Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Sevast.) by UPLC-ESI-qTOF-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction of Compounds for Qualitative Analysis
3.4. Identification of Iridoids by UPLC-qTOF-MS/MS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oszmiański, J.; Kucharska, A.; Gasiewicz, E. Usefulness of honeysuckle fruits for juice production. In Fruit and Vegetable Juices and Drinks Today and in the XXI Century; Michalczuk, L., Płocharski, W., Eds.; Research Institute of Pomology and Floriculture: Rytro, Poland, 1999; pp. 251–259. [Google Scholar]
- Liu, C.; Zheng, X.; Shi, J.; Xue, J.; Lan, Y.; Jia, S. Optimising microwave vacuum puffing for blue honeysuckle snacks. Int. J. Food Sci. Technol. 2010, 45, 506–511. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of dried powder preparation process on polyphenolic content and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica). LWT Food Sci. Technol. 2016, 67, 214–222. [Google Scholar]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Tanaka, T.; Onoe, K.; Ohno, S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006, 82, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Palikova, I.; Heinrich, J.; Bednar, P.; Marhol, P.; Kren, V.; Cvak, L.; Valentová, K.; Ruzicka, F.; Holá, V.; Kolár, M.; et al. Constituents and antimicrobial properties of blue honeysuckle: A novel source for phenolic antioxidants. J. Agric. Food Chem. 2008, 56, 11883–11889. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap berries (Lonicera caerulea L.)—A critical review of antioxidant capacity and health-related studies for potential value-added products. Food Bioprocess. Technol. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, A.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L.) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Kohlmünzer, S. Pharmacognosy: Book for Students of Pharmacy; PZWL: Warszawa, Poland, 2007. [Google Scholar]
- Ren, L.; Xue, X.; Zhang, F.; Wang, Y.; Liu, Y.; Li, C.; Liang, X. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Zhang, X.L.; Xue, X.Y.; Zhang, F.F.; Xu, Q.; Liang, X.M. Structural characterization of iridoid glucosides by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.L.; Chen, L.M.; Wang, Y.M.; Liu, X.Q.; Zhang, Q.W.; Gao, H.M.; Wang, Z.M.; Xiao, W.; Wang, Z.Z. Influence of sulfur fumigation on the chemical constituents and antioxidant activity of buds of Lonicera japonica. Molecules 2014, 19, 16640–16655. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Chen, C.Y.; Li, P. Structural characterization and identification of iridoid glycosides, saponins, phenolic acids and flavonoids in Flos Lonicerae Japonicae by a fast liquid chromatography method with diode-array detection and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 3227–3242. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.; Grygorieva, O. Iridoids and anthocyanins of cornelian cherry fruits (Cornus mas L.) and blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica). In Agrobiodiversity for Improving Nutrition, Health and Life Quality Part II; Brindza, J., Klymenko, S., Eds.; Slovak University of Agriculture in Nitra: Nitra, Slovakia, 2015; pp. 395–398. [Google Scholar]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Machida, K.; Asano, J.; Kikuchi, M. An iridoid glucoside from Lonicera caeruea. Phytochemistry 1995, 40, 603–604. [Google Scholar] [CrossRef]
- Machida, K.; Asano, J.; Kikuchi, M. Caeruleoside A and B, bis-iridoid glucosides from Lonicera-caerulea. Phytochemistry 1995, 39, 111–114. [Google Scholar] [CrossRef]
- Ye, J.; Su, J.; Chen, K.; Liu, H.; Yang, X.; He, Y.; Zhang, W. Comparative investigation on chemical constituents of flower bud, stem and leaf of Lonicera japonica Thunb. by HPLC-DAD-ESI-MS/ MS and GC-MS. J. Anal. Chem. 2014, 69, 777–784. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Huang, X.; Zhao, F.L.; Tang, Y.L.; Yin, L. Study on the chemical markers of Caulis Lonicerae japonicae for quality control by HPLC-QTOF/MS/MS and chromatographic fingerprints combined with chemometrics methods. Anal. Methods 2015, 7, 2064–2076. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Bowers, M.D. Iridoid and secoiridoid glycosides in a hybrid complex of bush honeysuckles (Lonicera spp., Caprifolicaceae): Implications for evolutionary ecology and invasion biology. Phytochemistry 2013, 86, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Anikina, E.V.; Syrchina, A.I.; Vereshchagin, A.L.; Larin, M.F.; Semenov, A.A. Bitter iridoid glucoside from the fruit of Lonicera caerulea. Chem. Nat. Compd. 