HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities
Abstract
:1. Introduction
2. Results
2.1. Optimization of UPLC Analysis
2.2. Selection of HSCCC Experimental Conditions
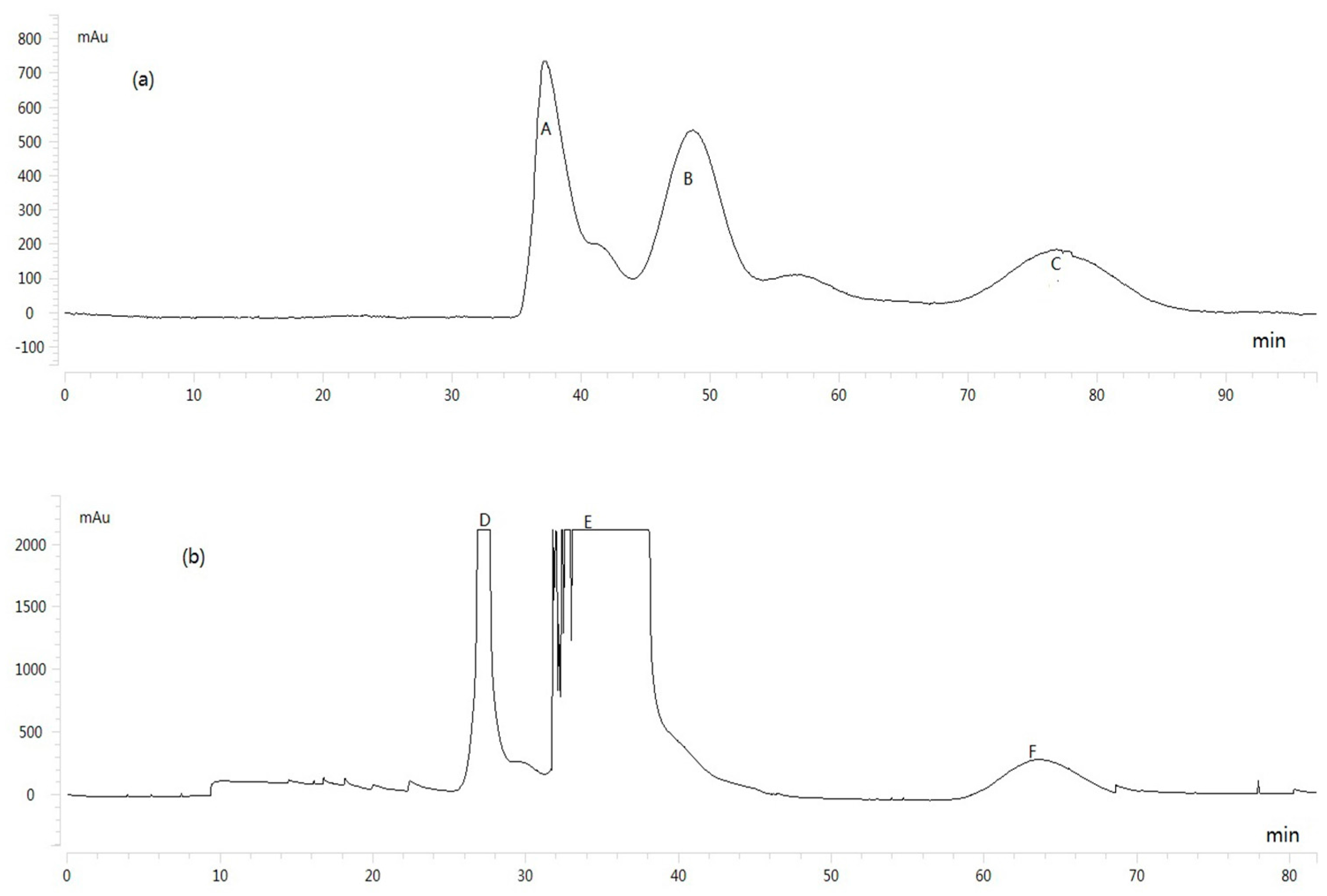
2.3. HSCCC Separation of Sample
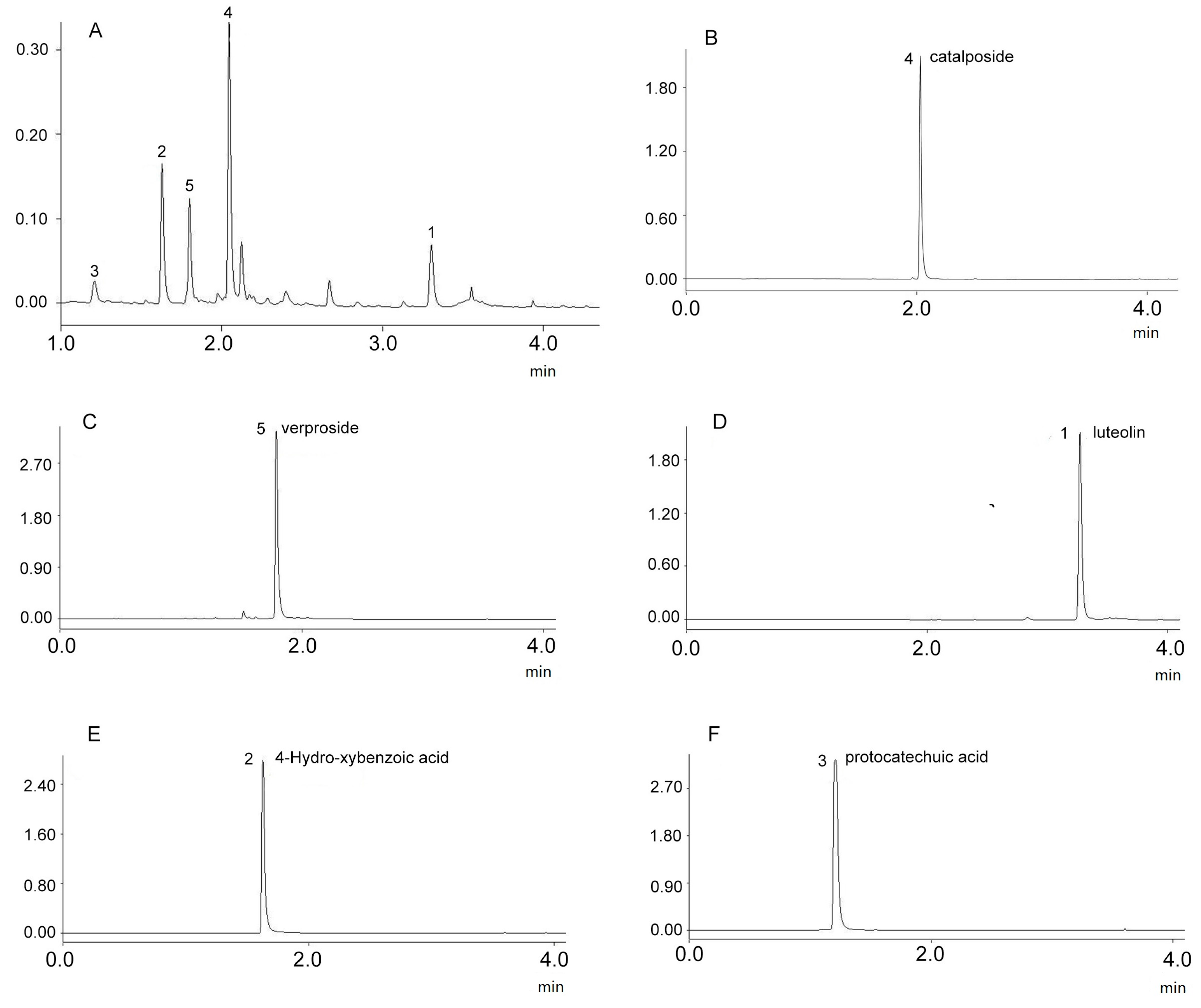
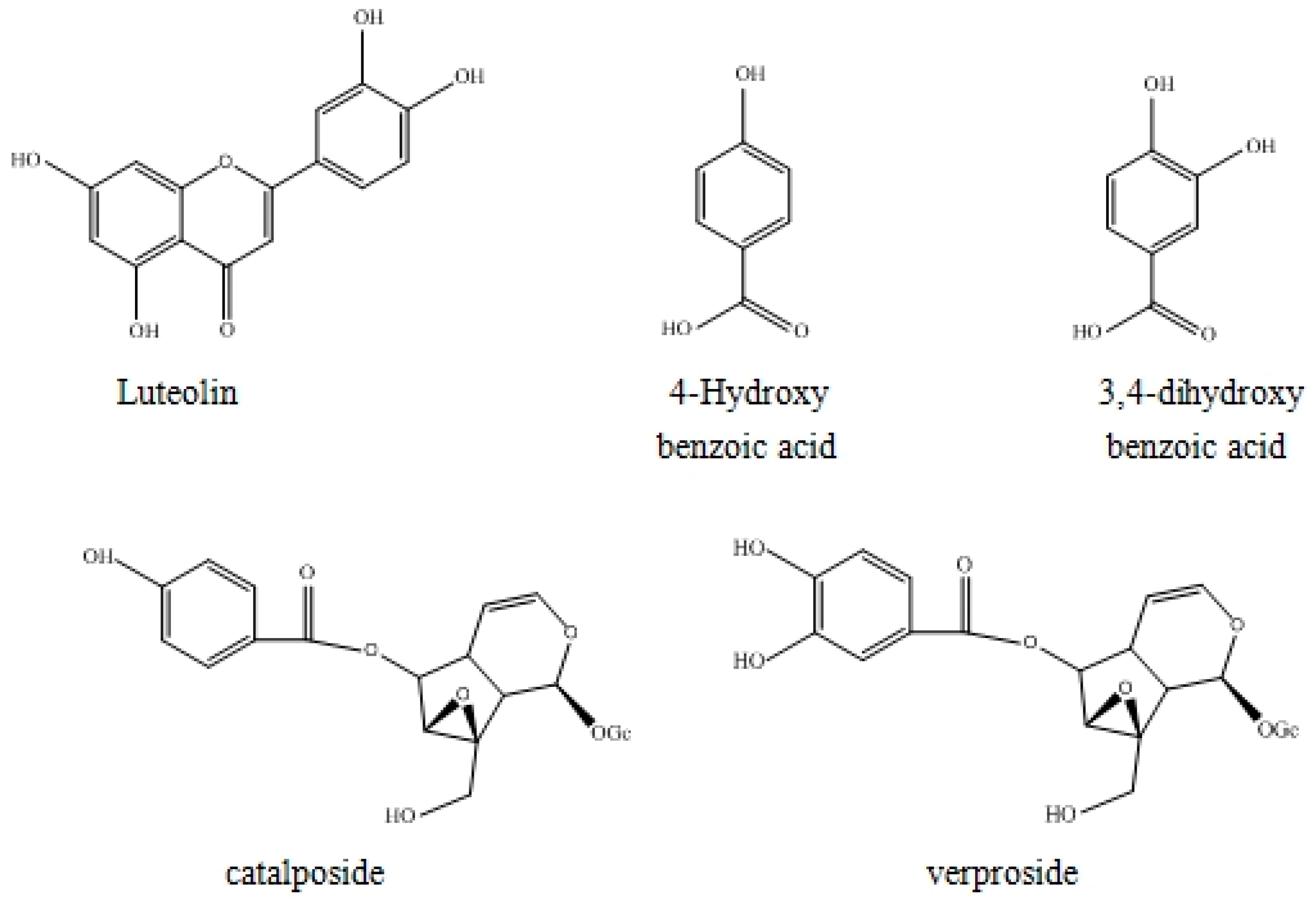
2.4. Structure Identification
2.5. Antioxidant Activity
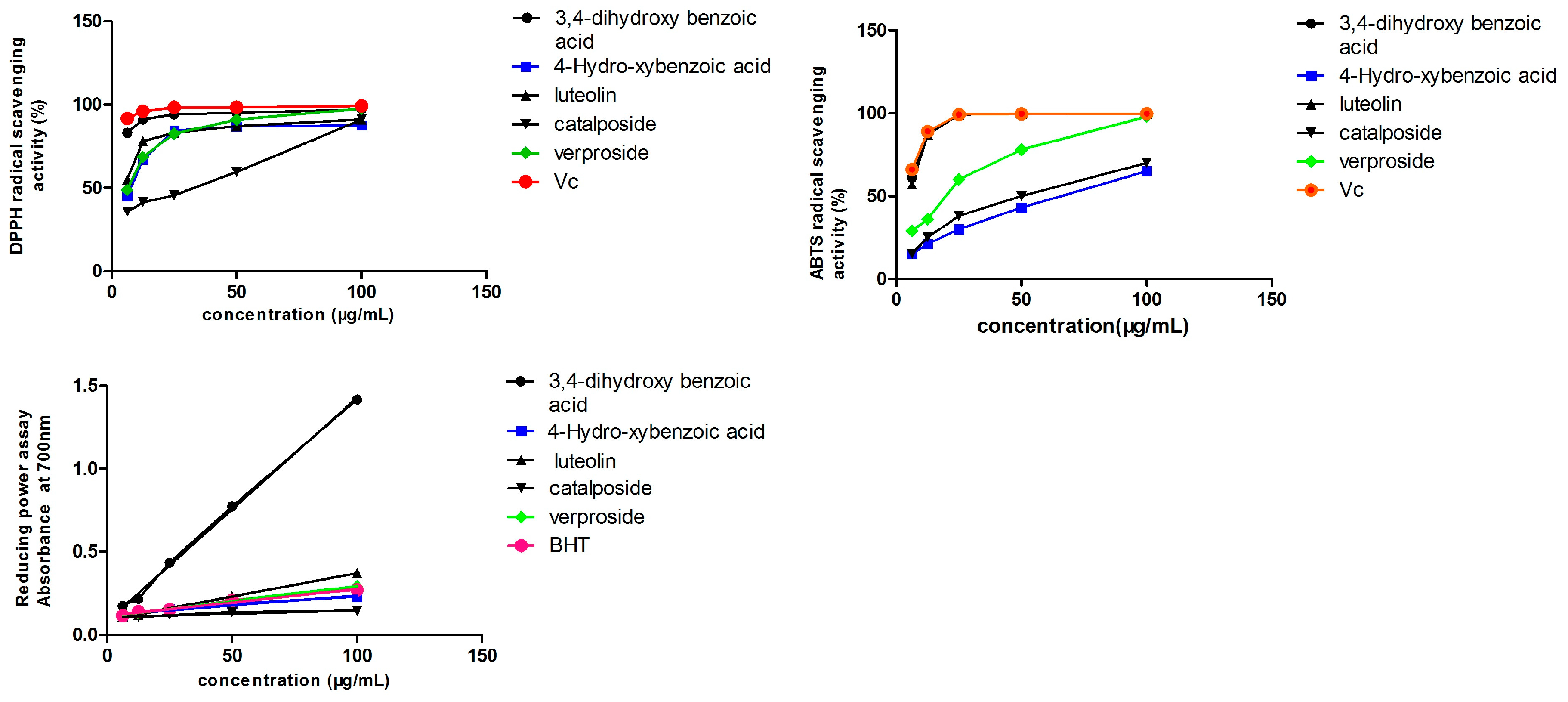
2.5.1. DPPH• Scavenging Capacity
2.5.2. ABTS Radical-Scavenging Capacity
2.5.3. Reducing Power Assay
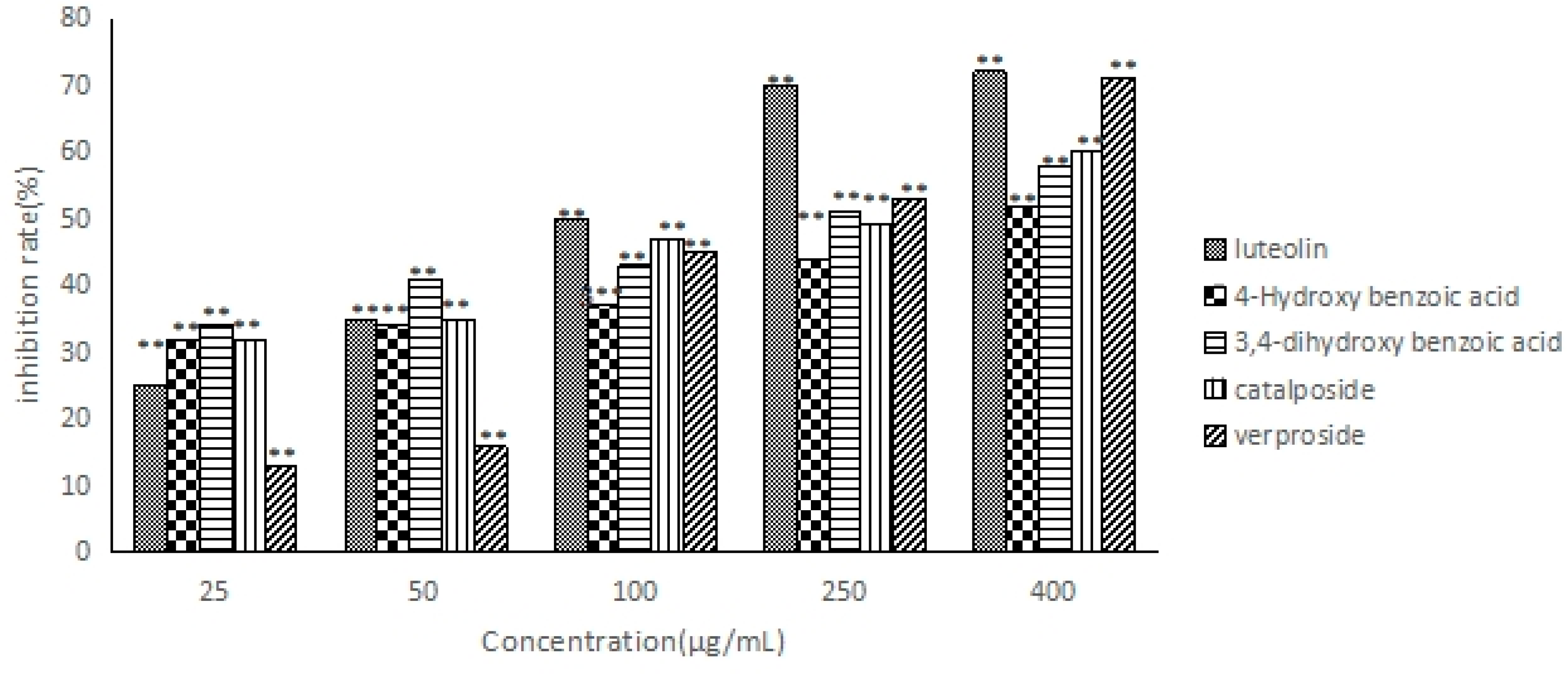
2.6. The Anti-Hepatocarcinoma Activity of Five Compounds
3. Discussion
4. Materials and Methods
4.1. HSCCC Apparatus
4.2. Reagents and Materials
4.3. Preparation of the Crude Extract
4.4. Separation and Purification by HSCCC
4.4.1. Selection of Two-Phase Solvent System
4.4.2. Preparation of Two-Phase Solvent System and Sample Solution
4.4.3. HSCCC Separation
4.5. Purification of Compounds 1–3 by Preparative HPLC
4.6. Peak Identification
4.6.1. UPLC-PDA Analysis
4.6.2. MS Analysis
4.6.3. NMR Analysis
4.7. In Vitro Antioxidant and Anti-Hepatocarcinoma Activity
4.7.1. DPPH• Scavenging Capacity [33]
4.7.2. ABTS Radical-Scavenging Capacity
4.7.3. Reducing Power Assay [35]
4.7.4. In Vitro Anti-Hepatocarcinoma Activity [2,12]
Cell Culture
