Evaluation of PVP/Au Nanocomposite Fibers as Heterogeneous Catalysts in Indole Synthesis
Abstract
:1. Introduction
2. Results and Discussion
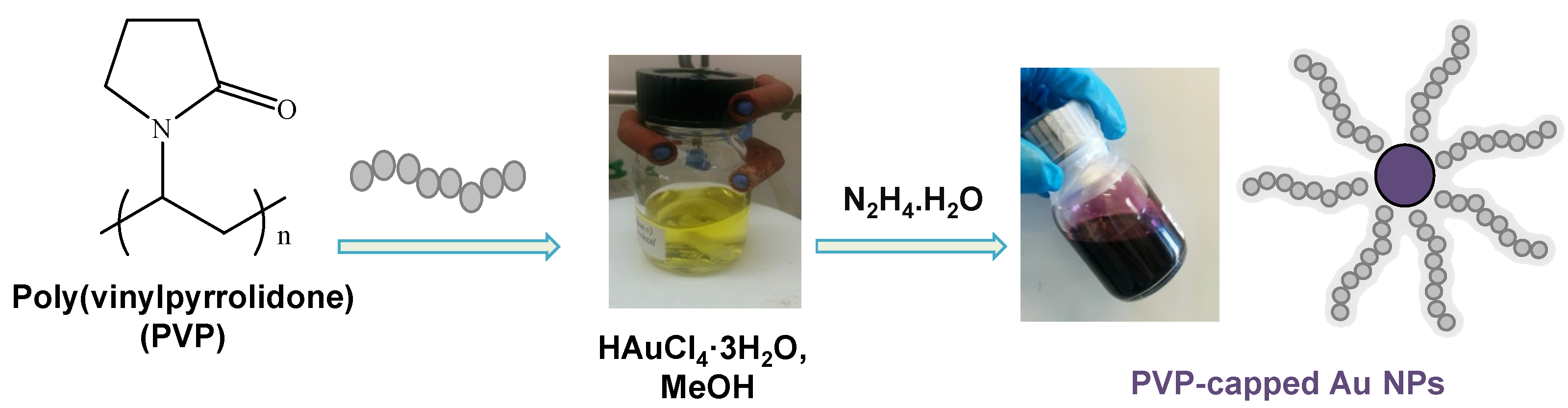
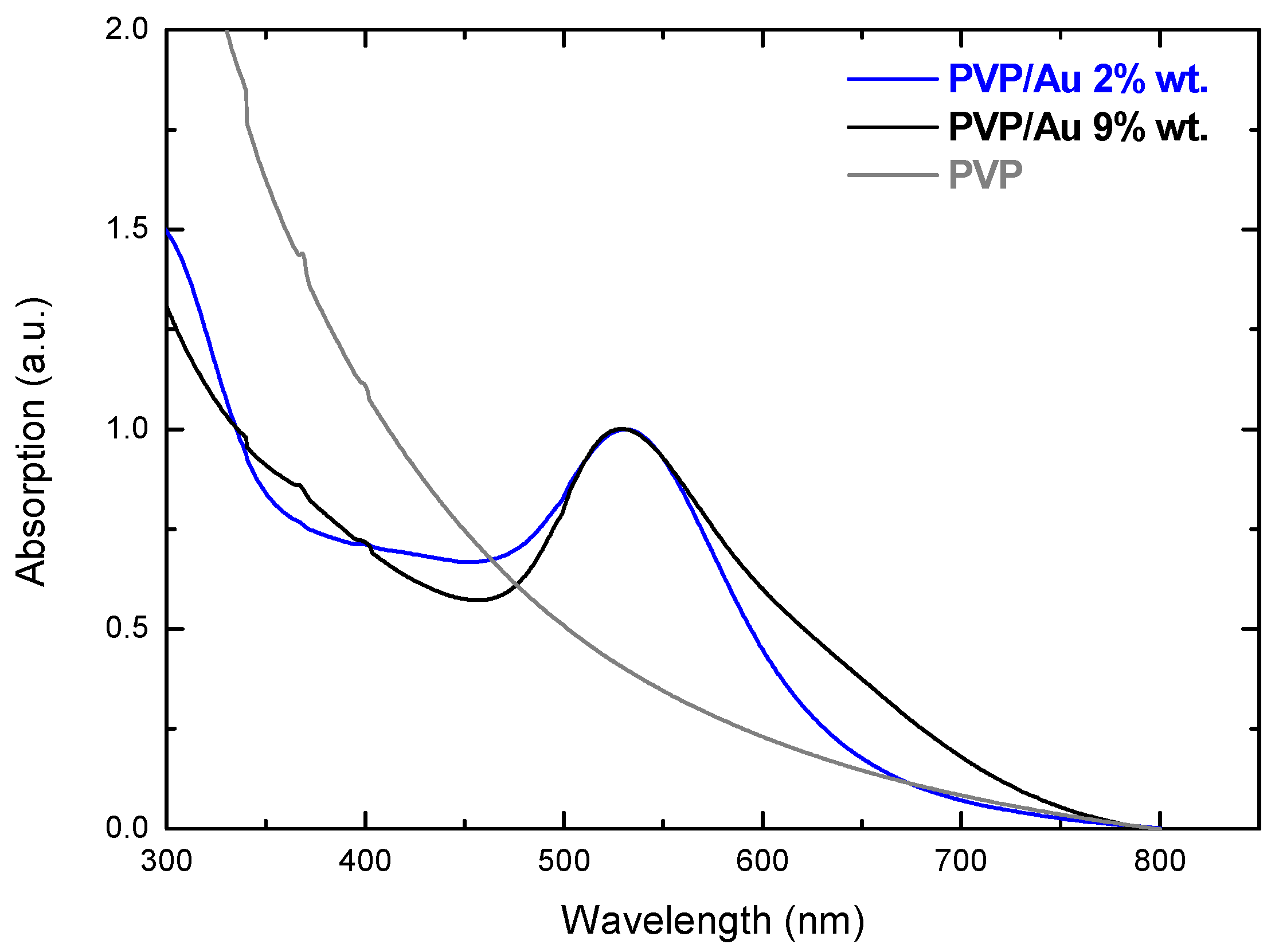
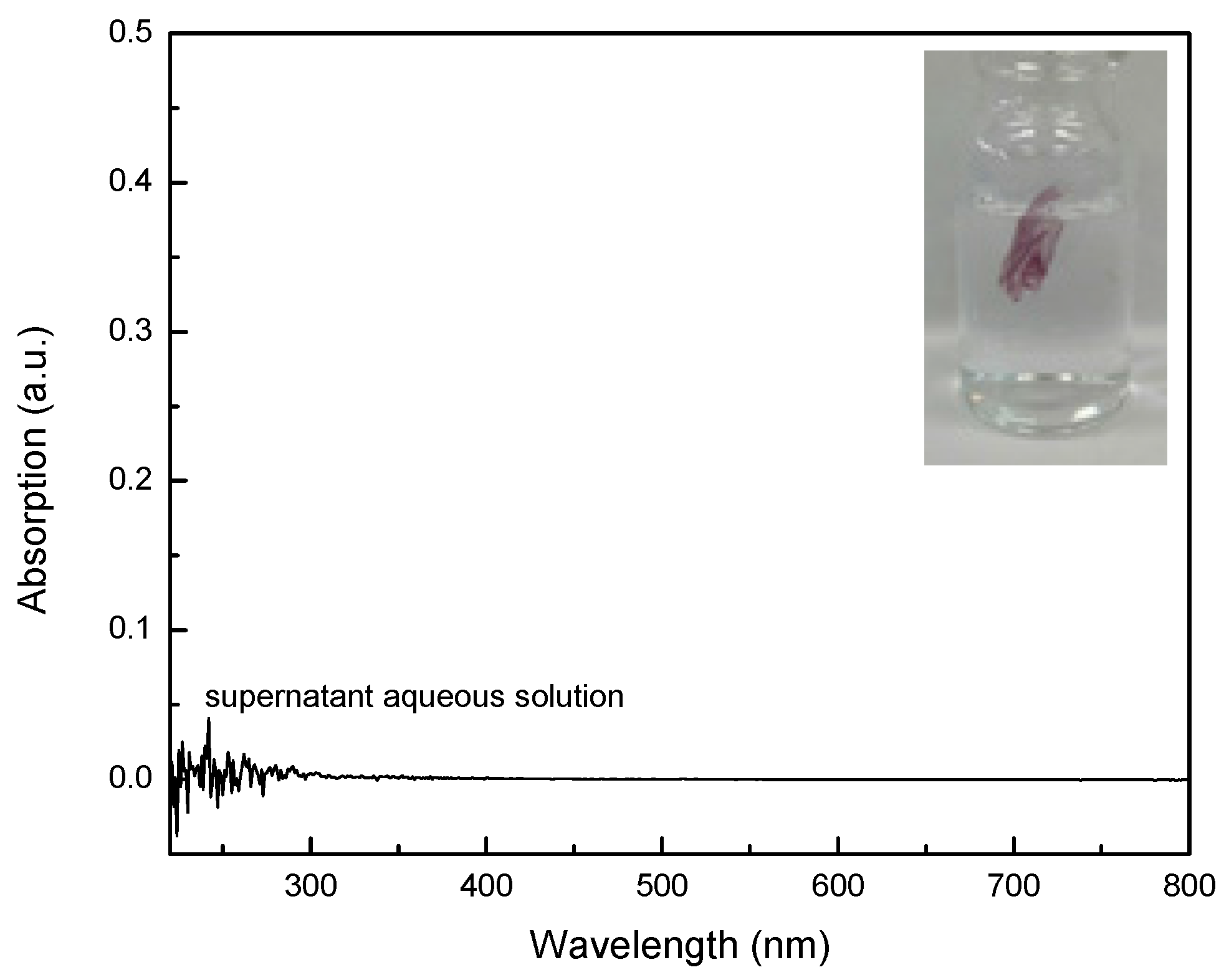
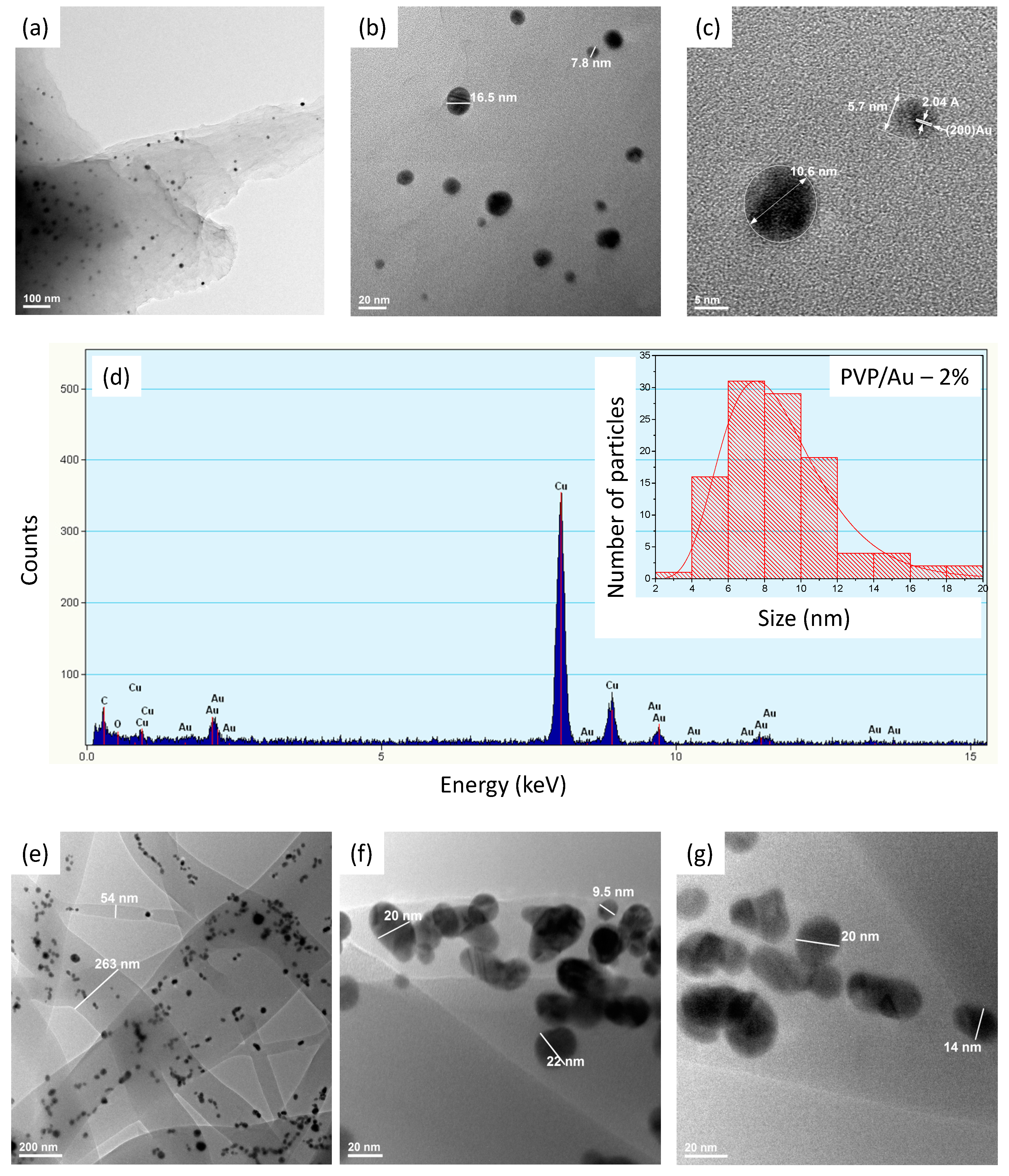
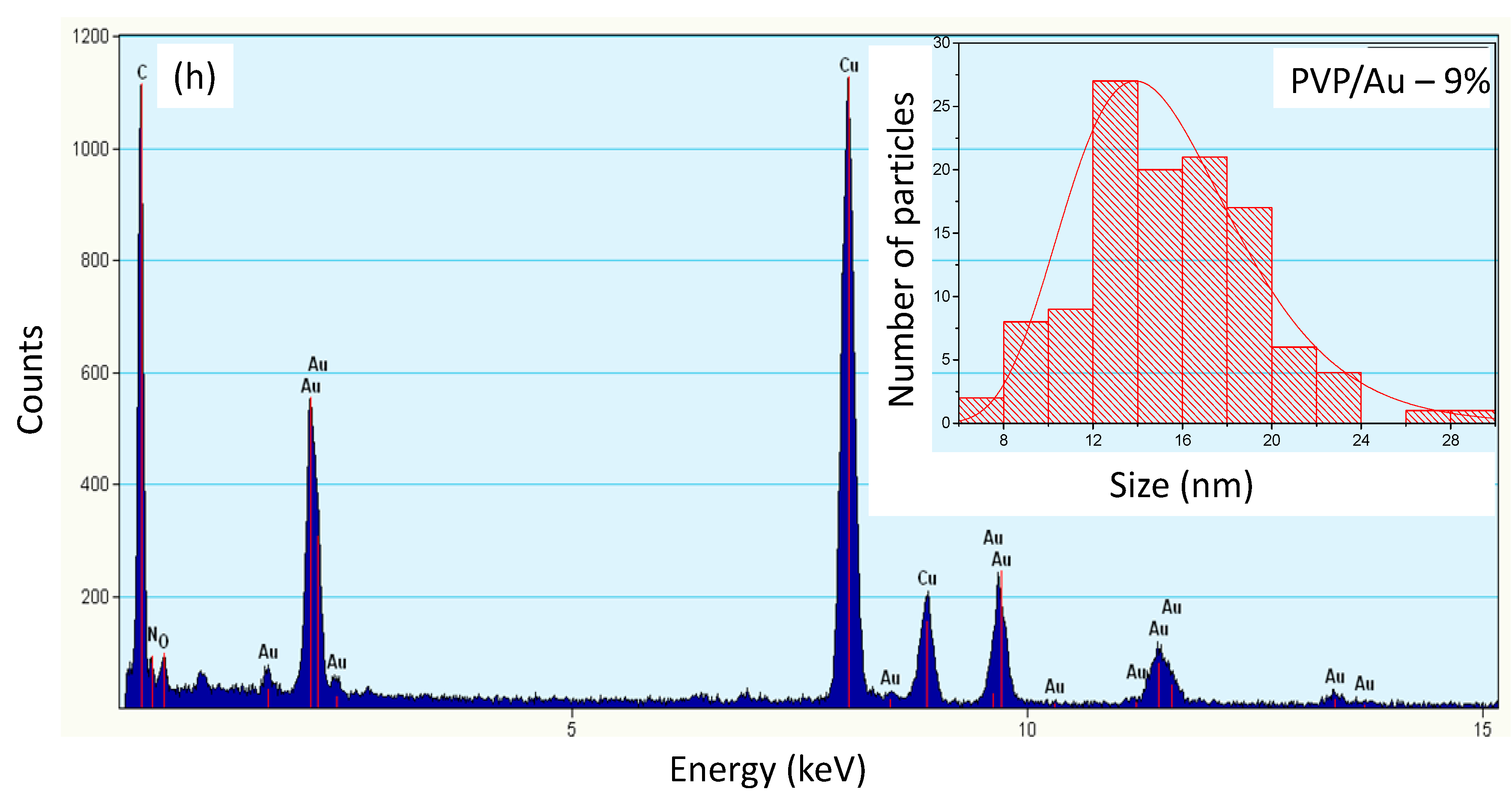
2.1. PVP/Au Colloidal Nanohybrids
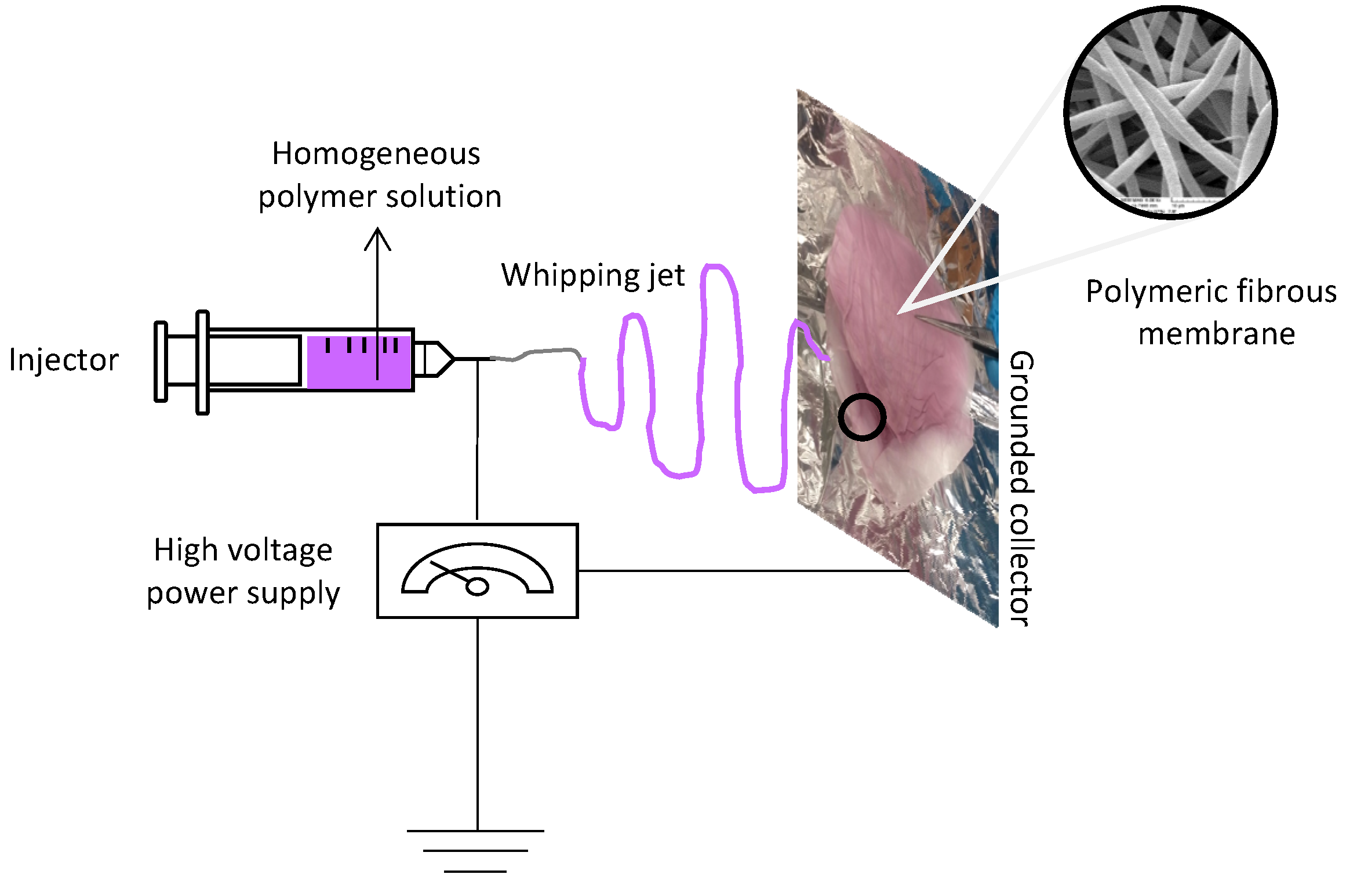
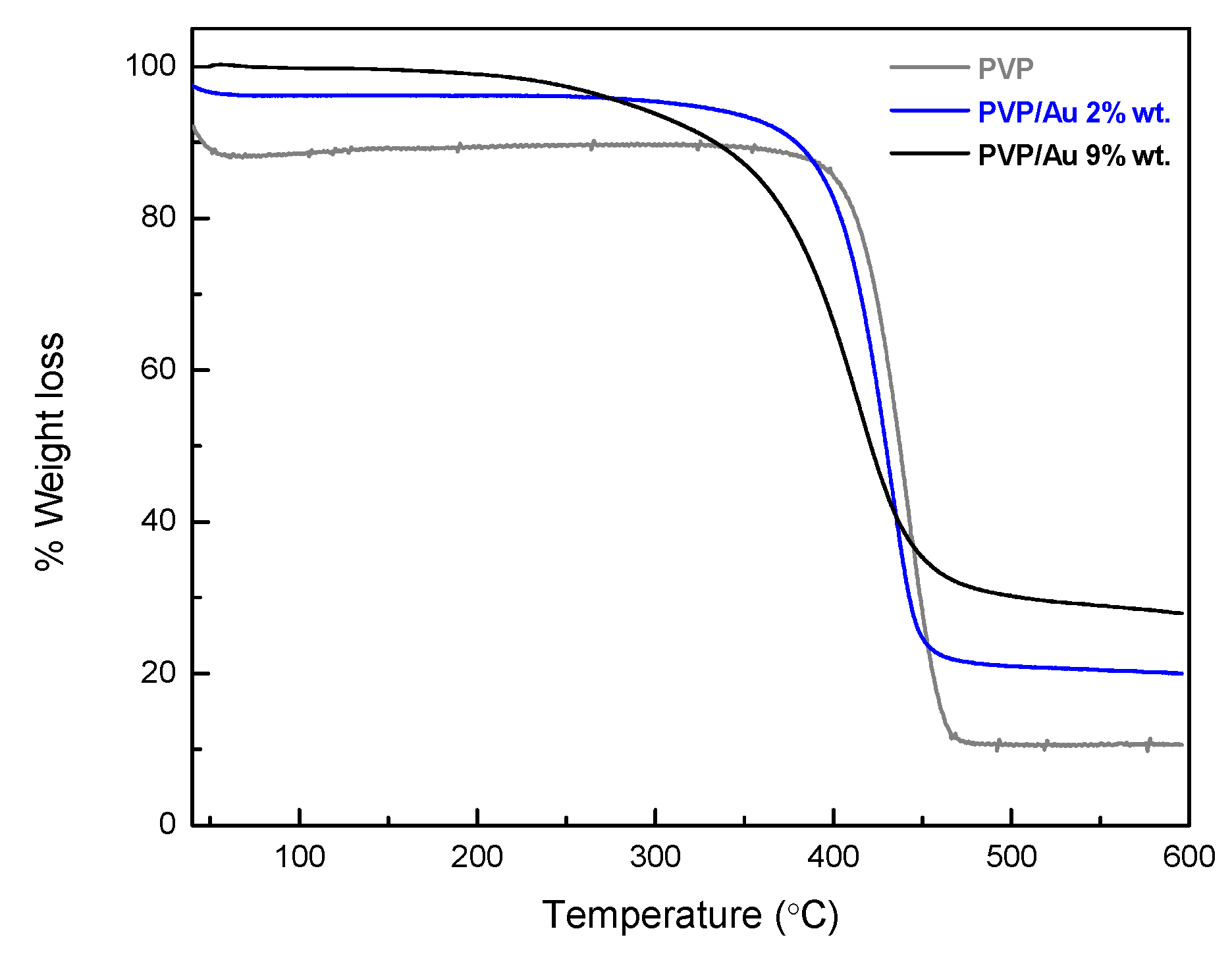
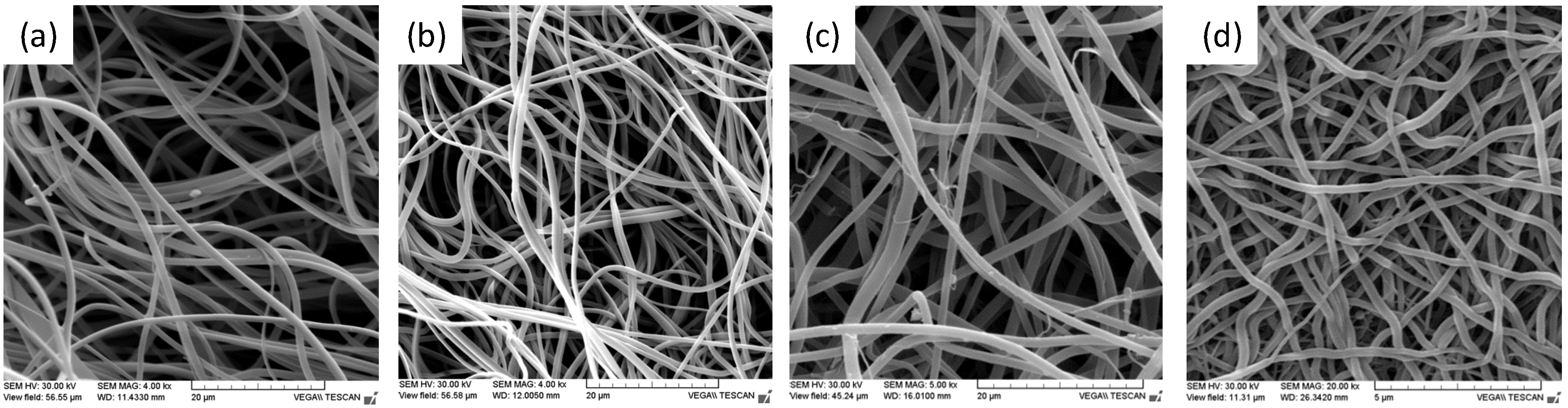
2.2. Membrane Fabrication
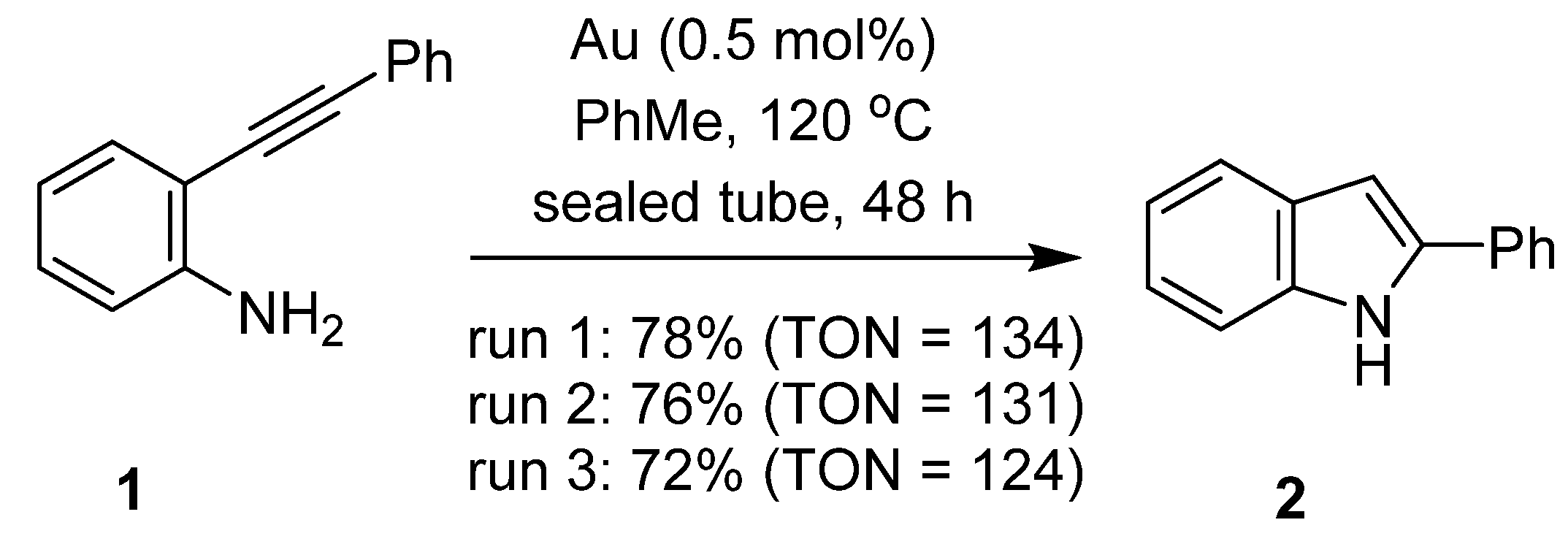
2.3. Catalysis
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Synthesis of PVP/Au Colloidal Nanohybrids
3.3. Fabrication of Electrospun PVP/Au Fibrous Membranes
3.4. Membrane Crosslinking
3.5. Catalysis
3.6. Characterization Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krasia-Christoforou, T. Organic-inorganic polymer hybrids: Synthetic strategies and applications. In Hybrid and Hierarchical Composite Materials; Chang-Soo, K., Sano, T., Randow, C., Eds.; Springer: Basel, Switzerland, 2015; pp. 11–63. [Google Scholar]
- Haruta, M. Catalysis of gold nanoparticles deposited on metal oxides. CATTECH 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Didier, A. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.; Hashmi, K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar]
- Guo, L.; Bai, J.; Li, C.; Meng, Q.; Liang, H.; Sun, W.; Li, H.; Liu, H. A novel catalyst containing palladium nanoparticles supported on PVP composite nanofiber films: Synthesis, characterization and efficient catalysis. Appl. Surf. Sci. 2013, 283, 107–114. [Google Scholar] [CrossRef]
