Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Acetylcholinesterase by Extracts from TCM Plants
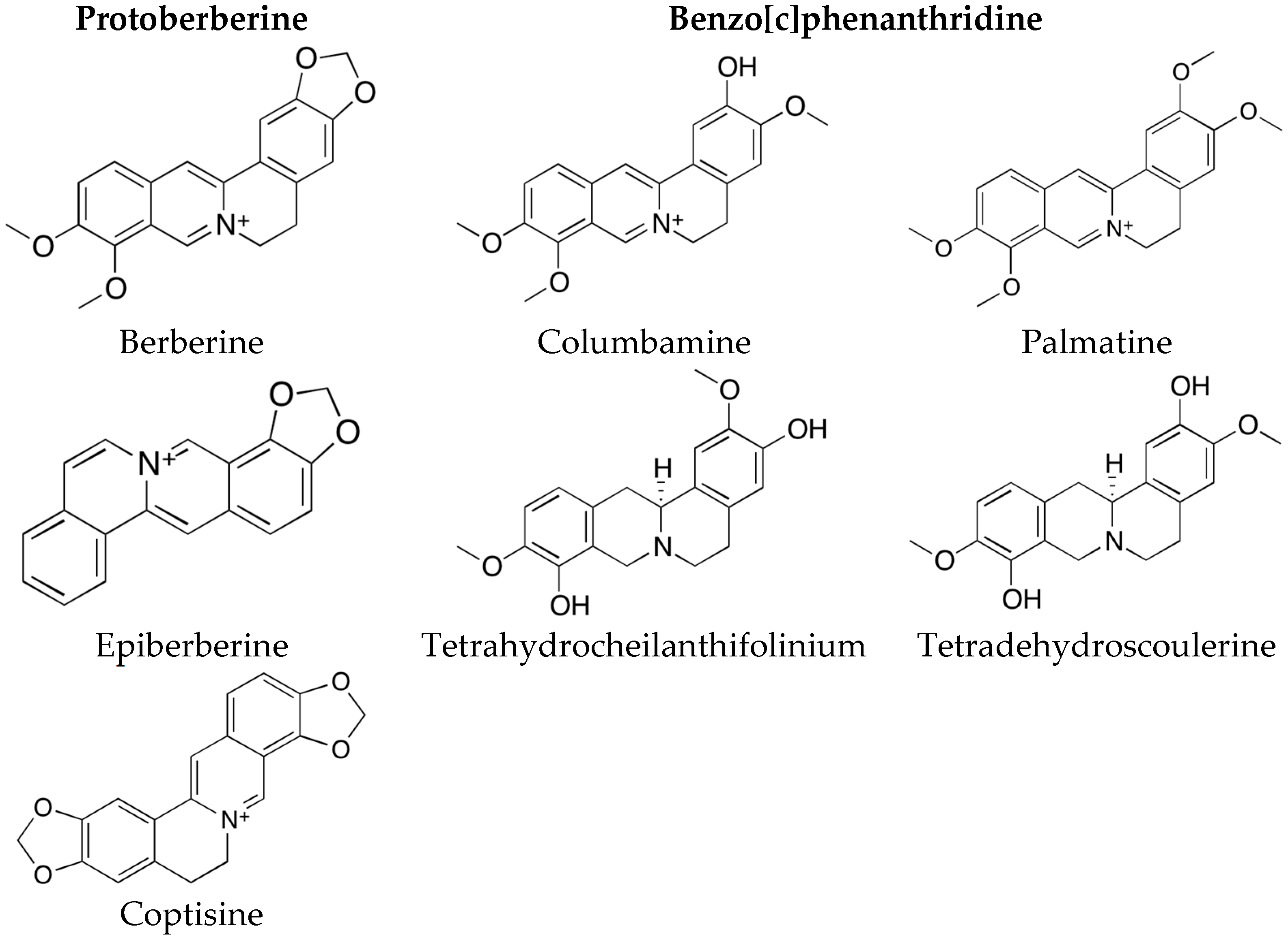
2.2. Phytochemical Analysis of Most Active Extracts
2.3. Inhibition of Acetylcholinesterase by Pure Substances
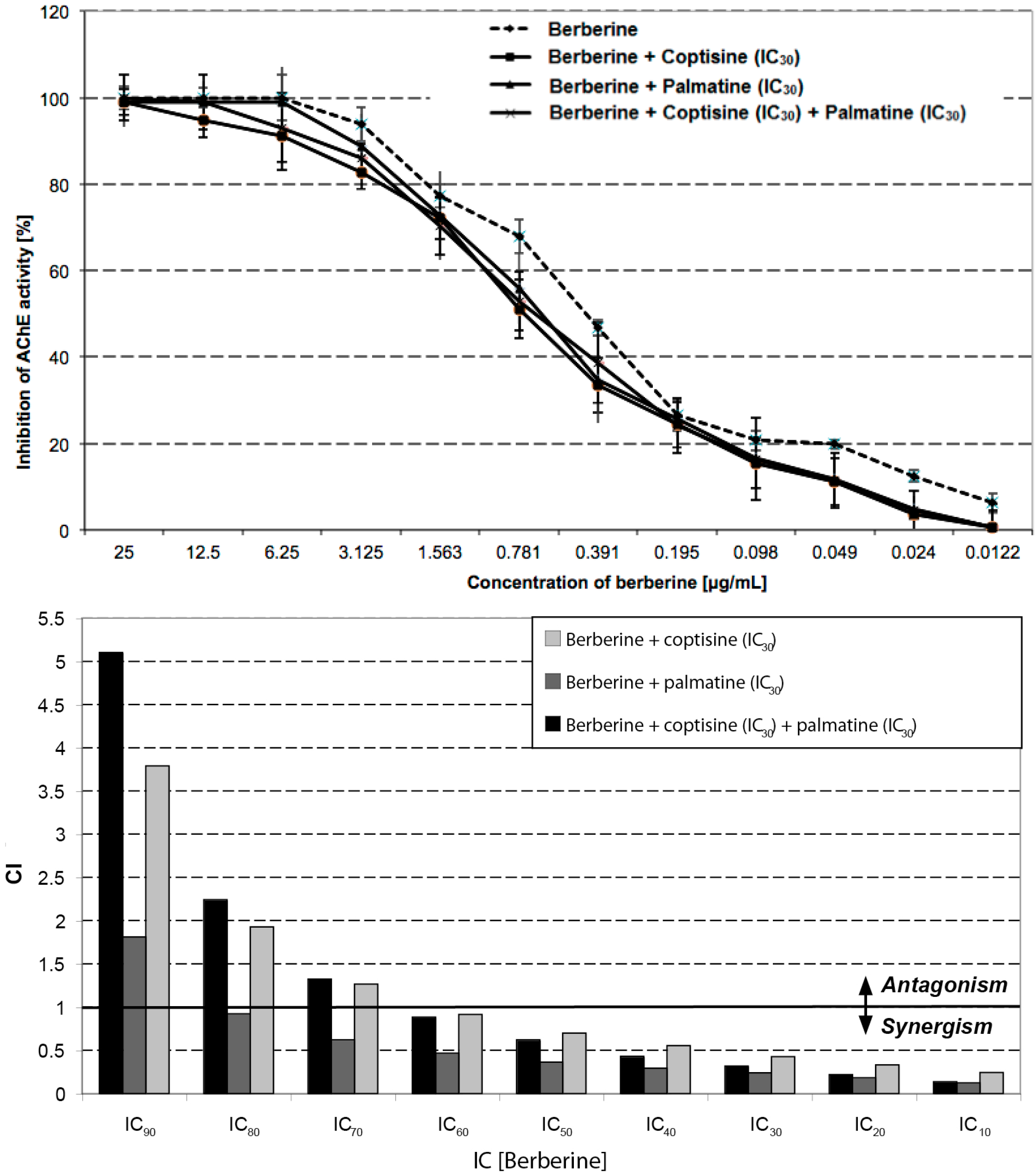
2.4. Inhibition of ACh is Based on Synergism
3. Discussion
4. Materials and Methods
4.1. TCM Plants
4.2. Chemicals
4.3. DNA Barcoding
4.4. Preparation of TCM Extracts
4.5. Cytotoxicity / MTT Assay
4.6. AChE Assay
4.7. Phytochemical Analysis
4.8. Evaluation of Synergistic Effects
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2015. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 10 August 2016).
- Czech, C.; Tremp, G.; Pradier, L. Presenilins and Alzheimer’s disease: Biological functions and pathogenic mechanisms. Prog. Neurobiol. 2000, 60, 363–384. [Google Scholar] [CrossRef]
- Cummings, J.L. Cognitive and behavioral heterogeneity in Alzheimer’s disease: Seeking the neurobiological basis. Neurobiol. Aging 2000, 21, 845–861. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol. Appl. Neurobiol. 1978, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P.; et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 2004, 363, 2105–2115. [Google Scholar] [PubMed]
- Giacobini, E. Modulation of brain acetylcholine levels with cholinesterase inhibitors as a treatment of Alzheimer disease. Keio J. Med. 1987, 36, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Torrero, D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Farlow, M.R.; Relkin, N.; Beach, T.G. Do cholinergic therapies have disease-modifying effects in Alzheimer’s disease? Alzheimer’s Dement. 2006, 2, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar] [PubMed]
- Ehret, M.J.; Chamberlin, K.W. Current Practices in the Treatment of Alzheimer Disease: Where is the Evidence After the Phase III Trials? Clin. Ther. 2015, 37, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Gmunder, F.; Hamburger, M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Wollen, K.A. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010, 15, 223–244. [Google Scholar] [PubMed]
