2.1. Optimization of LC-MS/MS Conditions
To identify and quantify the Sheng-Mai-San marker ingredients, a ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method was developed. The electrospray ionization (ESI-MS) spectra of the experimental and marker compounds were acquired in both ESI(+) and ESI(−) ionization modes simultaneously. Analytes were quantified in selective reaction monitoring (SRM) mode: ginsenoside Rb
1,
m/
z 1131.78 [M + Na]
+ →
m/
z 789.41 (CE 56 eV); ginsenoside Rb
2, 1101.78 [M + Na]
+ →
m/
z 789.40 (collision energy (CE) 54 eV); ginsenoside Rc, 1101.78 [M + Na]
+ →
m/
z 789.46 (CE 58 eV); ginsenoside Rd, 969.65 [M + Na]
+ →
m/
z 789.40 (CE 54 eV); ginsenoside Re, 969.65 [M + Na]
+ →
m/
z 789.41 (CE 44 eV); ginsenoside Rg
1, 823.63 [M + Na]
+ →
m/
z 643.34 (CE 36 eV); ginsenoside Rh
1, 637.48 [M − H]
− →
m/
z 475.32 (CE 24 eV); ginsenoside compound K, 645.54 [M + Na]
+ →
m/
z 465.32 (CE 30 eV); ophiopogonin D, 877.61 [M + Na]
+ →
m/
z 447.20 (CE 50 eV); schizandrin, 433.29 [M + H]
+ →
m/
z 384.21 (CE 20 eV); 5-methoxyflavone, 253.17 [M + H]
+ →
m/
z 238.02 (CE 24 eV); digoxin 779.48 [M − H]
− →
m/
z 649.27 (CE 34 eV) (
Table 1;
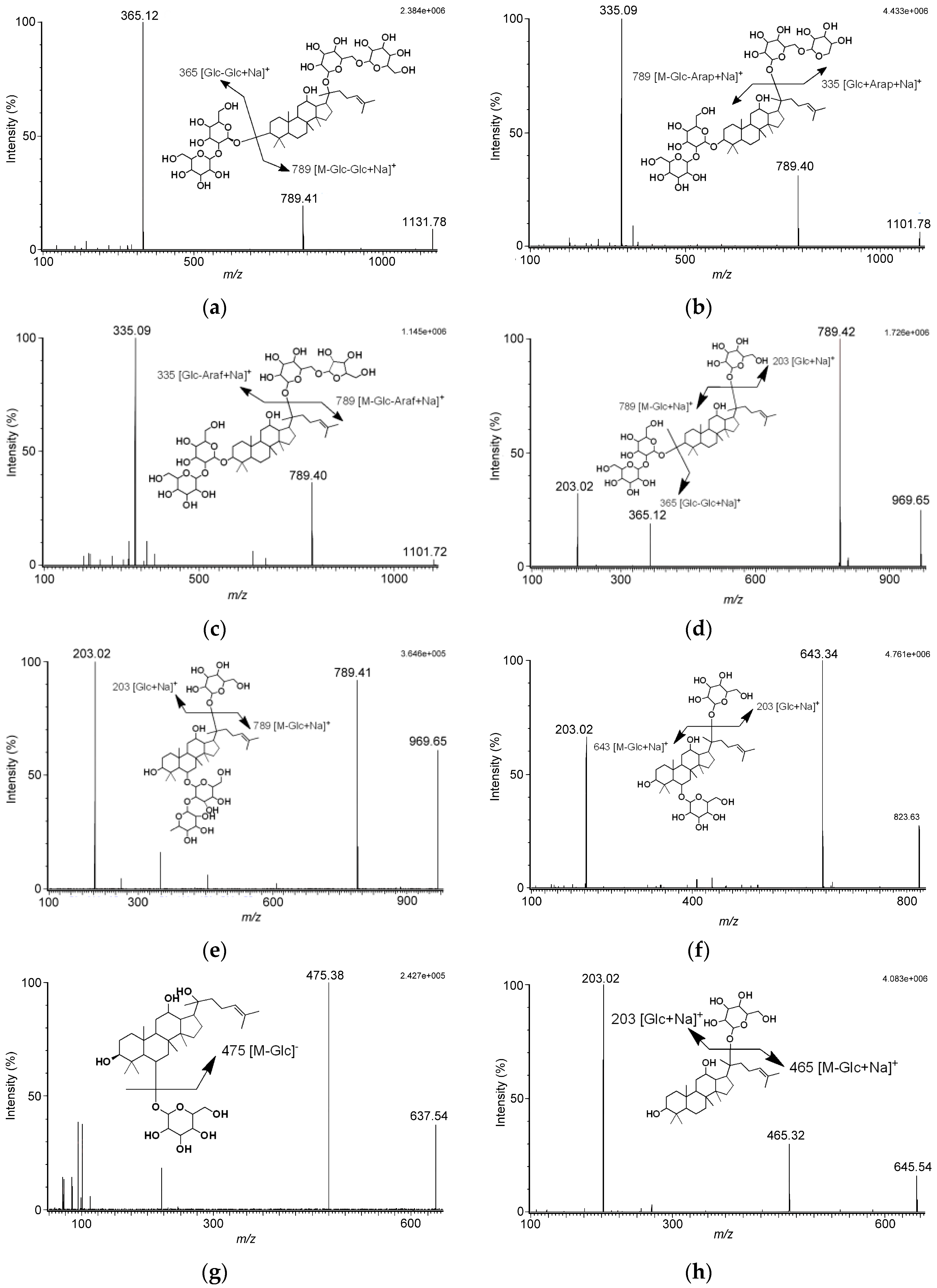