- Sahiner, N. Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci. 2013, 38, 1329–1356. [Google Scholar] [CrossRef]
- Diaz, M.; Barrera, A.; Lopez-Cuenca, S.; Martínez-Salazar, S.Y.; Rabelero, M.; Ceja, I.; Fernández, V.V.A.; Aguilar, J. Size-controlled gold nanoparticles inside polyacrylamide microgels. J. Appl. Polym. Sci. 2016, 133, 43560. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Wu, X.-W.; Huang, Q.; Shen, J.-S.; Zhang, H.-W. In situ synthesized gold nanoparticles in hydrogels for catalytic reduction of nitroaromatic compounds. Appl. Surf. Sci. 2015, 331, 210–218. [Google Scholar] [CrossRef]
- Wu, Q.; Cheng, H.; Chang, A.; Xu, W.; Lu, F.; Wu, W. Glucose-mediated catalysis of Au nanoparticles in microgels. Chem. Commun. 2015, 51, 16068–16071. [Google Scholar] [CrossRef] [PubMed]
- Arabatzis, I.M.; Stergiopoulos, T.; Andreeva, D.; Kitova, S.; Neophytides, S.G.; Falaras, P. Characterization and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Sonawane, R.S.; Dongare, M.K. Sol–gel synthesis of Au/TiO2 thin films for photocatalytic degradation of phenol in sunlight. J. Mol. Catal. A Chem. 2006, 243, 68–76. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Baeck, S.-H.; Cuenya, B.R.; McFarland, E.W. Catalytic activity of supported Au nanoparticles deposited from block copolymer micelles. J. Am. Chem. Soc. 2003, 125, 7148–7149. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Yuranov, I.; Suvorova, E.I.; Buffat, P.A.; Kiwi-Minsker, L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation. J. Catal. 2004, 224, 8–17. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Zou, P.; Zhang, P.; Zhang, M.; Mu, J.; Guo, Z.; Li, X.; Wang, C.; Liu, Y. In situ assembly of well-dispersed gold nanoparticles on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. Chem. Commun. 