- Hostettmann, K.; Borloz, A.; Urbain, A.; Marston, A. Natural product inhibitors of acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent Knowledge on Medicinal Plants as Source of Cholinesterase Inhibitors for the Treatment of Dementia. Mini Rev. Med. Chem. 2015, 16, 605–618. [Google Scholar] [CrossRef]
- Kaufmann, D.; Kaur Dogra, A.; Wink, M. Myrtenal inhibits acetylcholinesterase, a known Alzheimer target. J. Pharm. Pharmacol. 2011, 63, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Main, A.R.; Hastings, F.L. Carbamylation and binding constants for the inhibition of acetylcholinesterase by physostigmine (eserine). Science 1966, 154, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Li, A.R.; Zhu, Y.; Li, X.N.; Tian, X.J. Antimicrobial activity of four species of Berberidaceae. Fitoterapia 2007, 78, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Dong, Y.; Sheng, G.; Dong, X.; Sun, X.; Fu, J. Isolation and structure determination of anti-influenza component from Mahonia Bealei. J. Ethnopharmacol. 2006, 108, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Wang, X.L.; Xue, S.G. Extraction of berberine hydrochloride from Mahonia bealei (Fort.) Carr. Zhongguo Zhong Yao Za Zhi 1993, 18, 347–348. [Google Scholar] [PubMed]
- Zhao, T.F.; Wang, X.K.; Rimando, A.M.; Che, C.T. Folkloric medicinal plants: Tinospora sagittata var. cravaniana and Mahonia bealei. Planta Med. 1991, 57, 505–505. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, Y.; Liu, H.; Yan, Y.; Li, J. Determination of the alkaloid content in different parts of some Mahonia plants by HPCE. Pharm. Acta Helv. 2000, 74, 387–391. [Google Scholar] [CrossRef]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Bae, H.J.; Min, B.S.; Choi, J.S. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch. Pharm. Res. 2008, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Ma, Y.M.; Shi, R.; Wang, T.M.; Zhang, N.; Wang, C.H.; Yang, Y. Identification of the toxic constituents in Rhizoma Coptidis. J. Ethnopharmacol. 2010, 128, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E.; Wink, M. The plants in alphabetical order. In Medicinal Plants of the World, 1st ed.; Timber Press: London, UK, 2004; p. 238. [Google Scholar]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar]