Cell Proliferation Inhibition Assay
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, K.; Li, X.; Liu, A.; Jia, Z. Chemical constituents of V eron ka ciliate as a psychrophyte from Northwest China. Acta Bot. Boreali Occident. Sin. 2002, 23, 633–636. [Google Scholar]
- Yin, L.; Wei, L.; Fu, R.; Ding, L.; Guo, Y.; Tang, L.; Chen, F. Antioxidant and hepatoprotective activity of Veronica ciliata Fisch. extracts against carbon tetrachloride-induced liver injury in mice. Molecules 2014, 19, 7223–7236. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lu, Q.; Tan, S.; Ding, L.; Guo, Y.; Chen, F.; Tang, L. Bioactivity-guided isolation of antioxidant and anti-hepatocarcinoma constituents from Veronica ciliata. Chem. Cent. J. 2016, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Choi, B.M.; Oh, G.S.; Pae, H.O.; Kim, J.D.; Oh, H.; Lee, H.S. Catalposide protects Neuro 2A cells from hydrogen peroxide-induced cytotoxicity via the expression of heme oxygenase-1. Toxicol. Lett. 2003, 145, 46–54. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, S.C.; Choi, E.Y.; Kim, K.S.; Oh, J.M.; Lee, H.J.; Lee, M.H. Catalposide, a compound isolated from Catalpa Ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. Inflamm. Bowel. Dis. 2004, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Sung, M.H.; Ryu, H.W.; Lee, J.; Kim, H.S.; In, H.J.; Lee, Y. Verproside