2011, 47, 3906–3908. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of nanofibers from polymer solutions and melts. Adv. Appl. Mech. 2007, 41, 43–195. [Google Scholar]
- Zhang, Z.; Jiang, Y.; Chi, M.; Wang, C. Fabrication of Au nanoparticles supported on CoFe2O4 nanotubes by polyaniline assisted self-assembly strategy and their magnetically recoverable catalytic properties. Appl. Surf. Sci. 2016, 363, 578–585. [Google Scholar] [CrossRef]
- Moreno, I.; Navascues, N.; Irusta, S.; Santamaria, J. Electrospun Au/CeO2 nanofibers: A highly accessible low-pressure drop catalyst for preferential CO oxidation. J. Catal. 2015, 329, 479–489. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.-S.; Li, J.; Yang, P. Morphology adjustment of one dimensional CeO2 nanostructures via calcination and their composite with Au nanoparticles towards enhanced catalysis. RSC Adv. 2015, 5, 37585–37591. [Google Scholar] [CrossRef]
- Wang, X.; Choi, J.; Mitchell, D.R.G.; Truong, B.Y.; Kyratzis, L.I.; Caruso, A.R. Enhanced photocatalytic activity: Macroporous electrospun mats of mesoporous Au/TiO2 nanofibers. ChemCatChem 2013, 5, 2646–2654. [Google Scholar] [CrossRef]
- Anka, F.H.; Perera, S.D.; Ratanatawanate, C.; Balkus, J.K. Polyacrylonitrile gold nanoparticle composite electrospun fibers prepared by in situ photoreduction. Mater. Lett. 2012, 75, 12–15. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Green and one-step synthesis of gold nanoparticles incorporated into electrospun cyclodextrin nanofibers. RSC Adv. 2013, 3, 10197–10201. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.L.; Joonsuk, O.; Youngkwan, L.; Ji, H.Y.; Jaehuyn, H.; Jong, J.P.; Jong, M.K.; Jae-Do, N. Immobilization of gold nanoparticles on poly(methyl methacrylate) electrospun fibers exhibiting solid-state surface plasmon effect. Surf. Interface Anal. 2012, 44, 318–321. [Google Scholar]
- Deniz, A.E.; Vural, H.A.; Ortac, B.; Uyar, T. Gold nanoparticle/polymer nanofibrous composites by laser ablation and electrospinning. Mater. Lett. 2011, 65, 2941–2943. [Google Scholar] [CrossRef]
- Kundu, S.; Gill, R.S.; Saraf, R.F. Electrospinning of PAH nanofiber and deposition of Au NPs for nanodevice fabrication. J. Phys. Chem. C. 2011, 115, 15845–15852. [Google Scholar] [CrossRef]
- Hui, C.; Anindarupa, C.; Xiong, L.; Feroz, H.; Jianhua, Z.; Lauren, A.; Genevieve, K.; Lei, Z.; Qun, H. A multifunctional gold nanoparticle/polyelectrolyte fibrous nanocomposite prepared from electrospinning process. Mater. Express 2011, 1, 154–159. [Google Scholar]
- Kim, J.K.; Ahn, H. Fabrication and characterization of polystyrene/gold nanoparticle composite nanofibers. Macromol. Res. 2008, 16, 163–168. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Yang, S.; Du, J.; Wang, S.; Zheng, J.; Wang, Y.; Yang, Q.; Chen, X.; Jing, X. A simple and effective route for the preparation of poly(vinylalcohol) (PVA) nanofibers containing gold nanoparticles by electrospinning method. Solid State Commun. 2007, 1, 292–295. [Google Scholar] [CrossRef]
- Kim, G.M.; Wutzler, A.; Radusch, H.J.; Michler, G.H.; Simon, P.; Sperling, R.A.; Parak, W.J. One-dimensional arrangement of gold nanoparticles by electrospinning. Chem. Mater. 2005, 17, 4949–4957. [Google Scholar] [CrossRef]