1989, 24, 512–513. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Szumny, A.; Misztal, K. Iridoids and phenolic compounds of honeysuckle fruits. In Plant—The Source of Research Material; Bogucka-Kocka, A., Szymczak, G., Kocki, J., Sowa, I., Eds.; Book of Abstracts: 3rd International Conference and Workshop; POLIHYMNIA: Lublin, Poland, 2013; p. 148. [Google Scholar]
- Madhusudanan, K.P.; Raj, K.; Bhaduri, A.P. Effect of metal cationization on the low energy collision-induced dissociation of loganin, epi-loganin, and ketologanin studied by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2000, 35, 901–911. [Google Scholar] [CrossRef]
- Kumar, S.; Sati, O.P.; Semwal, V.D.; Nautiyal, M.; Sati, S.; Taked, Y. Iridoid glycosides from Lonicera quinquelocularis. Phytochemistry 2000, 53, 499–501. [Google Scholar] [CrossRef]
- Kanchanapooma, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar] [CrossRef]
- Jensen, S.R.; Klaer, A.; Nielsen, B.J. Loniceroside (secologanin) in Cornus officinalis and C. mas. Phytochemistry 1973, 12, 2064–2065. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the analyzed frozen fruits are available from the authors.

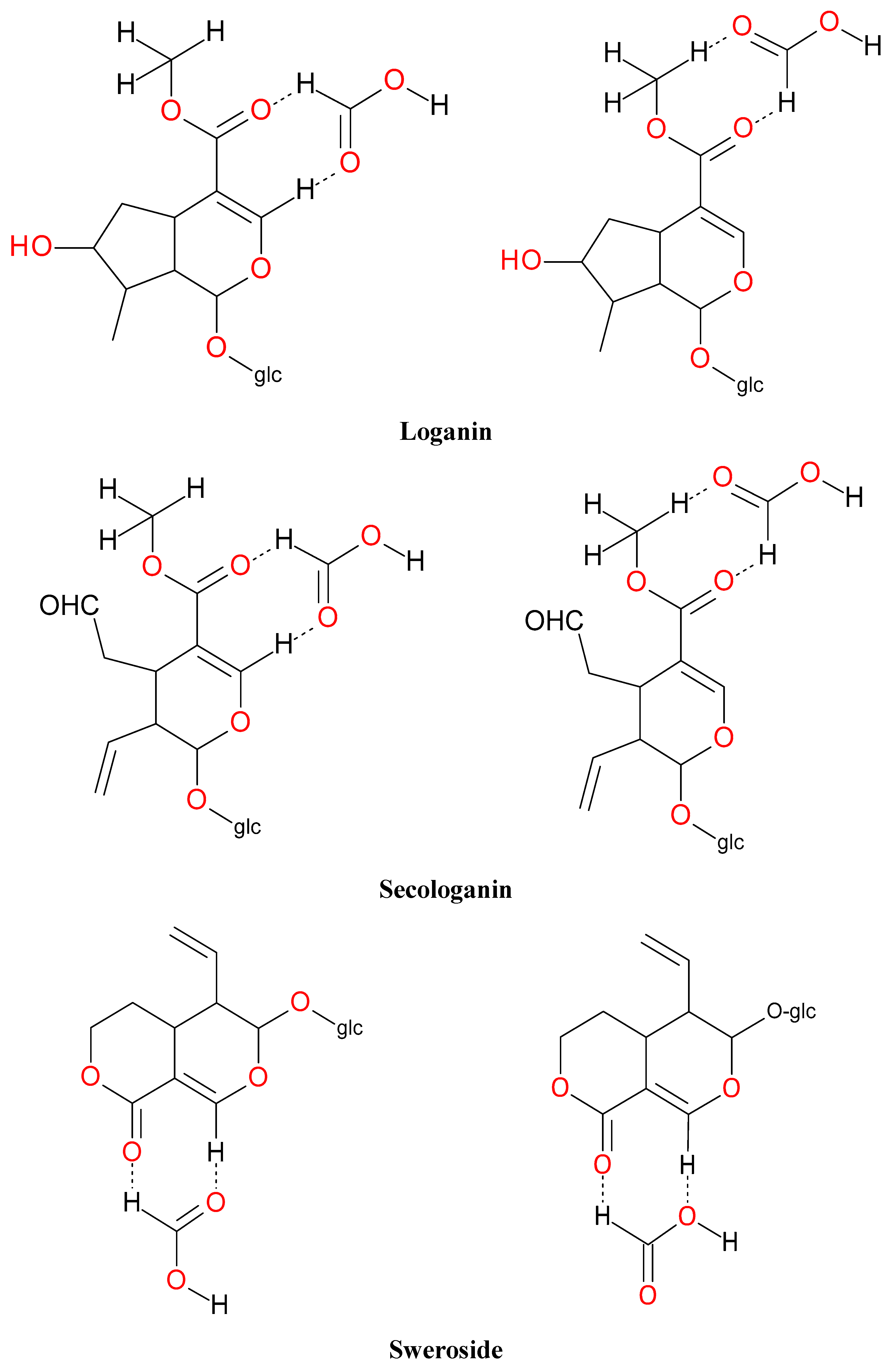

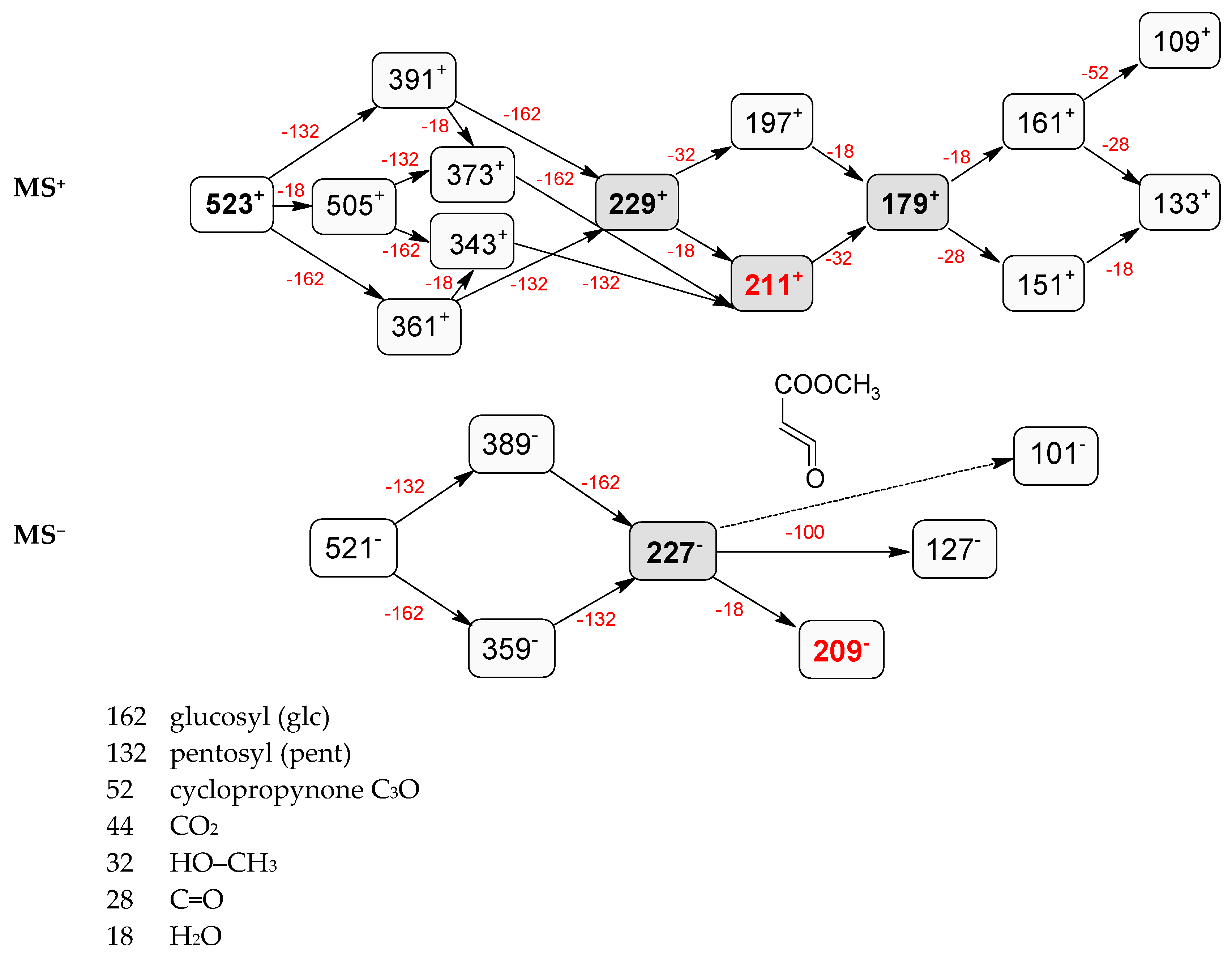
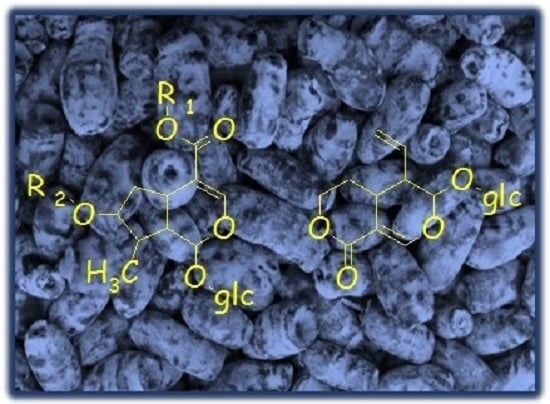
