Optimization of the Production of 1-Phenylethanol Using Enzymes from Flowers of Tea (Camellia sinensis) Plants
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Extraction of Crude 1PE Synthetic Enzymes from Tea Flowers
3.2. The Optimization of Production Conditions of 1PE Using Crude 1PE Synthetic Enzymes from Tea Flowers
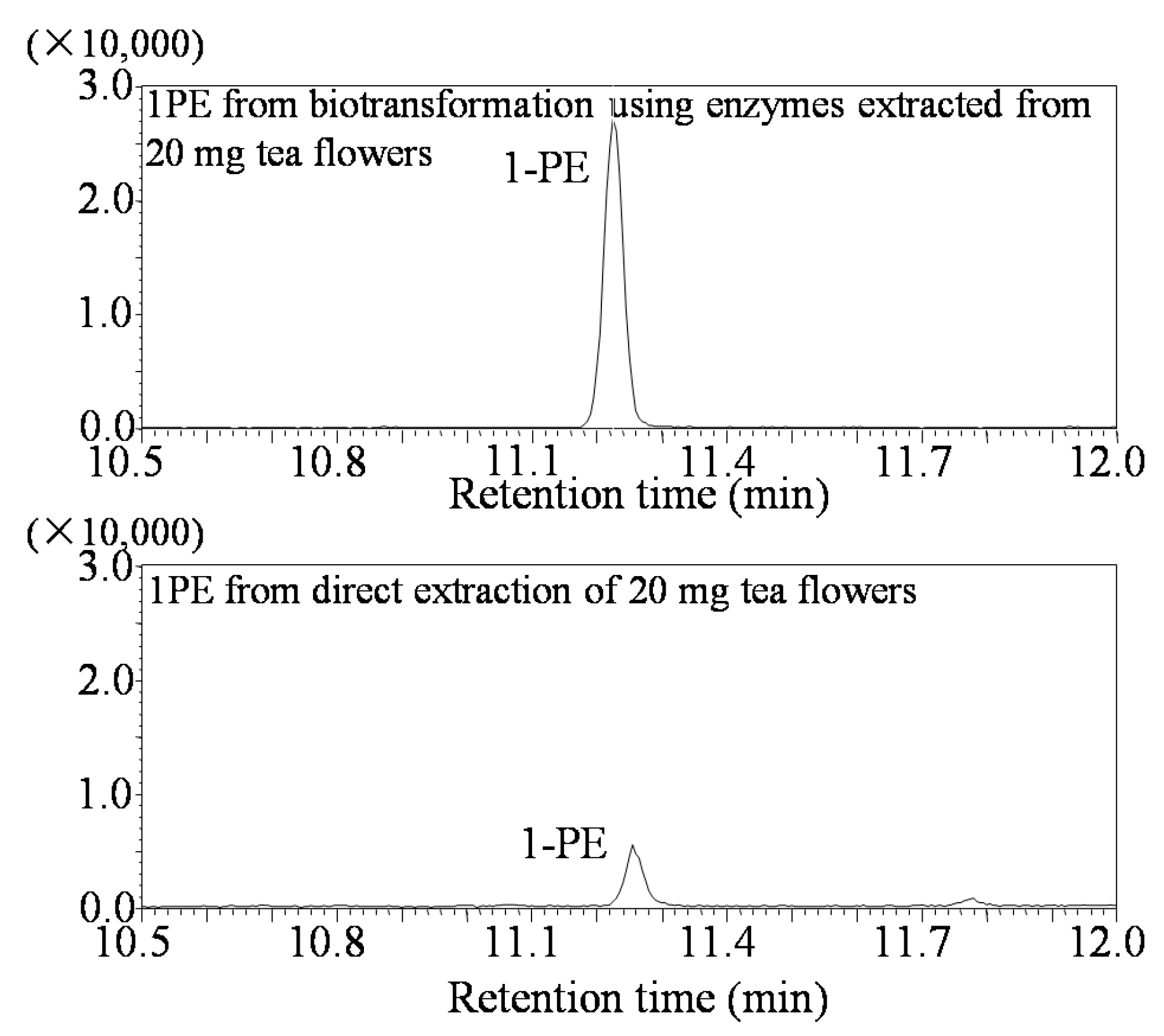
3.3. Analysis of (R)-1PE and (S)-1PE Products from Biotransformation Using Enzymes from Tea Flowers
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Dong, F.; Fu, X.M.; Watanabe, N.; Su, X.G.; Yang, Z.Y. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Büchel, C.; Gais, S.; Born, J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007, 315, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Tea Flower Web Reference. Available online: http://baike.baidu.com/link?url=kuRD4sm6LdTlTDfdYAMwJQaa_2QPoUi4_Hh8EWySpXiGJax6pFevl1IhtGcjqEqz9ZuShxXFoNeNccPNv47ME_ (accessed on 1 December 2016).