- Lemes, L.F.; de Andrade Ramos, G.; de Oliveira, A.S.; da Silva, F.M.; de Castro Couto, G.; da Silva Boni, M.; Guimaraes, M.J.; Souza, I.N.; Bartolini, M.; Andrisano, V.; et al. Cardanol-derived AChE inhibitors: Towards the development of dual binding derivatives for Alzheimer's disease. Eur. J. Med. Chem. 2016, 108, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.; Jantan, I. Synthetic Curcumin Analogs as Inhibitors of beta-Amyloid Peptide Aggregation: Potential Therapeutic and Diagnostic Agents for Alzheimer's Disease. Mini. Rev. Med. Chem. 2015, 15, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Na, M.; Dat, N.T.; Ngoc, T.M.; Youn, U.; Kim, H.J.; Min, B.S.; Lee, J.; Bae, K. Cholinesterase inhibitory and anti-amnesic activity of alkaloids from Corydalis turtschaninovii. J. Ethnopharmacol. 2008, 119, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Durairajan, S.S.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Huang, L.; Li, X.S.; Huang, J.D.; Li, M. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging 2012, 33, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Wang, C.Y.; Ho, K.J.; Lu, F.J.; Chang, T.C.; Lee, W.S. Magnolol induces apoptosis in human leukemia cells via cytochrome c release and caspase activation. Anticancer Drugs 2003, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Lee, B.J.; Nam, S.Y.; Lee, S.I.; Kim, Y.H.; Kim, K.H.; Oh, K.W.; Hong, J.T. Inhibitory effect of ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on memory impairment and neuronal toxicity induced by beta-amyloid. Pharmacol. Biochem. Behav. 2010, 95, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, D.Y.; Han, S.B.; Kim, Y.H.; Kim, K.H.; Hwang, B.Y.; Kang, J.K.; Lee, B.J.; Oh, K.W.; Hong, J.T. Inhibitory effect of ethanol extract of Magnolia officinalis on memory impairment and amyloidogenesis in a transgenic mouse model of Alzheimer’s disease via regulating beta-secretase activity. Phytother. Res. 2012, 26, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Goto, H.; Kogure, T.; Kohta, K.; Shintani, T.; Itoh, T.; Terasawa, K. Extract prepared from the bark of Cinnamomum cassia Blume prevents glutamate-induced neuronal death in cultured cerebellar granule cells. Phytother. Res. 2000, 14, 466–468. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Bruning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [PubMed]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the crude TCM plant extracts are available from the authors.