- Gill, R.S.; Saraf, R.F.; Kundu, S. Self-assembly of gold nanoparticles on poly(allylamine hydrochloride) nanofiber: A new route to fabricate “necklace” as single electron devices. ACS Appl. Mater. Interfaces 2013, 5, 9949–9956. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Zhang, R.; Reinhard, B.M.; Fujii, M.; Perotto, G.; Marelli, B.; Omenetto, F.G.; Negro, L.D. Enhanced photoluminescence of Si nanocrystals-doped cellulose nanofibers by plasmonic light scattering. Appl. Phys. Lett. 2015, 107, 041111. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, M.; Wu, W.; Xu, H.; Cheng, S.; Fan, L.J. Polyacrylonitrile/noble metal/SiO2 nanofibers as substrates for the amplified detection of picomolar amounts of metal ions through plasmon-enhanced fluorescence. Nanoscale 2015, 7, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, A.; Spadaro, D.; Magri, D.; Moffa, M.; Gucciardi, P.G.; Persano, L.; Maragò, O.M.; Pisignano, D. Surface-enhanced Raman spectroscopy in 3D electrospun nanofiber mats coated with gold nanorods. Anal. Bioanal. Chem. 2016, 408, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Lv, K.-P.; Huang, H.-T.; Cong, H.P.; Yu, S.H. Co-assembly of Au nanorods with Ag nanowires within polymer nanofiber matrix for enhanced SERS property by electrospinning. Nanoscale 2012, 4, 5348–5355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Zhang, X.; Xie, G.; Su, Z.; Wei, G. Nanoporous carbon nanofibers decorated with platinum nanoparticles for non-enzymatic electrochemical sensing of H2O2. Nanomaterials 2015, 5, 1891–1905. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun doping of carbon nanotubes and platinum nanoparticles into the β-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Ouyang, Z.; Li, J.; Wang, J.; Li, Q.; Ni, T.; Zhang, X.; Wang, H.; Li, Q.; Su, Z.; Wei, G. Fabrication, characterization and sensor application of electrospun polyurethane nanofibers filled with carbon nanotubes and silver nanoparticles. J. Mater. Chem. B 2013, 1, 2415–2424. [Google Scholar] [CrossRef]
- Qin, Q.-H.; Na, H.; Zhang, C.; Yu, Q.; Zhang, X.-Q.; Zhang, H.-X. Preparation of Au nanoparticles immobilized cross-linked poly(4-vinylpyridine) nanofibers and their catalytic application for the reduction of 4-nitrophenol. J. Nanosci. Nanotechnol. 2015, 15, 3909–3912. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Facile immobilization of gold nanoparticles into electrospun polyethyleneimine/polyvinyl alcohol nanofibers for catalytic applications. J. Mater. Chem. 2011, 21, 4493–4501. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopa, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Aihua, H.; He, A.; Liu, W.; Jiang, X.; Zheng, J.; Han, C.C.; Hsiao, B.S.; Chu, B.; et al. Chemical crosslinking and biophysical properties of electrospun hyaluronic acid based ultra-thin fibrous membranes. Polymer 2009, 50, 3762–3769. [Google Scholar] [CrossRef]
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, F.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. Cross-linking chitosan nanofibers. Biomacromolecules 2007, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S. Mechanical properties of composite hydrogels. Polym. J. 1989, 21, 847–850. [Google Scholar] [CrossRef]
- Savva, I.; Kalogirou, A.S.; Chatzinicolaou, A.; Papaphilippou, P.; Pantelidou, A.; Vasile, E.; Vasile, E.; Koutentis, P.A.; Krasia-Christoforou, T. PVP-crosslinked electrospun membranes with embedded Pd and Cu2O nanoparticles as effective heterogeneous catalytic supports. RSC Adv. 2014, 4, 44911–44921. [Google Scholar] [CrossRef]
