoside | 6.43 ± 0.17 | 28.50 ± 0.71 | 17.91 ± 0.45 | 17.53 ± 0.42 |
| Quercetin-3-O-β-d-glucuronide | 0.46 ± 0.01 | tr. | tr. | tr. |
| Subtotal | 14.51 | 49.27 | 51.80 | 38.75 |
| Ellagitannins | ||||
| Tellimagrandin I1 | 1.32 ± 0.03 | 7.61 ± 0.19 | 9.30 ± 0.25 | 4.56 ± 0.10 |
| Tellimagrandin I2 | 2.28 ± 0.06 | 6.58 ± 0.19 | 10.65 ± 0.29 | 6.71 ± 0.15 |
| Tellimagrandin II | 0.59 ± 0.02 | 2.27 ± 0.06 | 2.73 ± 0.07 | 2.02 ± 0.04 |
| Rugosin B1 | 2.42 ± 0.07 | 8.24 ± 0.25 | 7.69 ± 0.19 | 5.35 ± 0.12 |
| Rugosin B2 | 2.92 ± 0.09 | 12.41 ± 0.32 | 22.44 ± 0.67 | 12.36 ± 0.29 |
| Rugosin E1 | 1.75 ± 0.05 | 9.18 ± 0.23 | 13.42 ± 0.39 | 5.80 ± 0.12 |
| Rugosin E2 | 1.03 ± 0.03 | 3.28 ± 0.09 | 5.07 ± 0.14 | 2.52 ± 0.06 |
| Rugosin D | 0.78 ± 0.02 | 15.62 ± 0.42 | 19.92 ± 0.52 | 8.75 ± 0.20 |
| Subtotal | 13.09 | 65.19 | 91.22 | 48.07 |
| Other classes | ||||
| Protocatechuic acid | 0.78 ± 0.02 | 1.24 ± 0.03 | 1.32 ± 0.03 | 0.73 ± 0.01 |
| Gallic acid | 11.80 ± 0.34 | 25.76 ± 0.67 | 26.17 ± 0.68 | 19.25 ± 0.40 |
| Ellagic acid | 10.92 ± 0.32 | 26.72 ± 0.77 | 22.64 ± 0.61 | 15.27 ± 0.36 |
| Methyl salicylate | 0.71 ± 0.02 | 0.79 ± 0.02 | 0.97 ± 0.03 | 0.92 ± 0.02 |
| Caffeic acid | 1.08 ± 0.03 | 3.88 ± 0.12 | 7.66 ± 0.22 | 4.20 ± 0.09 |
| 1,3-Di-O-caffeoylquinic acid | 6.18 ± 0.17 | 11.24 ± 0.34 | 12.47 ± 0.31 | 10.43 ± 0.23 |
| Subtotal | 31.47 | 69.63 | 71.23 | 50.80 |
| Total | 59.07 | 184.09 | 214.25 | 137.62 |
| Compound | RI | MI a | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|---|---|
| Simple phenols | ||||||
| Benzaldehyde | 956 | i, ii, iii | 0.9 | 3.2 | 2.9 | 2.3 |
| Benzyl alcohol | 1031 | i, ii, iii | 0.5 | 3.0 | 2.2 | 1.4 |
| Salicylaldehyde | 1043 | i, ii, iii | 9.0 | 45.9 | 25.7 | 35.7 |
| Ethyl benzoate | 1170 | i, ii, iii | 0.3 | 0.8 | 0.9 | 0.3 |
| Methyl salicylate | 1192 | i, ii, iii | 73.9 | 20.7 | 14.2 | 18.4 |
| Ethyl salicylate | 1386 | i, ii, iii | 5.2 | 2.4 | 0.3 | 1.1 |
| Vanillin | 1400 | i, ii, iii | tr. | 0.3 | 5.9 | 0.9 |
| Benzyl salicylate | 1872 | i, ii, iii | 4.4 | 1.2 | 0.3 | 6.3 |
| Subtotal | 94.2 | 77.5 | 52.4 | 66.4 | ||
| Monoterpenes | ||||||
| Linalool | 1098 | i, ii, iii | 0.2 | 2.2 | 5.7 | 2.3 |
| α-Terpineol | 1190 | i, ii, iii | 0.3 | 1.1 | 0.9 | 2.2 |
| Geraniol | 1252 | i, ii, iii | 0.1 | 0.5 | 1.3 | 0.3 |
| Subtotal | 0.6 | 3.8 | 7.9 | 4.8 | ||
| Sesquiterpenes | ||||||
| β-Caryophyllene | 1420 | i, ii, iii | 0.4 | 1.5 | 1.9 | 1.6 |
| Humulene | 1455 | i, ii, iii | 0.2 | 1.3 | 1.1 | 0.9 |
| Germacrene D | 1483 | i, ii, iii | 0.2 | 0.9 | 1.2 | 1.2 |
| β-(E)-Ionone | 1489 | i, ii, iii | 0.2 | 1.9 | 2.7 | 2.3 |
| δ-Amorphene | 1510 | i, ii | 0.3 | 0.4 | 0.3 | 0.4 |
| Caryophyllene oxide | 1587 | i, ii | tr. | 0.1 | tr. | tr. |
| (E)-Asarone | 1686 | i, ii | 0.1 | 0.2 | 0.3 | 0.6 |
| Subtotal | 1.4 | 6.3 | 7.5 | 7.0 | ||
| Aliphatic compounds | ||||||
| Decanal | 1205 | i, ii, iii | tr. | 0.9 | 2.9 | 1.6 |
| Dodecanal | 1408 | i, ii, iii | tr. | 0.2 | 1.3 | 0.3 |
| Tetradecanal | 1610 | i, ii | tr. | 0.3 | 0.9 | 0.4 |
| Pentadecanal | 1712 | i, ii | 0.1 | 1.0 | 2.2 | 1.9 |
| Hexadecanal | 1816 | i, ii | 2.9 | 1.3 | 6.3 | 5.2 |
| Heptadecanal | 1919 | i, ii | 0.2 | 5.7 | 9.4 | 6.9 |
| n-Docosane | 2200 | i, ii | tr. | 0.2 | 0.9 | 0.3 |