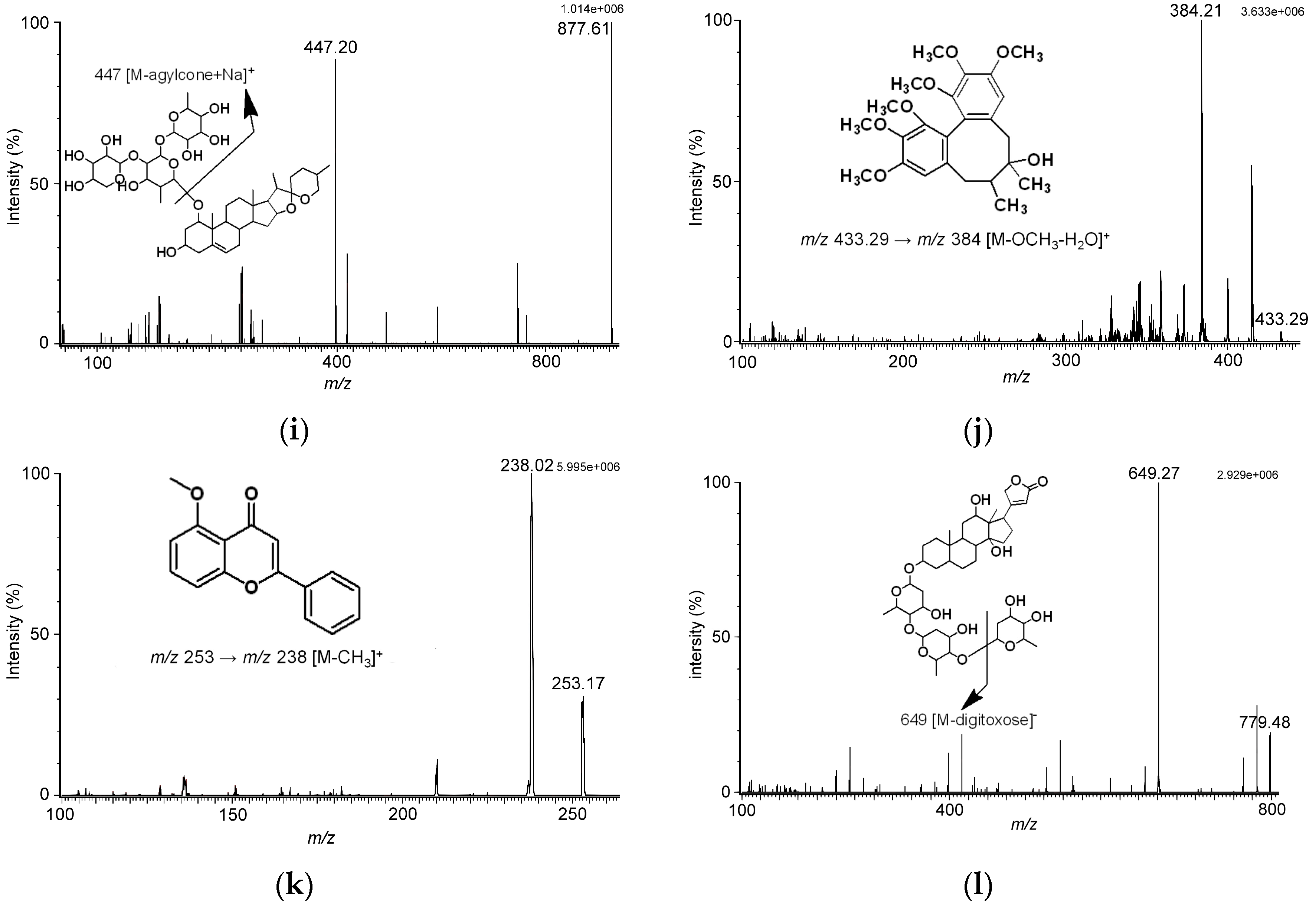
Figure 1). Analyzing the tandem mass spectrometry (MS/MS) spectra of analytes revealed fragmentation pattern of ginsenosides lost monosaccharide or disaccharide units, that were the characteristic fragments for the triterpene type of ginsenosides. As the MS/MS spectra in
Figure 1 show, we found no matter whether the MS conditions were set in positive or negative ion mode, the cleavage pathway of ginsenosides occurred at the glycosidic bond.
The fragmentation patterns of ginsenosides obtained in our study were the same as the fragmentation patterns of ginsenosides reported by Perrault and Costello [
21,
22]. The fragmentation pattern of ophiopogonin D indicated the precursor ion of
m/
z 877, [M + Na]
+, lost the agylcone to yield the product ion of
m/
z 447, [M − agylcone + Na]
+. Besides, the fragmentation pattern of schizandrin suggested the loss of a methyl group and a water molecule occurred, giving the product ion of
m/
z 384, [M − OCH
3 − H
2O]
+.
The analyte separation was optimized by varying the gradient elution, proper column, and flow rate, which were considered to be pivotal factors. The chromatographic conditions provided good resolution, appropriate ionization, and coelution. Optimization of LC-MS/MS conditions is required to provide good sensitivity, selectivity, and peak symmetry (