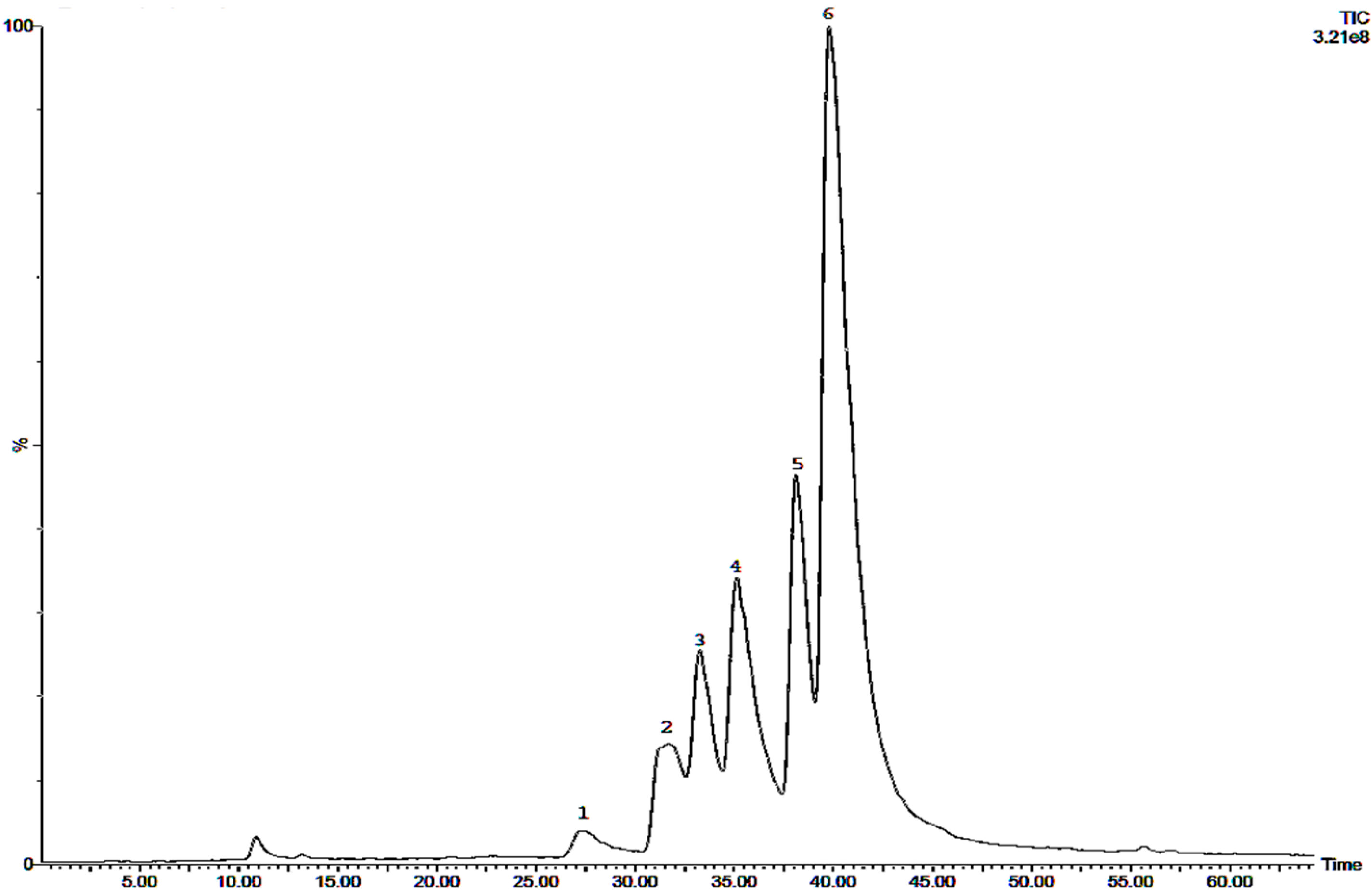
| Sample | IC50 AChE Inhibition (mg/mL) | IC50 COS7 (µg/mL) | Ratio IC50 COS7/AChE |
|---|---|---|---|
| Berberis bealei MeOH | 34.10 ± 4.89 | 35.37 ± 4.21 | 1.0 |
| Berberis bealei CH2Cl2 | 9.99 ± 1.18 | 13.36 ± 1.76 | 1.3 |
| Berberis bealei H2O | 87.77 ± 4.11 | 270.0 ± 13.5 | 3.1 |
| Coptis chinensis MeOH | 0.031 ± 0.002 | 3.72 ± 0.74 | 120 |
| Coptis chinensis CH2Cl2 | 8.13 ± 0.90 | 39.57 ± 4.87 | 4.9 |
| Coptis chinensis H2O | 2.5 ± 0.61 | 118.3 ± 7.4 | 47 |
| Phellodendron chinense MeOH | 8.03 ± 0.98 | 85.52 ± 11.90 | 10 |
| Phellodendron chinense CH2Cl2 | 6.34 ± 1.37 | 71.33 ± 6.87 | 11 |
| Phellodendron chinense H2O | 84.83 ± 1.84 | 282.9 ± 15.3 | 3.3 |
| Berberine | 1.48 ± 0.07 | - | - |
| Coptisine | 1.27 ± 0.06 | - | - |
| Palmatine | 5.21 ± 0.48 | - | - |
| Physostigmine | 2.24 ± 0.27 | - | - |
| Galantamine | 4.33 ± 0.21 | - | - |
| Family/Plant | Chinese Name | DNA | AChE (IC50 (µg/mL)) | COS7 (IC50 (µg/mL)) | ||||
|---|---|---|---|---|---|---|---|---|
| MeOH | CH2Cl2 | H2O | MeOH | CH2Cl2 | H2O | |||
| Acanthaceae | - | - | - | - | - | - | - | - |
| Andrographis paniculata | Chuan Xin Lian | Family | NA | NA | NA | 344.7 ± 13.3 | 104.7 ± 5.2 | 255.6 ± 11.9 |
| Amaranthaceae | - | - | - | - | - | - | - | - |
| Celiosa cristata | Ji Guan hua | Genus | NA | NA | NA | 28.43 ± 2.87 | 136.00 ± 8.40 | 263.9 ± 12.3 |
| Apiaceae | - | - | - | - | - | - | - | - |
| Bupleurum chinense | Chai Hu | Genus | NA | NA | NA | 358.7 ± 19.8 | 87.12 ± 3.67 | 15.60 ± 11.76 |
| Bupleurum marginatum | Nan Chai Hu | Genus | NA | NA | NA | 576.0 ± 31.2 | 67.41 ± 5.23 | 350.7 ± 17.5 |
| Centella asiatica | Lei Gong Gen | Species | NA | NA | NA | 392.8 ± 78.2 | 64.97 ± 4.85 | 325.8 ± 12.1 |
| Saposhnikovia divaricata | Fang Feng | Genus | NA | NA | NA | 1575 ± 147 | 46.00 ± 2.64 | 153.0 ± 7.7 |
| Selinum monnieri | She Chuang Zi | Family | NA | NA | NA | 120.0 ± 11.7 | 37.02 ± 2.39 | 339.7 ± 11.1 |
| Araliaceae | - | - | - | - | - | - | - | - |
| Eleutherococcus senticosus | Ci Wu Jia | Species | NA | NA | NA | 190.1 ± 18.4 | 61.49 ± 5.98 | 130.5 ± 7.5 |
| Panax ginseng | Ren Shen | Species | NA | NA | NA | 510.8 ± 29.5 | 47.76 ± 5.82 | 151.7 ± 8.9 |
| Panax notoginseng | San Qi | Species | NA | NA | NA | 229.5 ± 19.5 | 6.47 ± 0.89 | 182.3 ± 9.1 |
| Arecaceae | - | - | - | - | - | - | - | - |
| Areca catechu | Bing Lang | n/a | NA | NA | NA | 31.02 ± 2.69 | 117.02 ± 7.33 | 16.60 ± 2.01 |
| Apogynaceae | - | - | - | - | - | - | - | - |
| Cyanchum paniculatum | Liao Diao Zhu | Genus | NA | NA | NA | 227.7 ± 16.9 | 114.25 ± 6.78 | 220.7 ± 7.6 |
| Asparagaceae | - | - | - | - | - | - | - | - |
| Polygonatum humile | Huan Jjing | Species | NA | NA | NA | 147.4 ±15.4 | 53.94 ± 4.67 | 298.4 ± 13.6 |
| Asteraceae | - | - | - | - | - | - | - | - |
| Arcticum lappa | Niu Bang | Species | NA | NA | NA | 1813 ± 225 | 344.25 ± 12.31 | 355.5 ± 12.6 |