- Lin, Y.S.; Wu, S.S.; Lin, J.K. Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. J. Agric. Food Chem. 2003, 51, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Xu, Y.; Jie, G.L.; He, P.M.; Tu, Y.Y. Study on the antioxidant activity of tea flowers (Camellia sinensis). Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 148–152. [Google Scholar] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Yamamoto, K.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A–C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Nakamura, S.; Kato, Y.; Matsuhira, K.; Matsuda, H. Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of Chinese tea plant (Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Miyake, S.; Miki, Y.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Quantitative analysis of acylated oleananetype triterpene saponins, chakasaponins I–III and floratheasaponins A–F, in the flower buds of Camellia sinensis from different regional origins. J. Nat. Med. 2012, 66, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Tu, Y.Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT Food Sci. Technol. 2009, 42, 1439–1443. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.; Zeng, X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Dong, F.; Baldermann, S.; Murata, A.; Tu, Y.Y.; Asai, T.; Watanabe, N. Isolation and identification of spermidine derivatives in flowers of tea (Camellia sinensis) plants and their distributions in floral organs. J. Sci. Food Agric. 2012, 92, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.Y.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of l-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 2012, 169, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, F.; Kunimasa, A.; Zhang, Y.; Cheng, S.; Lu, J.; Zhang, L.; Murata, A.; Mayer, F.; Fleischmann, P.; et al. Occurrence of glycosidically conjugated 1-phenylethanol and its hydrolase β-primeverosidase in tea (Camellia sinensis) flowers. J. Agric. Food Chem. 2014, 62, 8042–8050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Gui, J.D.; Dong, F.; Cheng, S.; Mei, X.; Zhang, L.Y.; Li, Y.Q.; Su, X.G.; Baldermann, S.; et al. Molecular cloning and characterization of a short chain dehydrogenase showing activity with volatile compounds isolated from Camellia sinensis. Plant Mol. Biol. Rep. 2015, 33, 253–263. [Google Scholar] [CrossRef]
- Dong, F.; Zhou, Y.; Zeng, L.T.; Peng, Q.Y.; Zhang, L.; Su, X.G.; Watanabe, N.; Yang, Z.Y. Elucidation of differential accumulation of 1-phenylethanol in flowers and leaves of tea (Camellia sinensis) plants. Molecules 2016, 21, 