inhibits TNF-α-induced MUC5AC expression through suppression of the TNF-α/NF-κB pathway in human airway epithelial cells. Cytokine 2016, 77, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relation to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Kim, S.; Song, N.J.; Chang, S.H.; Hwang, Y.J.; Yang, D.K.; Park, K.W. Antiadipogenic and proosteogenic effects of luteolin, a major dietary flavone, are mediated by the induction of DnaJ (Hsp40) Homolog, Subfamily B, Member 1. J. Nutr. Biochem. 2016, 30, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dong, X.; Xu, D.; Meng, J.; Fu, X.; Wang, X.; Liu, Y. Preparative separation of main ustilaginoidins from rice false smut balls by high-speed counter-current chromatography. Toxins 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; He, J.; Zhu, S.; Zhao, W.; Zhang, Y.; Ito, Y.; Sun, W. Preparation of main iridoid glycosides in Fructus corni by macroporous resin column chromatography and countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 983–999. [Google Scholar] [PubMed]
- Tong, S.; Chen, L.; Zhang, Q.; Liu, J.; Yan, J.; Ito, Y. Separation of catalpol from Rehmannia glutinosa Libosch. by high-speed counter-current chromatography. J. Chromatogr. Sci. 2015, 53, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Pei, L.X.; Wang, K.B.; Sun, Y.S.; Wang, J.M.; Zhang, Y.L.; Ji, B.Y. Preparative isolation of two prenylated biflavonoids from the roots and rhizomes of Sinopodophyllum emodi by Sephadex LH-20 column and high-speed counter-current chromatography. Molecules 2015, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, L.; Gao, Y.; Zhao, B.; Wang, D.; Duan, W.; Yu, Z. Isolation of isochlorogenic acid isomers in flower buds of Lonicera japonica by high-speed counter-current chromatography and preparative high performance liquid chromatography. J. Chromatogr. B 2015, 981, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, S.; Ding, L.; Yuan, Y.; Zhu, P.; Epstein, S.; Yan, X. Efficient preparation of streptochlorin from Marine streptomyces sp. syylwhs-1-4 by combination of response surface methodology and high-speed counter-current chromatography. Molecules 2016, 21, 693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, L.; Wang, W.; Xia, Q.; Zhou, X.; Wang, Y.; Lu, T. Separation and purification of gardecin from Gardenia jasminoides Ellis by high-speed countercurrent chromatography. Sep. Sci. Technol. 2015, 50, 1899–1905. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Luo, H.; Yang, M. Simultaneous determination of three alkaloids in Huperzia serrata by UPLC-PDA and UPLC-Q/TOF-MS. Anal. Methods 2015, 7, 1770–1776. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Deng, L.; Cai, S.; Liu, J.; Li, W.; Chen, P. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed counter-current chromatography. Phytochem. Anal. 2015, 26, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Han, J.J.; Wu, R.J. High-speed countercurrent chromatography isolation of flavans from Ixeris chinensis and their identification by NMR spectroscopy. J. Sep. Sci. 2016, 39, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, Q.; Sun, C.; Li, J.; Yang, P.; Wang, X. Preparative separation of phenolic compounds from Chimonanthus praecox flowers by high-speed counter-current chromatography using a stepwise elution mode. Molecules 2016, 21, 1016. [Google Scholar] [CrossRef] [PubMed]
- Boersma, M.G.; van der Woude, H.; Bogaards, J.; Boeren, S.; Vervoort, J.; Cnubben, N.H.; Rietjens, I.M. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem. Res. Toxicol. 2002, 15, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Özgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Aşçı, A.; Kaya, Y.; Seçen, H. Relationship between chemical structure and antioxidant activity of luteolin and its glycosides isolated from Thymus sipyleus subsp. sipyleus var. Sipyleus. Rec. Nat. Prod. 2011, 5, 12–21. [Google Scholar] [CrossRef]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.N. Carbon-13 nuclear magnetic resonance of biologically important aromatic acids. I. Chemical shifts of benzoic acid and derivatives. J. Am. Chem. Soc. 1972, 94, 8564–8568. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, Y.; Guo, Y.; Peng, T.; Chen, F. Hepatoprotection using Brassica rapa var. rapa L. seeds and its bioactive compound, sinapine thiocyanate, for CCl 4-induced liver injury. J. Funct. Foods 2016, 22, 73–81. [Google Scholar] [CrossRef]
- Zhou, D.; Ruan, J.; Cai, Y.; Xiong, Z.; Fu, W.; Wei, A. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exilis (Hance) Ching. J. Ethnopharmacol. 2010, 129, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jiang, M.H.; Jiang, J.G.; Zhang, R.F.; Zhang, M.W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Food Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Pradhan, S.C. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J. Pharmacol. Exp. Ther. 2012, 3, 149–155. [Google Scholar]
- Esatbeyoglu, T.; Winterhalter, P. Preparation of dimeric procyanidins B1, B2, B5, and B7 from a polymeric procyanidin fraction of black chokeberry (Aronia melanocarpa). J. Agric. Food Chem. 2010, 58, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Cao, D.; Song, F.; Fan, G.; Wu, F. Preparative separation of nine flavonoids from Pericarpium Citri Reticulatae by preparative-HPLC and HSCCC. Sep. Sci. Technol. 2016, 51, 807–815. [Google Scholar] [CrossRef]
- He, K.; Zou, Z.; Hu, Y.; Yang, Y.; Xiao, Y.; Gao, P.; Ye, X. Purification of α-glucosidase from mouse intestine by countercurrent chromatography coupled with a reverse micelle solvent system. J. Sep. Sci. 2016, 39, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Han, R.W.; Zheng, N.; Yu, Z.N.; Wang, J.; Xu, X.M.; Qu, X.Y.; Wang, J.Q. Simultaneous determination of 38 veterinary antibiotic residues in raw milk by UPLC-MS/MS. Food Chem. 2015, 181, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Laszlo, J.A.; Evans, K.O. Antioxidant properties of feruloyl glycerol derivatives. Ind. Crops Prod. 2012, 36, 217–221. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.T.; Guo, Y.R.; Huang, Q.L.; Peng, T.; Xu, Y.; Chen, F. Antioxidant and anti-inflammatory activities of the phenolic extracts of Sapium sebiferum (L.) Roxb. leaves. J. Ethnopharmacol. 2013, 147, 517–524. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, B.; Ban, X.; Tian, J.; Zhu, L.; Wang, Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 2012, 141, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds catalposide and verproside are available from the authors.