| Artemisia annua | Huang Hua Hao | n/a | NA | NA | NA | 201.1 ± 8.7 | 34.57 ± 3.18 | 288.6 ± 11.4 |
| Artemisia capillaris | Yin Chen Hao | Genus | NA | NA | NA | 215.4 ± 9.3 | 29.49 ± 2.56 | 201.9 ± 9.5 |
| Centipeda minima | Ebu Shi Cao | n/a | NA | NA | NA | 54.21 ± 4.98 | 10.44 ± 1.70 | 55.64 ± 4.62 |
| Chrysanthemum indicum | Ye Ju Hua | Genus | NA | NA | NA | 287.2 ± 9.8 | 63.58 ± 5.78 | 320.9 ± 14.3 |
| Chrysanthemum morifolium | Ju Hua | Genus | NA | NA | NA | 166.7 ± 8.4 | 42.88 ± 3.96 | 760.4 ± 28.3 |
| Eclipta prostata | Han Lian Cao | Species | NA | NA | NA | 186.1 ± 12.8 | 112.06 ± 9.65 | 291.7 ± 13.9 |
| Senecio scandens | Qian Li Guang | Genus | NA | NA | NA | 126.2 ± 12.2 | 143.54 ± 9.64 | 114.2 ± 6.7 |
| Siegesbeckia orientalis | Xi Xian Cao | Family | NA | NA | NA | 84.4 ± 7.5 | 17.78 ± 1.94 | 159.2 ± 7.4 |
| Taraxum officinale | Pu Gong Ying | Species | NA | NA | NA | 485.3 ± 17 | 177.16 ± 8.45 | 156.9 ± 6.3 |
| Berberidaceae | - | - | - | - | - | - | - | - |
| Berberis bealei | Shi Da Gong Lao | Species | 34.10 ± 4.89 | 9.99 ± 1.18 | 87.77 ± 4.11 | 35.37 ± 4.21 | 13.36 ± 1.76 | 270.0 ± 13.5 |
| Dysosma versipellis | Ba Jiao Lian | n/a | NA | NA | NA | 54.90 ± 4.69 | 49.95 ± 5.29 | 1276 ± 39 |
| Epimedium koreanum | Yin Yang Huo | Species | NA | NA | NA | 0.37 ± 0.03 | 3.60 ± 0.56 | 140.8 ± 6.2 |
| Brassicaceae | - | - | - | - | - | - | - | - |
| Isatis indigotica rhizome | Ban Langen | Family | NA | NA | NA | 324.4 ± 17.8 | 42.38 ± 4.54 | 557.2 ± 27.2 |
| Isatis indigotica leaf | Daq Qing Ye | Family | NA | NA | NA | 90.62 ± 11.37 | 0.64 ± 0.07 | 93.51 ± 4.73 |
| Capsella bursa-pastoris | Ji Cai | Species | NA | NA | NA | 29.82 ± 3.87 | 120.82 ± 7.89 | 234.2 ± 11.6 |
| Caprifoliaceae | - | - | - | - | - | - | - | - |
| Lonicera confusa | Ren Dong Teng | Genus | NA | NA | NA | 118.9 ± 12.8 | 58.99 ± 5.13 | 446.8 ± 21.2 |
| Crassulacea | - | - | - | - | - | - | - | - |
| Sedum rosea | Hong Jing Tian | Species | NA | NA | NA | 87.42 ± 7.43 | 74.67 ± 6.54 | 61.97 ± 4.12 |
| Cupressaceae | - | - | - | - | - | - | - | - |
| Platycladus orientalis | Ce Bai Ye | Species | NA | NA | NA | 158.7 ± 12.4 | 21.80 ± 2.91 | 97.78 ± 56.53 |
| Dryopteridaceae | - | - | - | - | - | - | - | - |
| Cyrtomium fortunei | Guang Zhong | Species | NA | NA | NA | 348.7 ± 26.5 | 132.13 ± 5.03 | 30.42 ± 2.45 |
| Ephedraceae | - | - | - | - | - | - | - | - |
| Ephedra sinica | Ma Huang | Species | NA | NA | NA | 36.76 ± 3.93 | 41.82 ± 4.85 | 69.15 ± 5.98 |
| Equisetaceae | - | - | - | - | - | - | - | - |
| Equisetum hiemale | Mu Zei | Species | NA | NA | NA | 243.5 ± 17.1 | 35.76 ± 3.50 | 265.9 ± 12.3 |
| Euphorbiaceae | - | - | - | - | - | - | - | - |
| Croton tiglium | Ba Dou | n/a | NA | NA | NA | 222.1 ± 18.4 | 225.98 ± 10.69 | 166.2 ± 5.7 |
| Fabaceae | - | - | - | - | - | - | - | - |