1106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Fu, X.M.; Mei, X.; Zhou, Y.; Du, B.; Tu, Y.Y.; Yang, Z.Y. Characterization of functional proteases from flowers of tea (Camellia sinensis) plants. J. Funct. Foods 2016, 25, 149–159. [Google Scholar] [CrossRef]
- Höffken, H.W.; Duong, M.; Friedrich, T.; Breuer, M.; Hauer, B.; Reinhardt, R.; Rabus, R.; Heider, J. Crystal structure and enzyme kinetics of the (S)-specific 1-phenylethanol dehydrogenase of the denitrifying bacterium Strain EbN1. Biochemistry 2006, 45, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakata, Y.; Cao, C.; Sugiyama, Y.; Asanuma, Y.; Kanamaru, S.; Matsuda, T. Acetophenone reductase with extreme stability against a high concentration of organic compounds or an elevated temperature. Appl. Microbiol. Biotechnol. 2013, 97, 10413–10421. [Google Scholar] [CrossRef] [PubMed]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Aracil, J.; Garcia, T.; Sanchez, N.; Matinez, M. Enzymatic synthesis of fatty esters. Part II. Optimization studies. Enzyme Microb. Technol. 1999, 25, 591–597. [Google Scholar]
- Beg, Q.K.; Sahai, V.; Gupta, R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 2003, 39, 203–209. [Google Scholar] [CrossRef]
- Ismail, A.; Linder, M.; Ghoul, M. Optimization of butylgalactoside synthesis by α-galactosidase from Aspergillus oryzae. Enzyme Microb. Technol. 1999, 25, 208–213. [Google Scholar] [CrossRef]