- Mu, X.-D.; Evans, D.G.; Kou, Y.A. General method for preparation of PVP-stabilized noble metal nanoparticles in room temperature ionic liquids. Catal. Lett. 2004, 97, 151–154. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-protected metal nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Ramachandra Rao, C.N.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar]
- Gorin, D.J.; Toste, D. Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Perea-Buceta, J.E.; Wirtanen, T.; Laukkanen, O.-V.; Mäkelä, M.K.; Nieger, M.; Melchionna, M.; Huittinen, N.; Lopez-Sanchez, J.A.; Helaja, J. Cycloisomerization of 2-alkynylanilines to indoles catalyzed by carbon-supported gold nanoparticles and subsequent homocoupling to 3,3′-biindoles. Angew. Chem. Int. Ed. 2013, 52, 11835–11839. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Barden, T.C. Indoles: Industrial, agricultural and over-the-counter uses. Top. Heterocycl. Chem. 2010, 26, 31–46. [Google Scholar]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.P.; Baek, T.J.; Seong, G.H. Gold nanoparticle aggregation-based highly sensitive DNA detection using atomic force microscopy. Anal. Bioanal. Chem. 2007, 388, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Savva, I.; Constantinou, D.; Marinica, O.; Vasile, E.; Vekas, L.; Krasia-Christoforou, T. Fabrication and characterization of superparamagnetic poly(vinyl pyrrolidone)/poly(l-lactide)/Fe3O4 electrospun membranes. J. Magn. Magn. Mater. 2014, 352, 30–35. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Harwood, L.M. “Dry-column” flash chromatography. Aldrichim. Acta 1985, 18, 25. [Google Scholar]