| Abrus cantonensis | Ji Gu Cao | Genus | NA | NA | NA | 733.1 ± 42.6 | 129.45 ± 7.65 | 575.2 ± 24.4 |
| Acacia catechu | Er Cha | n/a | NA | NA | NA | 34.86 ± 2.55 | 31.56 ± 3.78 | 35.71 ± 3.86 |
| Cassia tora | Jue Ming Zi | Genus | NA | NA | NA | 75.95 ± 6.50 | 189.15 ± 8.43 | 481.3 ± 19.4 |
| Desmodium styracifolium | Guang Jin Qian Cao | Genus | NA | NA | NA | 104.1 ± 9.35 | 139.86 ± 5.50 | 333.5 ± 13.9 |
| Glycyrrhiza inflata | Gan Cao | Species | NA | 333 ± 9 | NA | 126.8 ± 11.2 | 6.97 ± 1.43 | 583.9 ± 21.3 |
| Spatholobus suberectus | Ji Xue Teng | Genus | NA | NA | NA | 54.87 ± 4.89 | 154.66 ± 6.72 | 16.63 ± 1.32 |
| Sutherlandia frutescens | n/a | n/a | NA | NA | NA | 352.0 ± 29.5 | 259.37 ± 9.39 | 857.3 ± 26.8 |
| Geraniaceae | - | - | - | - | - | - | - | - |
| Geranium wilfordii | Loa Guan Cao | Genus | NA | NA | NA | 169.8 ± 16.4 | 17.02 ± 1.80 | 225.8 ± 9.6 |
| Ginkgoaceae | - | - | - | - | - | - | - | - |
| Ginkgo biloba | Yin Xing | Species | NA | 1003 ± 15 | NA | 260.1 ± 24.21 | 15.40 ± 1.34 | 450.8 ± 21.3 |
| Hypericaceae | - | - | - | - | - | - | - | - |
| Hypericum japonicum | Tian Ji Huang | Genus | NA | NA | NA | 100.9 ± 9.0 | 10.83 ± 0.53 | 151.8 ± 13.9 |
| Iridaceae | - | - | - | - | - | - | - | - |
| Belamcanda chinensis | She Gan | Species | NA | NA | NA | 319.5 ± 28.4 | 89.25 ± 7.32 | 222.1 ± 12.7 |
| Lamiaceae | - | - | - | - | - | - | - | - |
| Mentha haplocalyx | Bo He | Genus | NA | NA | NA | 147.8 ± 12.3 | 34.16 ± 3.35 | 285.7 ± 14.6 |
| Prunella vulgaris | Xia Ku Cao | Species | NA | NA | NA | 494.5 ± 45.7 | 90.48 ± 6.47 | 21.57 ± 2.90 |
| Scutellaria baicalensis | Huang Qin | Species | NA | NA | NA | 28.88 ± 2.65 | 287.98 ± 8.93 | 46.41 ± 3.96 |
| Lauraceae | - | - | - | - | - | - | - | - |
| Cinnamomum cassia | Gui Zhi | Genus | 1027 ± 16 | 953 ± 12 | NA | 108.4 ± 9.8 | 23.20 ± 2.76 | 453.9 ± 21.5 |
| Loranthaceae | - | - | - | - | - | - | - | - |
| Taxillus chinensis | Sang Ji Sheng | Family | NA | NA | NA | 378.2 ± 32.4 | 68.65 ± 7.42 | 181.7 ± 9.2 |
| Lythraceae | - | - | - | - | - | - | - | - |
| Punica granatum | Shi Liu | Species | NA | NA | NA | 218.6 ± 16.3 | 126.64 ± 8.64 | 8.608 ± 1.432 |
| Magnoliaceae | - | - | - | - | - | - | - | - |
| Magnolia officinalis | Hou Pu | Species | 320 ± 9 | 183 ± 6 | NA | 13.12 ± 0.97 | 5.45 ± 1.37 | 73.01 ± 5.42 |
| Melanthiaceae | - | - | - | - | - | - | - | - |
| Paris polyphylla | Qi Ye Yi Zhi Hua | Species | NA | NA | NA | 5.52 ± 0.39 | 24.07 ± 2.82 | 38.49 ± 2.47 |
| Myrisinaceae | - | - | - | - | - | - | - | - |
| Lysimachia christinae | Jin Qian Cao | Genus | NA | NA | NA | 436.3 ± 36.6 | 137.39 ± 6.39 | 152.2 ± 8.4 |
| Myrtaceae | - | - | - | - | - | - | - | - |
| Eucalyptus robusta | An Shu | n/a | NA | NA | NA | 15.21 ± 0.62 | n/a | 94.19 ± 5.99 |
| Oleaceae | - | - | - | - | - | - | - | - |
| Fraxinus chinensis | Qin Pi | n/a | NA | NA | NA | 38.72 ± 6.59 | 39.23 ± 4.11 | 193.2 ± 11.6 |