Figure 2).
2.2. Method Validation
The calibration curves showed good linearity in the ranges of 25–500 ng/mL for ginsenoside Rb
1, 50–500 ng/mL for ginsenoside Rb
2, 50–500 ng/mL for ginsenoside Rc, 10–500 ng/mL for ginsenoside Rd, 25–250 ng/mL for ginsenoside Re, 10–100 ng/mL for ginsenoside Rg
1, 50–500 ng/mL for ginsenoside Rh
1, 25–250 ng/mL for ginsenoside compound K, 10–100 ng/mL for ophiopogonin D, and 50–500 ng/mL for schizandrin. The calibration curves and correlation coefficients (
r2) were as follows:
y = 0.0059
x − 0.047 (
r2 = 0.999, ginsenoside Rb
1),
y = 0.0156
x + 0.6078 (
r2 = 0.998, ginsenoside Rb
2),
y = 0.0235
x − 0.9627 (
r2 = 0.998, ginsenoside Rc),
y = 0.0108
x − 0.0355 (
r2 = 0.999, ginsenoside Rd),
y = 0.011
x − 0.0143 (
r2 = 0.998, ginsenoside Re),
y = 0.0018
x − 0.0121 (
r2 = 0.999, ginsenoside Rg
1),
y = 0.002
x − 0.0305 (
r2 = 0.999, ginsenoside Rh
1),
y = 0.0285
x + 0.0203 (
r2 = 0.999, ginsenoside compound K),
y = 0.0309
x + 0.0775 (
r2 = 0.998, ophiopogonin D), and
y = 0.0126
x − 0.2304 (
r2 = 0.999, schizandrin) (
Table 2).