| Peak No. | tR (min) | Ion | MS1 (m/z) | MS2 (m/z) | Identification |
|---|---|---|---|---|---|
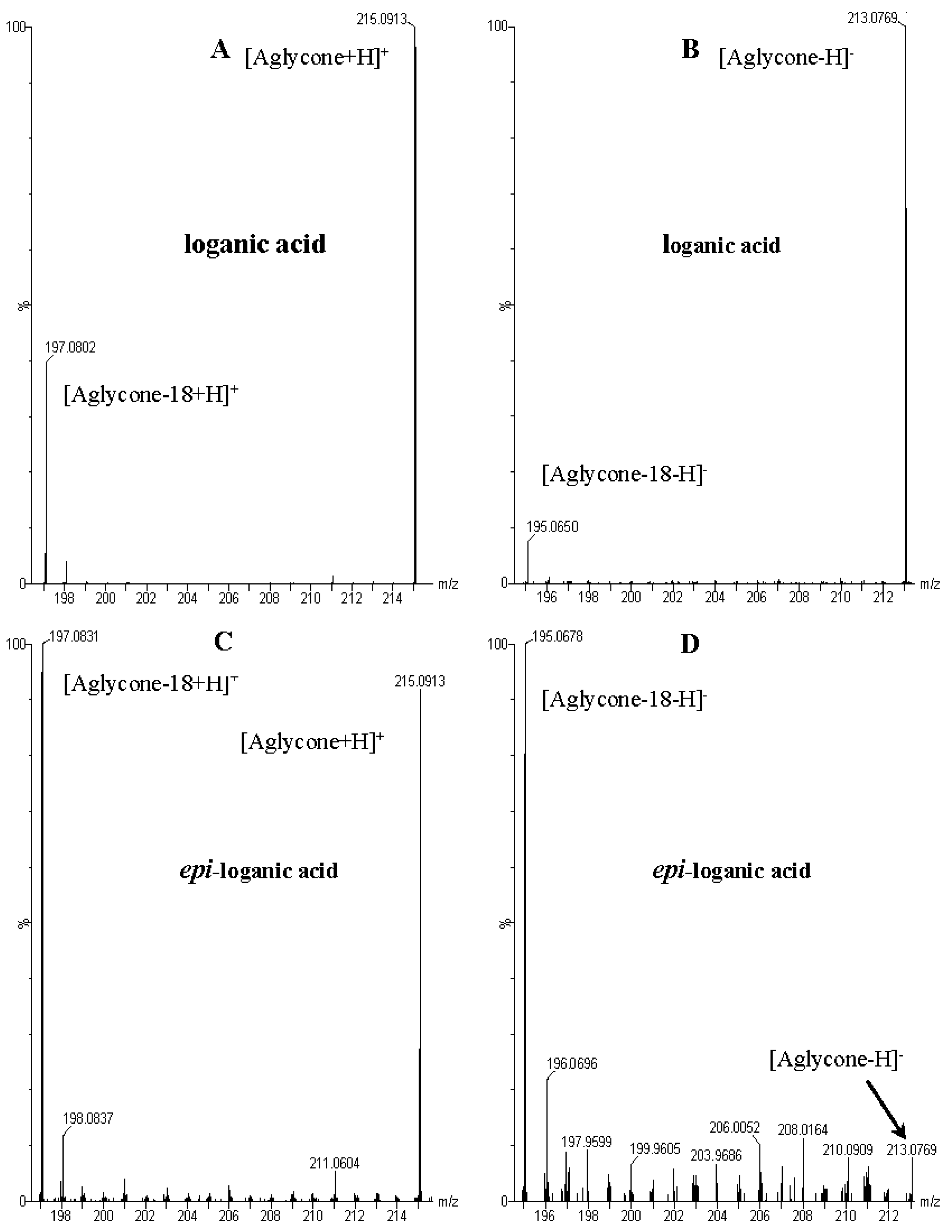
| 1 | 3.73 | − | 375.1276 [M − H]− 751.2630 [2M − H]− 1127.4083 [3M − H]− | 213.0769 [M − 162 − H]−, 195.0650 [M − 162 − 18 − H]−, 169.0855 [M − 162 − 44 − H]−, 151.0771 [M − 162 − 18 − 44 − H]−, 125.0608 [M − 162 − 18 − 44 − 26 − H]−, 101.0232 | loganic acid |
| + | 377.1440 [M + H]+ 753.2834 [2M + H]+ | 215.0913 [M − 162 + H]+, 197.0831 [M − 162 − 18 + H]+, 179.0701 [M − 162 − 18 − 18 + H]+, 151.0394 [M − 162 − 18 − 18 − 28 + H]+, 161.0598 [M − 162 − 18 − 18 − 18 + H]+, 133.0659 [M − 162 − 18 − 18 − 28 − 18 + H]+, 109.0671 [M − 162 − 18 − 18 − 18 − 52 + H]+ | |||
| 2 | 4.27 | − | 375.1276 [M − H]− 751.2630 [2M − H]− | 213.0769 [M − 162 − H]−, 195.0650 [M − 162 − 18 − H]−, 169.0855 [M − 162 − 44 − H]−, 151.0771 [M − 162 − 44 − 18 − H]−, 125.0608 [M − 162 − 18 − 44 − 26 − H]−, 01.0232 | 7-epi-loganic acid |
| + | 377.1440 [M + H]+ 753.2834 [2M + H]+ | 215.0913 [M − 162 + H]+, 197.0831 [M − 162 − 188 + H]+, 179.0701 [M − 162 − 18− 18 + H]+, 151.0394 [M − 162 − 18 − 18 − 28 + H]+, 161.0598 [M − 162 − 18 − 18 − 18 + H]+, 133.0659 [M − 162 − 18 − 18 − 28 − 18 + H]+, 109.0671 [M − 162 − 18 − 18 − 18 − 52 + H]+ | |||
| 3 | 4.53 | − | 507.1746 [M − H]− 1015.3484 [2M − H]− | 375.1356 [M − 132 − H]−, 345.0806 [M − 162 − H]−, 213.0769 [M − 132 − 162 − H]−, 195.0650 [M − 162 − 18 − H]−, 169.0855 [M − 132 − 162 − 44 − H]−, 151.0746 [M − 132 − 162 − 44 − 18 − H]−, 125.0608 [M − 132 − 162 − 18 − 44 − 26 − H]−, 101.0232 | loganic acid 7-O-pentoside |