- Çelik, D.; Bayraktar, E.; Mehmetoğlu, Ü. Biotransformation of 2-phenylethanol to phenylacetaldehyde in a two-phase fed-batch system. Biochem. Eng. J. 2004, 17, 5–13. [Google Scholar] [CrossRef]
- Lee, M.T.; Chen, W.C.; Chou, C.C. Maximization of cholesterol oxidase production by Rhodococcusequi no. 23 by using response surface methodology. Biotechnol. Appl. Biochem. 1998, 28, 229–233. [Google Scholar] [PubMed]
- Vohra, A.; Satyanarayana, T. Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochem. 2002, 37, 999–1004. [Google Scholar] [CrossRef]
- Beg, Q.K.; Saxena, R.K.; Gupta, R. Kinetic constants determination for an alkaline protease from Bacillus mojavensis using response surface methodology. Biotechnol. Bioeng. 2002, 78, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds including acetophenone, 1-phenylethanol, and [2H5]ring-acetophenone are available from the authors.
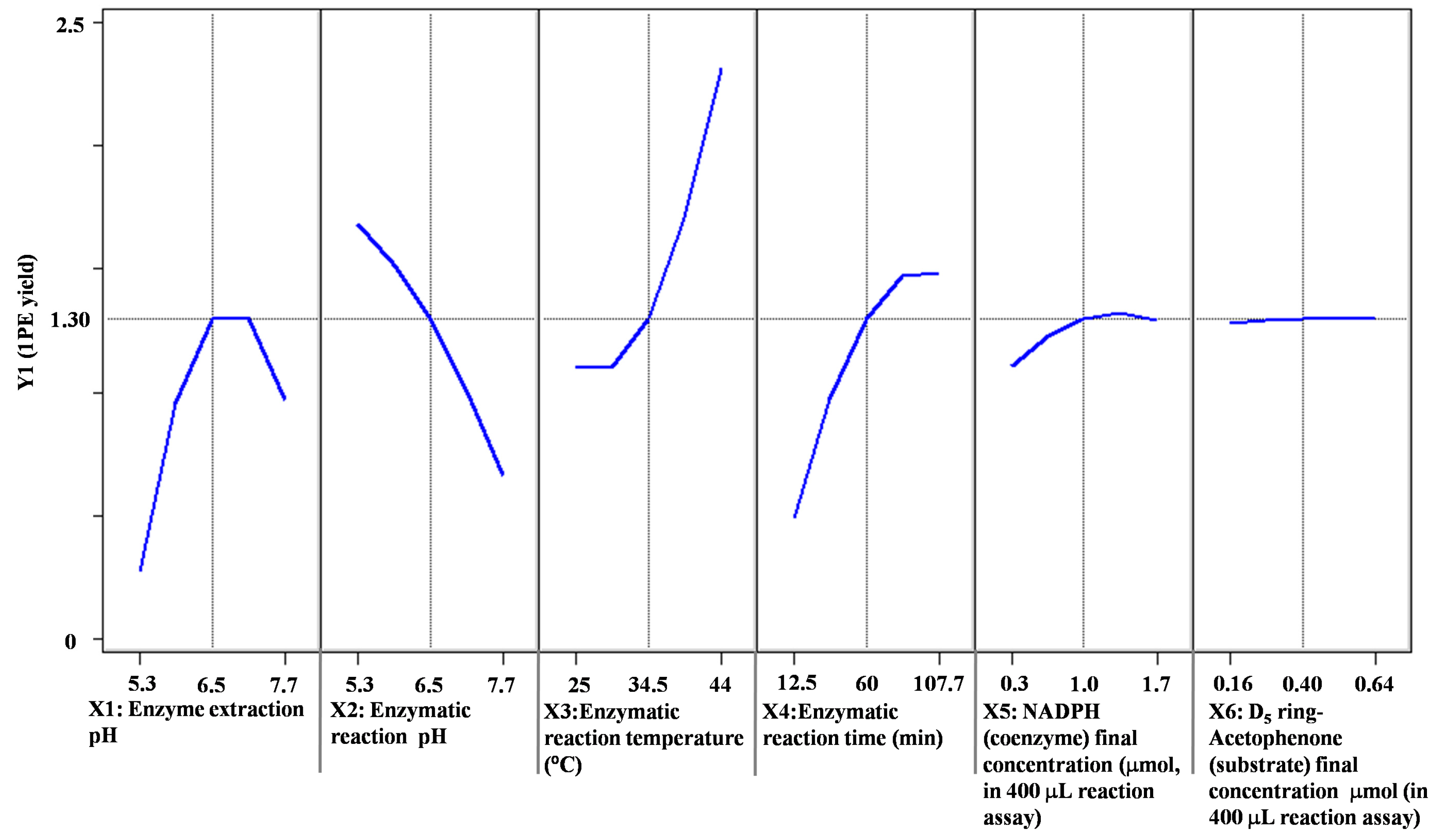
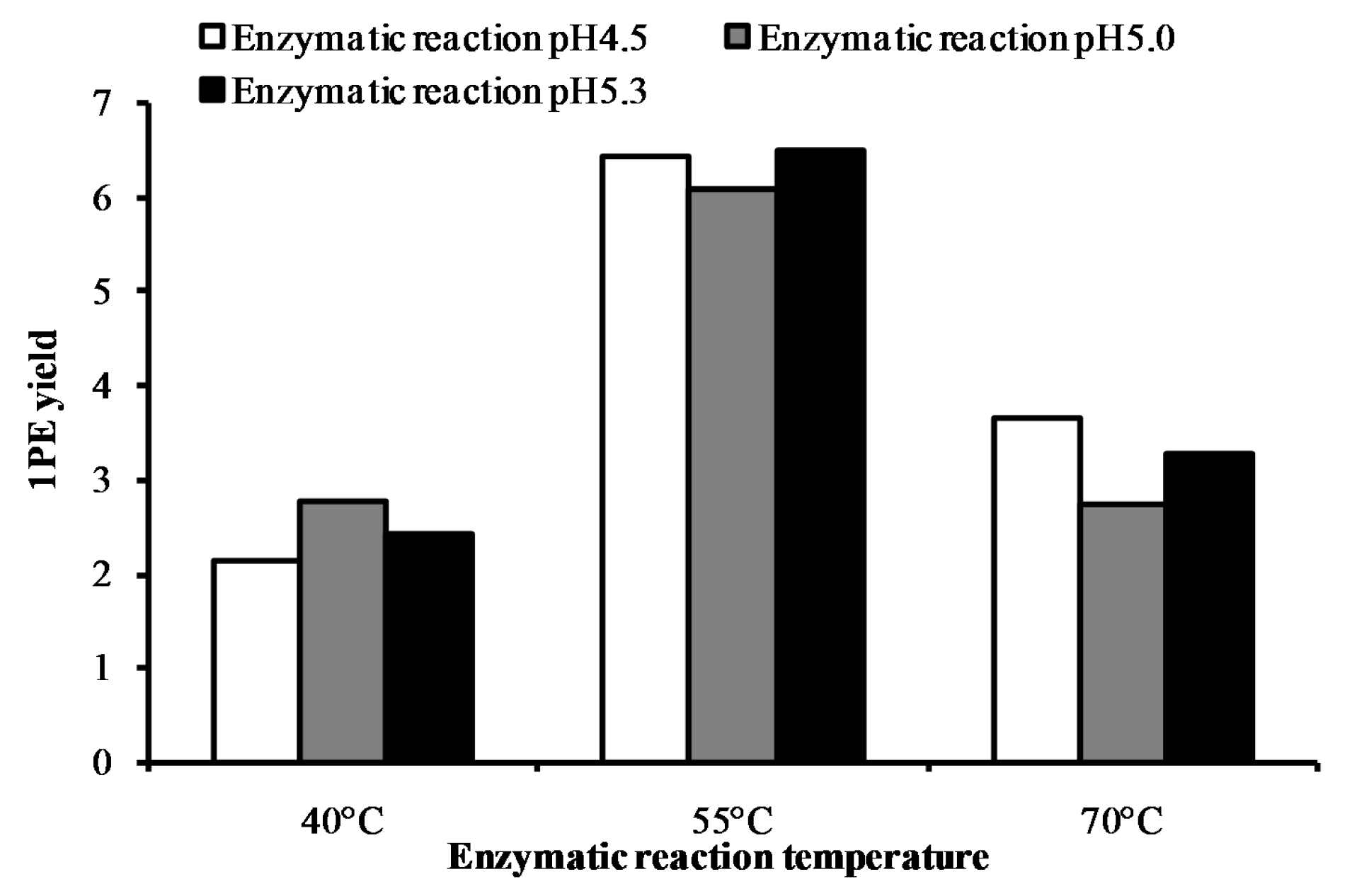
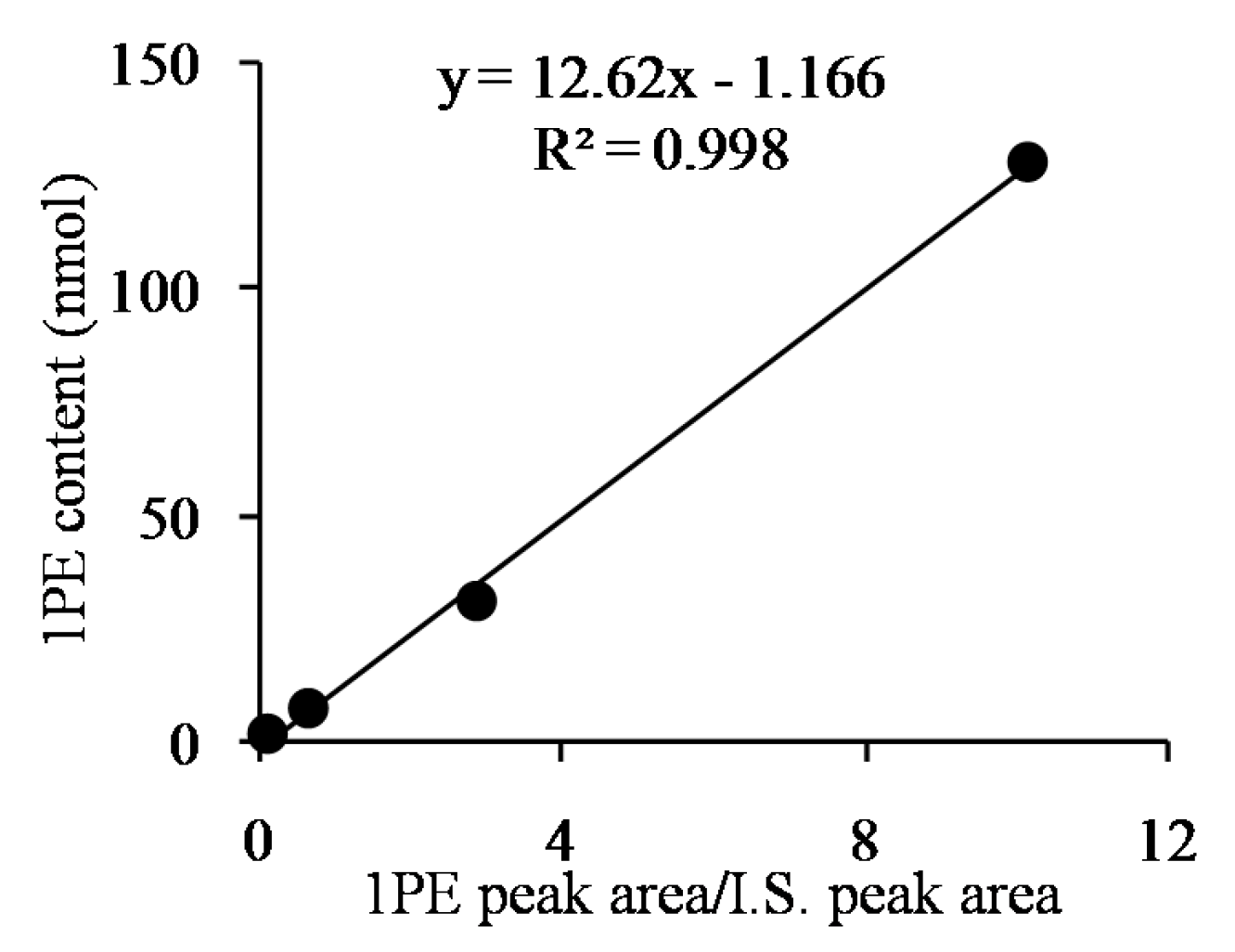
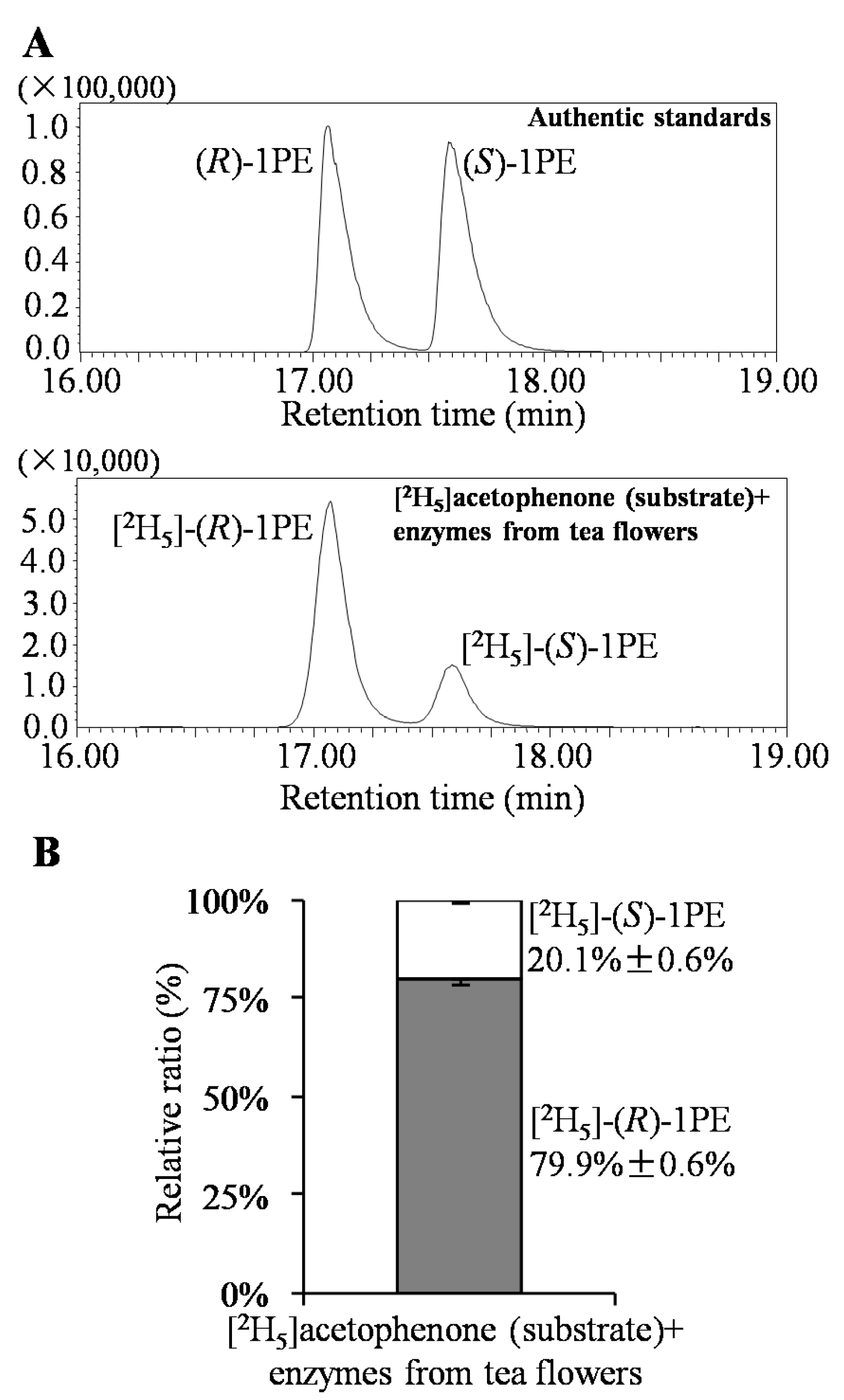
| Factors (Coded Level) | −2.37841 | −1 | 0 | 1 | 2.37841 |
|---|---|---|---|---|---|
| X1: Enzyme extraction pH | 5.3 | 6 | 6.5 | 7 | 7.7 |
| X2: Enzymatic reaction pH | 5.3 | 6 | 6.5 | 7 | 7.7 |
| X3: Enzymatic reaction temperature (°C) | 25 | 30 | 34 | 38 | 44 |