| n-Tricosane | 2300 | i, ii | tr. | 0.1 | 1.2 | 0.2 |
| n-Tetracosane | 2400 | i, ii | tr. | 0.1 | 0.3 | 0.7 |
| n-Pentacosane | 2500 | i, ii | tr. | 0.2 | 0.9 | 0.3 |
| Subtotal | 3.2 | 10.0 | 26.3 | 17.8 | ||
| Total | 99.4 | 97.6 | 94.1 | 96.0 | ||
| Yield, % b | 0.02 | 0.07 | 0.11 | 0.05 | ||
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Yield, mg/g | 11.75 | 13.03 | 17.07 | 17.24 |
| Total carbohydrate content, mg/g | 973.64 ± 30.47 | 982.50 ± 28.63 | 989.47 ± 31.20 | 980.52 ± 30.39 |
| Uronic acid content, mg/g | 102.27 ± 3.40 | 63.18 ± 2.24 | 79.30 ± 3.01 | 90.45 ± 3.43 |
| Protein content, mg/g | 23.18 ± 0.61 | 14.37 ± 0.35 | 12.60 ± 0.30 | 17.22 ± 0.49 |
| Reaction with I2 | positive | positive | positive | positive |
| Reaction with resorcinol | negative | negative | negative | negative |
| Reaction with Yariv reagent | positive | positive | positive | positive |
| Monosaccharide composition, mol % | ||||
| Ara | 10.5 | 4.2 | 5.1 | 6.3 |
| Gal | 48.8 | 43.0 | 46.4 | 40.5 |
| Glc | 23.4 | 36.4 | 29.0 | 33.6 |
| Fuc | 0.2 | 0.5 | 0.4 | 0.9 |
| Man | 7.0 | 6.3 | 6.9 | 6.0 |
| Rha | 0.8 | 2.5 | 3.1 | 1.6 |
| Xyl | 0.4 | 1.1 | 1.2 | 2.7 |
| GalA | 8.8 | 5.9 | 7.8 | 8.3 |
| Method | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Amy b | >100 | 74.80 ± 2.54 ii | 89.67 ± 2.78 | 85.52 ± 2.90 ii |
| Glu b | ~100 | 78.53 ± 2.28 | 71.35 ± 2.06 iv | 76.14 ± 2.74 iv |
| AGE c | 10.27 ± 0.41 v | 52.11 ± 1.87 vi | 61.18 ± 2.20 vi | 32.90 ± 1.22 v |
| Spiraeoside | Rugosin D | Methyl salicylate | Acarbose | |
| Amy b | >100 | 6.27 ± 0.21 i | >100 | 10.57 ± 0.34 i |
| Glu b | >100 | 4.82 ± 0.16 iii | >100 | 38.60 ± 1.34 iii |
| AGE d | <5 | 69.72 ± 2.37 | <5 | n.d. e |
| Method b | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| DPPH• | 23.72 ± 0.59 ii | 8.13 ± 0.21 ii | 7.33 ± 0.19 ii | 10.43 ± 0.26 ii |
| ABTS•+ | 9.76 ± 0.23 iv | 4.97 ± 0.11 iii | 4.08 ± 0.08 iii | 5.74 ± 0.12 iii |
| Br•− | 111.89 ± 2.12 v | 241.95 ± 4.35 vi | 259.64 ± 4.67 vi | 228.11 ± 4.33 v, vi |
| CBA | 21.16 ± 0.76 | 4.11 ± 0.15 ix | 3.53 ± 0.12 viii, ix | 4.55 ± 0.16 ix |
| Spiraeoside | Rugosin D | Methyl salicylate | Trolox | |
| DPPH• | 82.40 ± 2.14 | 5.36 ± 0.11 i | >100 | 7.40 ± 0.14 i |
| ABTS•+ | 7.02 ± 0.14 iii, iv | 0.70 ± 0.01 | 59.18 ± 1.24 | 4.27 ± 0.08 iii |
| Br•− | 1046.82 ± 19.88 vii | 1293.63 ± 24.58 vii | 331.53 ± 5.96 vi | 663.25 ± 12.98 |
| CBA | >100 | 2.62 ± 0.09 | >100 | 2.70 ± 0.10 viii |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Meadowsweet Teas as New Functional Beverages: Comparative Analysis of Nutrients, Phytochemicals and Biological Effects of Four Filipendula Species. Molecules 2017, 22, 16. https://doi.org/10.3390/molecules22010016
Olennikov DN, Kashchenko NI, Chirikova NK. Meadowsweet Teas as New Functional Beverages: Comparative Analysis of Nutrients, Phytochemicals and Biological Effects of Four Filipendula Species. Molecules. 2017; 22(1):16. https://doi.org/10.3390/molecules22010016
Chicago/Turabian StyleOlennikov, Daniil N., Nina I. Kashchenko, and Nadezhda K. Chirikova. 2017. "Meadowsweet Teas as New Functional Beverages: Comparative Analysis of Nutrients, Phytochemicals and Biological Effects of Four Filipendula Species" Molecules 22, no. 1: 16. https://doi.org/10.3390/molecules22010016








