Mechanochemical Lignin-Mediated Strecker Reaction
Abstract
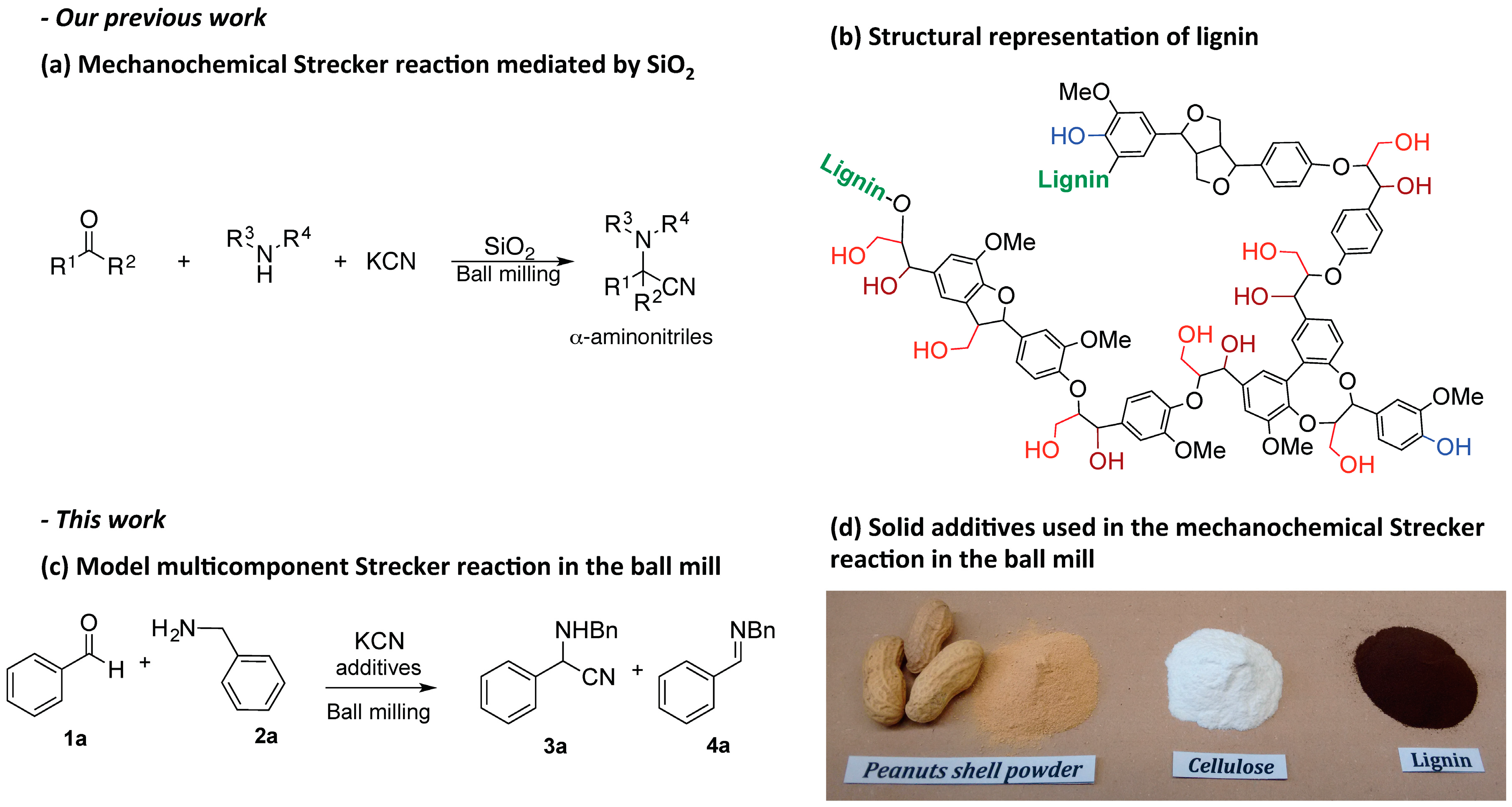
:1. Introduction
2. Results and Discussion
2.1. Lignocellulosic Biomass as Activator of the Mechanochemical Strecker Reaction
2.2. Investigation of the Role of the Kraft Lignin in the Mechanochemical Reaction
2.3. Scope of the Mechanochemical Strecker Reaction
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Monitoring of the reactions and purification of the products
3.2.2. Characterization of the Products 3, 4, 5 and 6 (NMR, IR Spectroscopy, MS Spectrometry)
3.2.3. Mechanochemical Synthesis
3.2.4. Capping of Kraft Lignin with Dimethyl Carbonate
3.2.5. 31P-NMR Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BWL | Beachwood lignin |
| C | Cellulose |
| CKL | Capped Kraft lignin |
| CL | Cherry lignin |
| DMC | Dimethyl carbonate |
| IS | Internal standard |
| KL | Kraft lignin |
| LAG | Liquid assisted grinding |
| OL | Oak lignin |
| PSP | Peanut shell powder |
References
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallization outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Hernández, J.G.; Friščić, T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015, 56, 4253–4265. [Google Scholar] [CrossRef]
- Hernández, J.G.; Juaristi, E. Recent efforts directed to the development of more sustainable asymmetric organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.G.; Frings, M.; Bolm, C. Mechanochemical enzymatic kinetic resolution of secondary alcohols under ball-milling conditions. ChemCatChem 2016, 8, 1769–1772. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Meine, N.; Rinaldi, R.; Schüth, F. Solvent-free catalytic depolymerization of cellulose to water-soluble oligosaccharides. ChemSusChem 2012, 5, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Boissou, F.; Sayoud, N.; de Oliveira Vigier, K.; Barakat, A.; Marinkovic, S.; Estrine, B.; Jérôme, F. Acid-assisted ball milling of cellulose as an efficient pretreatment process for the production of butyl glycosides. ChemSusChem 2015, 8, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Rechulski, M.D.K.; Käldström, M.; Richter, U.; Schüth, F.; Rinaldi, R. Mechanocatalytic depolymerization of lignocellulose performed on hectogram and kilogram scales. Ind. Eng. Chem. Res. 2015, 54, 4581–4592. [Google Scholar] [CrossRef]
- Polindara-García, L.A.; Juaristi, E. Synthesis of Ugi 4-CR and Passerini 3-CR adducts under Mechanochemical activation. Eur. J. Org. Chem. 2016, 2016, 1095–1102. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Solvent-free carbon-carbon bond formations in ball mills. Adv. Synth. Catal. 2007, 349, 2213–2233. [Google Scholar]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.G.; Turberg, M.; Schiffers, I.; Bolm, C. Mechanochemical Strecker reaction: Access to α-aminonitriles and tetrahydroisoquinolines under ball-milling conditions. Chem. Eur. J. 2016, 22, 14513–14517. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose nanocrystals as chiral inducers: Enantioselective catalysis and transmission electron microscopy 3D characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Peng, X.-W.; Zhong, L.-X.; Li, Y.; Sun, R.-C. Lignosulfonic acid: A renewable and effective biomass-based catalyst for multicomponent reactions. ACS Sustain. Chem. Eng. 2015, 3, 1366–1373. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D. Methylation of softwood kraft lignin with dimethyl carbonate. Green Chem. 2015, 17, 1077–1087. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Suneja, A.; Bisai, V.; Singh, V.K. Approach to isoindolinones, isoquinolinones, and THIQs via Lewis acid-catalyzed domino Strecker-lactamization/alkylations. Org. Lett. 2016, 18, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-A.; Cho, Y.-H.; Lee, Y.-S.; Cheon, C.-H. Formation of Amides from Imines via Cyanide-Mediated Metal-Free Aerobic Oxidation. J. Org. Chem. 2015, 80, 11993–11998. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; An, W.; Wang, Y.; Yu, A. Mechanisms of Metal-Free Aerobic Oxidation To Prepare Benzoxazole Catalyzed by Cyanide: A Direct Cyclization or Stepwise Oxidative Dehydrogenation and Cyclization? J. Org. Chem. 2016, 81, 10857–10862. [Google Scholar] [CrossRef] [PubMed]
- Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Cu(0), O2 and mechanical forces: A saving combination for efficient production of Cu–NHC complexes. Chem. Sci. [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of functionalized phenolic monomers through selective oxidation and C-O bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.




| OH | OH (mmol/g) | |||||
|---|---|---|---|---|---|---|
| Lignin | Aliphatic OH | 5-Substituted OH | Guaiacyl OH | p-Hydroxyphenyl OH | Total Phenolic OH | |
| KL | 1.79 | 0.90 | 1.77 | 0.17 | 2.84 | |
| CKL | 0.7 | 0.3 | 0.5 | 0.04 | 0.84 | |
| Entry | Carbonyl Compound | Amine | α-aminonitrile 3a–j | Imine 4a–j | Yield [3:4] (%) b |
|---|---|---|---|---|---|
 |  |  |  |  |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabral, S.; Turberg, M.; Wanninger, A.; Bolm, C.; Hernández, J.G. Mechanochemical Lignin-Mediated Strecker Reaction. Molecules 2017, 22, 146. https://doi.org/10.3390/molecules22010146
Dabral S, Turberg M, Wanninger A, Bolm C, Hernández JG. Mechanochemical Lignin-Mediated Strecker Reaction. Molecules. 2017; 22(1):146. https://doi.org/10.3390/molecules22010146
Chicago/Turabian StyleDabral, Saumya, Mathias Turberg, Andrea Wanninger, Carsten Bolm, and José G. Hernández. 2017. "Mechanochemical Lignin-Mediated Strecker Reaction" Molecules 22, no. 1: 146. https://doi.org/10.3390/molecules22010146