| X4: Enzymatic reaction time (min) | 12.5 | 40 | 60 | 80 | 107.5 |
| X5: NADPH (coenzyme) final concentration μmol (in 400 μL reaction assay) | 0.3 | 0.7 | 1 | 1.3 | 1.7 |
| X6: [2H5]ring-acetophenone (substrate) final concentration μmol (in 400 μL reaction assay) | 0.16 | 0.3 | 0.4 | 0.5 | 0.64 |
| Run | X1 | X2 | X3 | X4 | X5 | X6 | Y1 a (1PE Yield) |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | 0.758559371 |
| 2 | −1 | −1 | −1 | −1 | 1 | 1 | 0.846748345 |
| 3 | −1 | −1 | −1 | 1 | −1 | 1 | 1.153593462 |
| 4 | −1 | −1 | −1 | 1 | 1 | −1 | 1.150490973 |
| 5 | −1 | −1 | 1 | −1 | −1 | 1 | 1.025881547 |
| 6 | −1 | −1 | 1 | −1 | 1 | −1 | 1.154783234 |
| 7 | −1 | −1 | 1 | 1 | −1 | −1 | 1.586798695 |
| 8 | −1 | −1 | 1 | 1 | 1 | 1 | 1.452172855 |
| 9 | −1 | 1 | −1 | −1 | −1 | 1 | 0.447787063 |
| 10 | −1 | 1 | −1 | −1 | 1 | −1 | 0.508368068 |
| 11 | −1 | 1 | −1 | 1 | −1 | −1 | 0.765378069 |
| 12 | −1 | 1 | −1 | 1 | 1 | 1 | 0.631801149 |
| 13 | −1 | 1 | 1 | −1 | −1 | −1 | 0.620733457 |
| 14 | −1 | 1 | 1 | −1 | 1 | 1 | 0.770290718 |
| 15 | −1 | 1 | 1 | 1 | −1 | 1 | 1.05186958 |
| 16 | −1 | 1 | 1 | 1 | 1 | −1 | 1.269535781 |
| 17 | 1 | −1 | −1 | −1 | −1 | 1 | 0.828065574 |
| 18 | 1 | −1 | −1 | −1 | 1 | −1 | 0.953103788 |
| 19 | 1 | −1 | −1 | 1 | −1 | −1 | 1.110510204 |