| Solvent System (n-Hexane/n-Butanol/Water) | k-Values | ||||
|---|---|---|---|---|---|
| k1 | k2 | k3 | k4 | k5 | |
| 1.5:5:5 | 0.009 | 0.13 | 0.19 | 0.52 | 1 |
| 1.5:4:5 | 0.019 | 0.17 | 0.55 | 0.56 | 1.7 |
| 1.5:3:5 | 0.02 | 0.19 | 0.59 | 0.67 | 2.1 |
| 1.5:2:5 | 0.1 | 0.2 | 0.6 | 1.4 | 3.8 |
| 3:2:5 | 0.21 | 0.32 | 0.95 | 2.7 | 5.8 |
| Carbon | Compound | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | 121.95 | 123.8 | 92.9 | 95.6 | |
| 2 | 164.2 | 131.98 | 116.6 | ||
| 3 | 103.6 | 115.56 | 146.8 | 141.2 | 142.8 |
| 4 | 182.4 | 162.02 | 152.0 | 101.6 | 103.6 |
| 5 | 162.7 | 115.56 | 118.6 | 35.3 | 36.8 |
| 6 | 99 | 131.98 | 124.8 | 80.1 | 82.1 |
| 7 | 164.5 | 170.9 | 58.3 | 59.9 | |
| 8 | 94 | 65.7 | 67.2 | ||
| 9 | 158.1 | 41.7 | 43.7 | ||
| 10 | 104.7 | 58.5 | 61.8 | ||
| 1′ | 123.1 | 97.8 | 99.6 | ||
| 2′ | 113.5 | 73.3 | 75.6 | ||
| 3′ | 145.8 | 77.5 | 78.5 | ||
| 4′ | 149.4 | 70.4 | 72.4 | ||
| 5′ | 116 | 76.5 | 77.5 | ||
| 6′ | 119.5 | 61.5 | 63.9 | ||
| 1″ | 119.4 | 122.4 | |||
| 2″ | 131.6 | 116.6 | |||
| 3″ | 115.4 | 146.4 | |||
| 4″ | 162.4 | 152.4 | |||
| 5″ | 115.4 | 117.4 | |||
| 6″ | 131.6 | 124.6 | |||
| 7″ | 165.5 | 168.8 | |||
| Samples | IC50 (μg/mL) | Reducing Power | |
|---|---|---|---|
| DPPH | ABTS | ||
| luteolin | 4.168 | 5.587 | 0.0028 |
| 4-hydroxy benzoic acid | 6.838 | 58.78 | 0.0012 |
| 3,4-dihydroxy benzoic acid | 0.703 | 5.186 | 0.013 |
| catalposide | 20.16 | 43.83 | 0.0004 |
| verproside | 6.502 | 16.9 | 0.0018 |
| Vc | 0.57 | 4.645 | |
| BHT | 0.0016 | ||
| Compounds | HepG2 |
|---|---|
| luteolin | 102.356 ± 2.01 |
| 4-hydroxy benzoic acid | 444.759 ± 2.65 |
| 3,4-dihydroxy benzoic acid | 186.033 ± 2.27 |
| catalposide | 184.592 ± 2.27 |
| verproside | 177.147 ± 2.25 |
| 5-FU | 35.420 ± 1.56 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Sun, Y.; Shu, Y.; Tan, S.; Yin, L.; Guo, Y.; Tang, L. HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities. Molecules 2016, 21, 1234. https://doi.org/10.3390/molecules21091234
Lu Q, Sun Y, Shu Y, Tan S, Yin L, Guo Y, Tang L. HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities. Molecules. 2016; 21(9):1234. https://doi.org/10.3390/molecules21091234
Chicago/Turabian StyleLu, Qiuxia, Yiran Sun, Yueyue Shu, Shancai Tan, Li Yin, Yiran Guo, and Lin Tang. 2016. "HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities" Molecules 21, no. 9: 1234. https://doi.org/10.3390/molecules21091234