The limit of detection (LOD) for the herbal ingredients is shown in
Table 2. The data show that th limit of quantification (LOQ) for ginsenoside Rb
1, ginsenoside Rb
2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rg
1, ginsenoside Rh
1, ginsenoside compound K, ophiopogonin D, and schizandrin were 25, 50, 50, 10, 25, 10, 50, 25, 10 and 50 ng/mL, respectively. The precision and accuracy were evaluated by intra- and inter-day assays. Good linearity was achieved over the calibration range, with all coefficients of correlation being greater than 0.995. The relative standard deviation (RSD) values were found to be within the range of 0.45%–8.37% for intraday assays and 0.09%–12.06% for inter-day assays, with accuracy ranges of 4.44%–3.41% and 7.22%–11.01%, respectively. The results are summarized in
Table 3, which indicates that the precision and accuracy values were within the acceptable range.
2.3. Determination of the Markers for Sheng-Mai-San Preparations
To investigate the contents of the marker components in commercially available Sheng-Mai-San products from various pharmaceutical manufacturers, the most strongly detected product ion was selected for quantification analysis. The results demonstrate that, among the samples obtained from pharmaceutical manufacturers A–E and the decoction powder, the contents of ginsenoside Rb
1, ginsenoside Rb
2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rg
1, ginsenoside Rh
1, ophiopogonin D, and schizandrin were 9.44 to 346 μg/g, 6.57 to 277.6 μg/g, 6.53 to 544.8 μg/g, 19.58 to 244.8 μg/g, 26.46 to 738.7 μg/g, 4.60 to 114.6 μg/g, 33.38 to 550.6 μg/g, 2.65 to 16.36 μg/g, and 7.39 to 1532 μg/g, respectively (
Table 4). However, the ginsenoside compound K herbal ingredient was not detectable in any of the samples from the pharmaceutical manufacturers or the decoction powder. The ophiopogonin D herbal ingredient was only detectable in the samples from pharmaceutical manufacturers A, D and E. All established contents for the potential active ingredients in the samples are shown in
Table 5. LC-MS/MS with chemical profiling was conducted to rapidly evaluate the chemical consistency among Sheng-Mai-San herbal pharmaceutical products (SMS products). The composition ratios of this herbal pharmaceutical product from different manufacturers were consistently labeled, but samples A–E contained different amounts of the ten marker ingredients. The herbal origin, herbal growth environment, period of cultivation, decoction process, and even granulation process influenced the contents of the marker components. In this study, a developed and validated LC-MS/MS method was used to simultaneously determine ginsenoside Rb
1, ginsenoside Rb
2, ginsenoside Rc, ginsenoside Rd, ginsenoside Re, ginsenoside Rg
1, ginsenoside Rh
1, ginsenoside compound K, ophiopogonin D, and schizandrin in various brands of Sheng-Mai-San.