| 20 | 1 | −1 | −1 | 1 | 1 | 1 | 2.091175436 |
| 21 | 1 | −1 | 1 | −1 | −1 | −1 | 1.490051857 |
| 22 | 1 | −1 | 1 | −1 | 1 | 1 | 1.525848802 |
| 23 | 1 | −1 | 1 | 1 | −1 | 1 | 1.838977526 |
| 24 | 1 | −1 | 1 | 1 | 1 | −1 | 2.564189622 |
| 25 | 1 | 1 | −1 | −1 | −1 | −1 | 0.652233718 |
| 26 | 1 | 1 | −1 | −1 | 1 | 1 | 0.782094508 |
| 27 | 1 | 1 | −1 | 1 | −1 | 1 | 0.937807534 |
| 28 | 1 | 1 | −1 | 1 | 1 | −1 | 1.035549206 |
| 29 | 1 | 1 | 1 | −1 | −1 | 1 | 1.105724121 |
| 30 | 1 | 1 | 1 | −1 | 1 | −1 | 1.009265424 |
| 31 | 1 | 1 | 1 | 1 | −1 | −1 | 1.500473779 |
| 32 | 1 | 1 | 1 | 1 | 1 | 1 | 1.35519365 |
| 33 | −2.37841 | 0 | 0 | 0 | 0 | 0 | 0.481731606 |
| 34 | 2.378414 | 0 | 0 | 0 | 0 | 0 | 0.698984848 |
| 35 | 0 | −2.37841 | 0 | 0 | 0 | 0 | 1.570230689 |
| 36 | 0 | 2.378414 | 0 | 0 | 0 | 0 | 0.722668367 |
| 37 | 0 | 0 | −2.37841 | 0 | 0 | 0 | 0.896550121 |
| 38 | 0 | 0 | 2.378414 | 0 | 0 | 0 | 2.489739273 |
| 39 | 0 | 0 | 0 | −2.37841 | 0 | 0 | 0.548374842 |
| 40 | 0 | 0 | 0 | 2.378414 | 0 | 0 | 1.370597491 |
| 41 | 0 | 0 | 0 | 0 | −2.37841 | 0 | 1.263502722 |
| 42 | 0 | 0 | 0 | 0 | 2.378414 | 0 | 1.074987099 |
| 43 | 0 | 0 | 0 | 0 | 0 | −2.37841 | 1.103270031 |
| 44 | 0 | 0 | 0 | 0 | 0 | 2.378414 | 1.421635721 |
| 45 | 0 | 0 | 0 | 0 | 0 | 0 | 1.226729653 |
| 46 | 0 | 0 | 0 | 0 | 0 | 0 | 1.377231877 |