| Ophioglossacea | - | - | - | - | - | - | - | - |
| Ophioglossum vulgatum | Yi Zhi Jian | Species | NA | NA | NA | 68.70 ± 11.42 | 62.87 ± 6.58 | 344.0 ± 13.4 |
| Orchideaceae | - | - | - | - | - | - | - | - |
| Dendrobium loddigesii | Shi Hu | Species | NA | NA | NA | 61.65 ± 15.36 | 25.74 ± 2.94 | 104.3 ± 6.3 |
| Paeoniaceae | - | - | - | - | - | - | - | - |
| Paeonia lactiflora | Chi Shao | Genus | NA | NA | NA | 309.8 ± 24.7 | 34.06 ± 4.60 | 148.2 ± 6.1 |
| Pedaliaceae | - | - | - | - | - | - | - | - |
| Harpagophytum procumbens | n/a | n/a | NA | NA | NA | 217.2 ± 18.53 | 15.89 ± 2.25 | 242.9 ± 12.6 |
| Poaceae | - | - | - | - | - | - | - | - |
| Cymbopogon distans | Yun Xian Cao | Genus | NA | NA | NA | 17.88 ± 1.16 | 114.57 ± 8.63 | 257.8 ± 13.2 |
| Polygonaceae | - | - | - | - | - | - | - | - |
| Fallopia multiflora | He Shou Wu | Genus | NA | 523 ± 11 | NA | 48.87 ± 4.62 | 107.74 ± 7.94 | 61.32 ± 5.61 |
| Polygonum cuspidatum | Hu Zhang | Species | NA | NA | NA | 19.59 ± 2.23 | 2.85 ± 0.87 | 39.81 ± 3.84 |
| Rheum officinale | Da Huang | Species | NA | NA | NA | 3.53 ± 0.80 | n/a | 51.59 ± 5.48 |
| Ranunculaceae | - | - | - | - | - | - | - | - |
| Coptis chinensis | Huang Lian | Species | 0.031 ± 0.002 | 8.13 ± 0.90 | 2.5 ± 0.61 | 3.72 ± 0.74 | 39.57 ± 4.87 | 118.3 ± 7.4 |
| Rosaceae | - | - | - | - | - | - | - | - |
| Rosa chinensis | Yu Ji Hua | n/a | NA | NA | NA | 36.76 ± 3.45 | 141.55 ± 9.52 | 24.32 ± 2.86 |
| Rosa laevigata | Jin Ying Zi | n/a | NA | NA | NA | 13.30 ± 2.21 | 151.80 ± 9.79 | 93.61 ± 7.30 |
| Sanguisorba officinalis | Di Yu | Species | NA | NA | NA | 1100 ± 97 | 26.77 ± 3.58 | 20.55 ± 2.59 |
| Rubiaceae | - | - | - | - | - | - | - | - |
| Hedyotis diffusa | Bai Hua She She Cao | Genus | NA | NA | NA | 418.1 ± 34.8 | 45.35 ± 4.12 | 158.7 ± 9.2 |
| Rutaceae | - | - | - | - | - | - | - | - |
| Evodia lepta | San Cha Ku | Family | NA | NA | NA | 5.13 ± 0.75 | 42.06 ± 4.65 | 419.3 ± 19.4 |
| Evodia rutaecarpa | Wu Zhu Yu | n/a | NA | NA | NA | 427.7 ± 37.2 | 8.78 ± 1.78 | 1176 ± 34 |
| Phellodendron chinense | Huang Bai | Genus | 8.03 ± 0.98 | 6.34 ± 1.37 | 84.83 ± 1.84 | 85.52 ± 11.90 | 71.33 ± 6.87 | 282.9 ± 15.3 |
| Saururaceae | - | - | - | - | - | - | - | - |
| Houttuynia cordata | Yu Xing Cao | Species | NA | NA | NA | 63.95 ± 5.98 | 48.26 ± 5.19 | 633.3 ± 17.8 |
| Schisandraceae | - | - | - | - | - | - | - | - |
| Kadsura longipedunculata | Zi Ging Pi | Species | NA | NA | NA | 43.92 ± 2.59 | 1.88 ± 0.94 | 6.812 ± 1.678 |
| Selaginellaceae | - | - | - | - | - | - | - | - |
| Selaginella tamariscina | Juan Bai | Species | NA | NA | NA | 150.9 ± 13.34 | 98.83 ± 8.62 | 103.9 ± 6. |
| Valerianaceae | - | - | - | - | - | - | - | - |
| Patrinia scabiosaefolia | Bai Jiang | Genus | NA | NA | NA | 15.96 ± 2.69 | 38.77 ± 2.87 | 38.49 ± 3.73 |
| Verbenaceae | - | - | - | - | - | - | - | - |