- Brand, J.P.; Chevalley, C.; Waser, J. One-pot gold-catalyzed synthesis of 3-silylethynyl indoles from unprotected o-alkynylanilines. Beilstein J. Org. Chem. 2011, 7, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zille, M.; Stolle, A.; Wild, A.; Schubert, U.S. ZnBr2-mediated synthesis of indoles in a ball mill by intramolecular hydroamination of 2-alkynylanilines. RSC Adv. 2014, 4, 13126–13133. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.

| Electrospinning Conditions | |||||
|---|---|---|---|---|---|
| Entry | Au Loading (% wt) | Needle (G) | Voltage (KV) | Needle-to-Collector Distance (cm) | Flow Rate (mL/h) |
| 1 | 0 | 16 | 25.0 | 28 | 1.8 |
| 2 | 2 | 16 | 22.5 | 20 | 2.0 |
| 3 | 9 | 16 | 22.5 | 15 | 1.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savva, I.; Kalogirou, A.S.; Achilleos, M.; Vasile, E.; Koutentis, P.A.; Krasia-Christoforou, T. Evaluation of PVP/Au Nanocomposite Fibers as Heterogeneous Catalysts in Indole Synthesis. Molecules 2016, 21, 1218. https://doi.org/10.3390/molecules21091218
Savva I, Kalogirou AS, Achilleos M, Vasile E, Koutentis PA, Krasia-Christoforou T. Evaluation of PVP/Au Nanocomposite Fibers as Heterogeneous Catalysts in Indole Synthesis. Molecules. 2016; 21(9):1218. https://doi.org/10.3390/molecules21091218
Chicago/Turabian StyleSavva, Ioanna, Andreas S. Kalogirou, Mariliz Achilleos, Eugenia Vasile, Panayiotis A. Koutentis, and Theodora Krasia-Christoforou. 2016. "Evaluation of PVP/Au Nanocomposite Fibers as Heterogeneous Catalysts in Indole Synthesis" Molecules 21, no. 9: 1218. https://doi.org/10.3390/molecules21091218