| 47 | 0 | 0 | 0 | 0 | 0 | 0 | 1.242217123 |
| 48 | 0 | 0 | 0 | 0 | 0 | 0 | 1.291999792 |
| 49 | 0 | 0 | 0 | 0 | 0 | 0 | 1.273597247 |
| 50 | 0 | 0 | 0 | 0 | 0 | 0 | 1.249548763 |
| 51 | 0 | 0 | 0 | 0 | 0 | 0 | 1.224737373 |
| 52 | 0 | 0 | 0 | 0 | 0 | 0 | 1.28414853 |
| 53 | 0 | 0 | 0 | 0 | 0 | 0 | 1.263331053 |
| 54 | 0 | 0 | 0 | 0 | 0 | 0 | 1.250814756 |
| 55 | 0 | 0 | 0 | 0 | 0 | 0 | 1.216741671 |
| 56 | 0 | 0 | 0 | 0 | 0 | 0 | 1.308596416 |
| 57 | 0 | 0 | 0 | 0 | 0 | 0 | 1.225345486 |
| 58 | 0 | 0 | 0 | 0 | 0 | 0 | 1.360042105 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Zhou, Y.; Zeng, L.; Watanabe, N.; Su, X.; Yang, Z. Optimization of the Production of 1-Phenylethanol Using Enzymes from Flowers of Tea (Camellia sinensis) Plants. Molecules 2017, 22, 131. https://doi.org/10.3390/molecules22010131
Dong F, Zhou Y, Zeng L, Watanabe N, Su X, Yang Z. Optimization of the Production of 1-Phenylethanol Using Enzymes from Flowers of Tea (Camellia sinensis) Plants. Molecules. 2017; 22(1):131. https://doi.org/10.3390/molecules22010131
Chicago/Turabian StyleDong, Fang, Ying Zhou, Lanting Zeng, Naoharu Watanabe, Xinguo Su, and Ziyin Yang. 2017. "Optimization of the Production of 1-Phenylethanol Using Enzymes from Flowers of Tea (Camellia sinensis) Plants" Molecules 22, no. 1: 131. https://doi.org/10.3390/molecules22010131