| Verbena officinalis | Ma Bian Cao | Species | NA | NA | NA | 37.76 ± 4.42 | 145.91 ± 9.06 | 93.94 ± 5.97 |
| Violaceae | - | - | - | - | - | - | - | - |
| Viola yezoensis | Zi Hua Di Ding Cao | Genus | NA | NA | NA | 19.19 ± 1.45 | 60.87 ± 5.49 | 135.6 ± 6.4 |
| Zingiberaceae | - | - | - | - | - | - | - | - |
| Alpinia galanga | Hong Dou Kou | n/a | NA | NA | NA | 53.48 ± 4.92 | 5.76 ± 1.53 | 952.2 ± 31.2 |
| Alpinia oxaphylla | Yi Zhi Ren | Species | NA | NA | NA | 110.2 ± 9.5 | 30.40 ± 2.81 | 105.8 ± 7.5 |
| Sample | IC10 | IC20 | IC30 | IC40 | IC50 | IC60 | IC70 | IC80 | IC90 |
|---|---|---|---|---|---|---|---|---|---|
| Berberine | 0.27 | 0.51 | 0.76 | 1.08 | 1.48 | 2.02 | 2.85 | 4.32 | 8.09 |
| Berberine + coptisine IC30 | 0.051 | 0.11 | 0.18 | 0.28 | 0.4 | 0.59 | 0.9 | 1.5 | 3.22 |
| CI | 0.24 | 0.33 | 0.43 | 0.55 | 0.7 | 0.91 | 1.26 | 1.92 | 3.79 |
| Berberine + palmatine IC30 | 0.031 | 0.08 | 0.15 | 0.24 | 0.37 | 0.59 | 0.96 | 1.75 | 4.33 |
| CI | 0.12 | 0.18 | 0.24 | 0.29 | 0.36 | 0.47 | 0.62 | 0.92 | 1.81 |
| Berberine + coptisine IC30 + palmatine IC30 | 0.027 | 0.066 | 0.12 | 0.19 | 0.31 | 0.48 | 0.78 | 1.42 | 3.47 |
| CI | 0.14 | 0.22 | 0.32 | 0.43 | 0.62 | 0.88 | 1.32 | 2.24 | 5.1 |
| Coptisine | 0.6 | 0.79 | 0.95 | 1.11 | 1.27 | 1.46 | 1.69 | 2.04 | 2.68 |
| Coptisine + berberine IC30 | 0.12 | 0.26 | 0.44 | 0.67 | 1 | 1.49 | 2.3 | 3.91 | 8.67 |
| CI | 0.36 | 0.67 | 1.04 | 1.49 | 2.11 | 2.98 | 4.39 | 7.06 | 14.6 |
| Coptisine + palmatine IC30 | 0.13 | 0.3 | 0.52 | 0.82 | 1.23 | 1.86 | 2.9 | 5.01 | 11.4 |
| CI | 0.25 | 0.47 | 0.7 | 0.98 | 1.33 | 1.82 | 2.57 | 3.93 | 7.6 |
| Coptisine + berberine IC30 + palmatine IC30 | 0.13 | 0.29 | 0.47 | 0.73 | 1.08 | 1.6 | 2.44 | 4.11 | 8.97 |
| CI | 0.43 | 0.83 | 1.25 | 1.83 | 2.59 | 3.67 | 5.37 | 8.63 | 17.8 |
| Palmatine (µg/mL) | 1.73 | 2.6 | 3.4 | 4.25 | 5.21 | 6.39 | 7.97 | 10.4 | 15.7 |
| Palmatine + berberine IC30 | 0.25 | 0.67 | 1.28 | 2.18 | 3.54 | 5.77 | 9.81 | 18.7 | 49.6 |
| CI | 0.47 | 1.14 | 2.06 | 3.38 | 5.34 | 8.49 | 14.1 | 26.4 | 68.5 |
| Palmatine + coptisine IC30 | 0.37 | 0.96 | 1.82 | 3.08 | 5 | 8.11 | 13.7 | 26.1 | 68.8 |
| CI | 0.44 | 0.76 | 1.1 | 1.49 | 1.97 | 2.6 | 3.54 | 5.13 | 8.99 |
| Palmatine + berberine IC30 + coptisine IC30 | 0.43 | 1.06 | 1.95 | 3.2 | 5.05 | 7.97 | 13.1 | 24 | 59.8 |
| CI | 1.27 | 2.92 | 5.19 | 8.33 | 12.9 | 20.12 | 32.7 | 59.2 | 145 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules 2016, 21, 1161. https://doi.org/10.3390/molecules21091161
Kaufmann D, Kaur Dogra A, Tahrani A, Herrmann F, Wink M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules. 2016; 21(9):1161. https://doi.org/10.3390/molecules21091161
Chicago/Turabian StyleKaufmann, Dorothea, Anudeep Kaur Dogra, Ahmad Tahrani, Florian Herrmann, and Michael Wink. 2016. "Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target" Molecules 21, no. 9: 1161. https://doi.org/10.3390/molecules21091161