2.4. Evaluation of Additives for Raw Herbal Powder
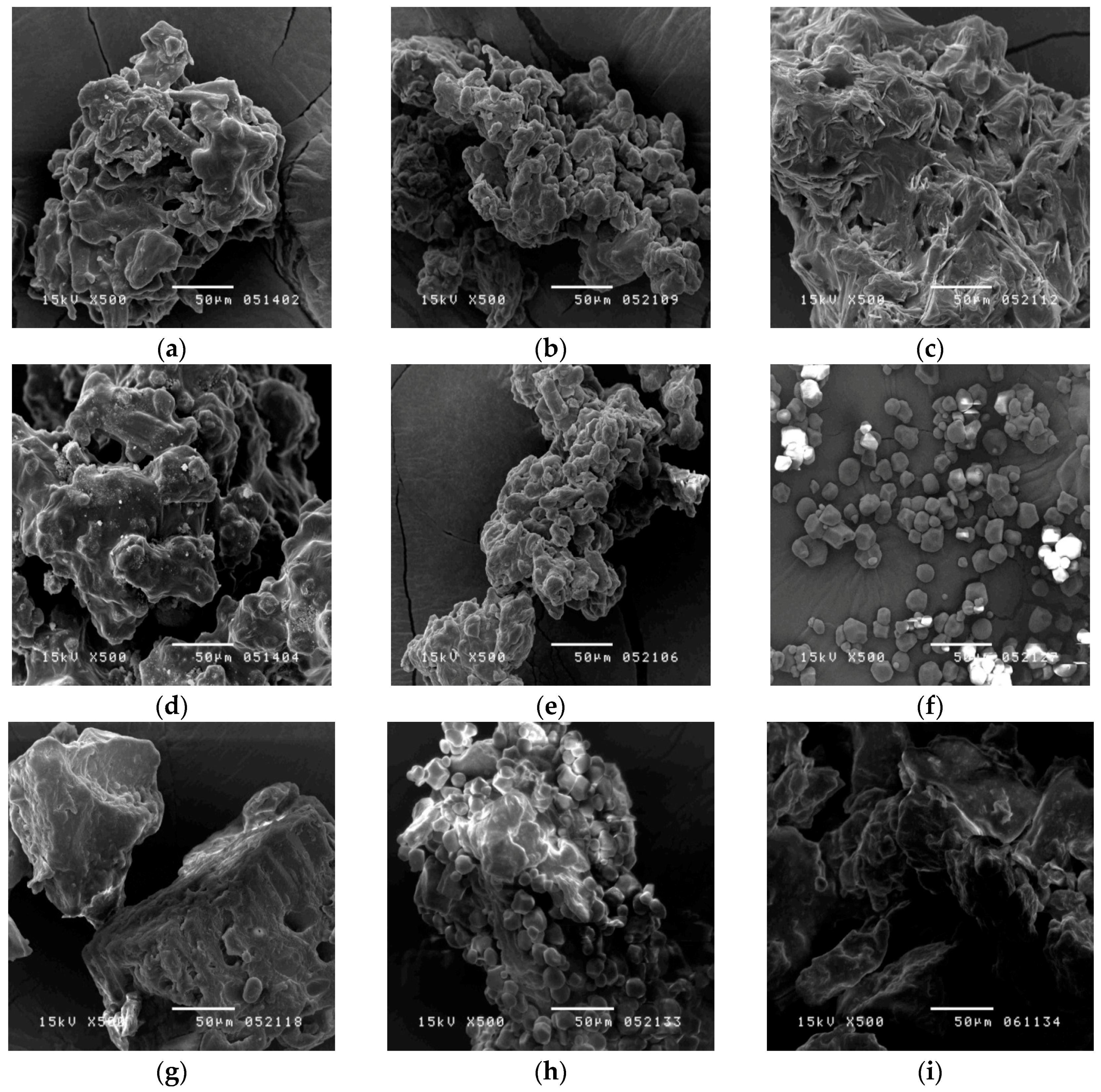
To observe the morphology of the samples, a scanning electron microscope (SEM) was used.
Figure 3a–e show the outer appearance of the herbal pharmaceutical products, which feature a rough and irregular surface with sharp layers. In comparison with the SMS products, it was found that cornstarch was granular, uniform, and polygonal in shape (
Figure 3f) [
23], whereas the raw herbal powder was rough, irregular, and lumpy (
Figure 3g). By SEM observation of the morphologies, the SMS products, starch, and raw herbal powder could be distinguished clearly. However, it is difficult to identify whether the raw herbal powder was added into the SMS products.
Congo red has a strong interaction with polysaccharides by noncovalent affinity and synthesizes a red complex [
24]. An Aperio ScanScope slide scanner was used for the observation and identification of the cellulose fibers via Congo red staining. The photographs show that Sheng-Mai-San products made from different manufacturers (
Figure 4a–e) and raw herbal powder (
Figure 4g) were red or pink but cornstarch was not (
Figure 4f). The results indicate that samples A to E contain fiber components, suggesting the possible use of raw herbal powder or cellulose fiber as additives.
Iodine-KI solution is used in a common test for starch. In the presence of iodine-KI, amylose in starch is responsible for the formation of a deep blue color. The triiodide ion complex slips inside the coil of starch, creating an intense blue-black color. The starch identification method was assessed by light microscopy photographs using iodine-KI reagent staining in this study. The photographs show that the SMS products made by different manufacturers (
Figure 5a–e) and cornstarch (
Figure 5f) were blue or violet. It was found that the starch contents of samples A–E could be clearly observed under the microscope using iodine-KI reagent staining, and Aperio ImageScope software could be used to calculate the number of particles of cornstarch (100–400 mm
2) or large starches from herbs (>400 mm
2) within a fixed area. The result indicates that brands A to E contain cornstarch in particle numbers of 115–1267 and particle sizes of 173.1–206.7 mm
2 (
Table 5). Specifically, comparing brand D and pure cornstarch, the number of cornstarch particles were similar, indicating that brand D has a high cornstarch content. The cornstarch content in the brand D powder was calculated as approximately 79%. The results also reveal that brand A contains not only cornstarch but also herbal starch. The raw herbal powder contains large starches and no cornstarch; the particles were 2535.9 ± 270.3 mm
2 in size, corresponding to herbal starch but being much larger than cornstarch.
Swelling, or an increase in volume, is observed as starch absorbs water. Determining the volume change of starch granules indicates the degree of hygroscopic swelling. Differences among starch granules may also result in different patterns of swelling power and solubility [
23]. Generally, cornstarch is added into commercial herbal powders as an excipient to increase the stability and dispersity. The results demonstrate that the solubility of herbal pharmaceutical powder produced by manufacturers A–E was 0.91%–6.66% at 55 °C, 2.67%–6.50% at 65 °C, 3.04%–6.27% at 75 °C, 3.35%–6.16% at 85 °C, and 3.06%–12.27% at 95 °C (
Table 6). Importantly, the SP of sample D was 0.91 to 12.27%, indicating that the SP increased with temperature. This finding is in agreement with the literature, which has reported that the SP of cornstarch is proportional to temperature [
25]. Solubility testing was used to evaluate the water solubility at different temperatures among the herbal samples made by different manufacturers. The results demonstrate that the solubility of herbal pharmaceutical powder produced by manufacturers A–E was 39.93%–68.02% at 55 °C, 43.07%–69.39% at 65 °C, 39.92%–65.14% at 75 °C, 45.91%–66.65% at 85 °C, and 47.23%–71.02% at 95 °C (
Table 7). Theoretically, the amylose and amylopectin molecules swell or dissolve gradually, and the water solubility of starch may increase with increasing temperature [
26,
27]. Therefore, the WSI will increase with the relative proportion of starch content. In samples A–C and E, no significant increase in the solubility was observed with increasing temperature. However, the WSI of sample D increased with temperature. These results suggest that sample D contains abundant starch.
The addition of raw herbal powder was confirmed by crude fiber analysis [
24]. The crude fiber content (%) of samples A, B, C, D, and E was 19.81% ± 0.61%, 19.60% ± 0.26%, 12.40% ± 0.18%, 0.26% ± 0.04%, and 19.23% ± 1.20%, respectively (
Table 8). Meanwhile, the crude fiber contents of the raw herbal powder and cornstarch/raw herbal powder (1:1) were 21.12% ± 0.61% and 9.52% ± 0.98%, respectively. Because raw herbal powder is mainly obtained from herbal plants, there is more cellulose fiber in the raw herbal powder than in the preparations. Excluding sample D, the crude fiber contents for the SMS products (A–C and E) were between the values for the raw herbal powder mixed with cornstarch (1:1) and the pure raw herbal powder, suggesting that these products all contain different proportions of raw herbal powder. In the literature, cornstarch was reported to contain almost no crude fiber [
23]. Therefore, sample D contained less than 50% raw herbal powder. According to the previous study, crude fiber analysis was considered as a creditable indicator for the determination of the raw herbal powder added to a compound [
24].