| + | 509.1876 [M + H]+ 1017.3707 [2M + H]+ | 491.1775 [M − 18 + H]+, 377.1493 [M − 132 + H]+, 359.1348 [M − 18 − 132 + H]+, 347.1344 [M − 162 + H]+, 329.1228 [M − 18 − 162 + H]+, 215.0913 [M − 132 − 162 + H]+, 197.0802 [M − 132 − 162 − 18 + H]+, 179.0701 [M − 132 − 162 − 18 − 18 + H]+, 161.0598 [M − 132 − 162 − 18 − 18 − 18 + H]+, 151.0771 [M − 132 − 162 − 18 − 18 − 28 + H]+, 133.0659 [M − 132 − 162 − 18 − 18 − 28 − 18 + H]+, 109.0671 [M − 132 − 162 − 18 − 18 − 18 − 52 + H]+ | |||
| 4 | 5.12 | − | 507.1746 [M − H]− 1015.3484 [2M − H]− | 357.1144 [M − 132 − 18 − H]−, 327.1092 [M − 162 − 18 − H]−, 195.0650 [M − 132 − 18 − 162 − H]−, 177.0549 [M − 132 − 18 − 162 − 18 − H]−, 151.0771 [M − 132 − 18 − 162 − 18 − 44 − H]−, 133.0654 [M − 132 − 18 − 162 − 18 − 44 − H]−, 125.0608 [M − 132 − 18 − 162 − 44 − 26 − H]− 101.0232 | 7-epi-loganic acid 7-O-pentoside |
| + | 509.1876 [M + H]+ 1017.3707 [2M + H]+ | 491.1775 [M − 18 + H]+, 377.1493 [M − 132 + H]+, 359.1348 [M − 18 − 132 + H]+, 347.1344 [M − 162 + H]+, 329.1228 [M − 18 − 162 + H]+, 215.0913 [M − 132 − 162 + H]+, 197.0802 [M − 132 − 162 − 18 + H]+, 179.0701 [M − 132 − 162 − 18 − 18 + H]+, 161.0598 [M − 132 − 162 − 18 − 18 − 18 + H]+, 151.0771 [M − 132 − 162 − 18 − 18 − 28 + H]+, 133.0659 [M − 132 − 162 − 18 − 18 − 28 − 18 + H]+, 109.0671 [M − 132 − 162 − 18 − 18 − 18 − 52 + H]+ | |||
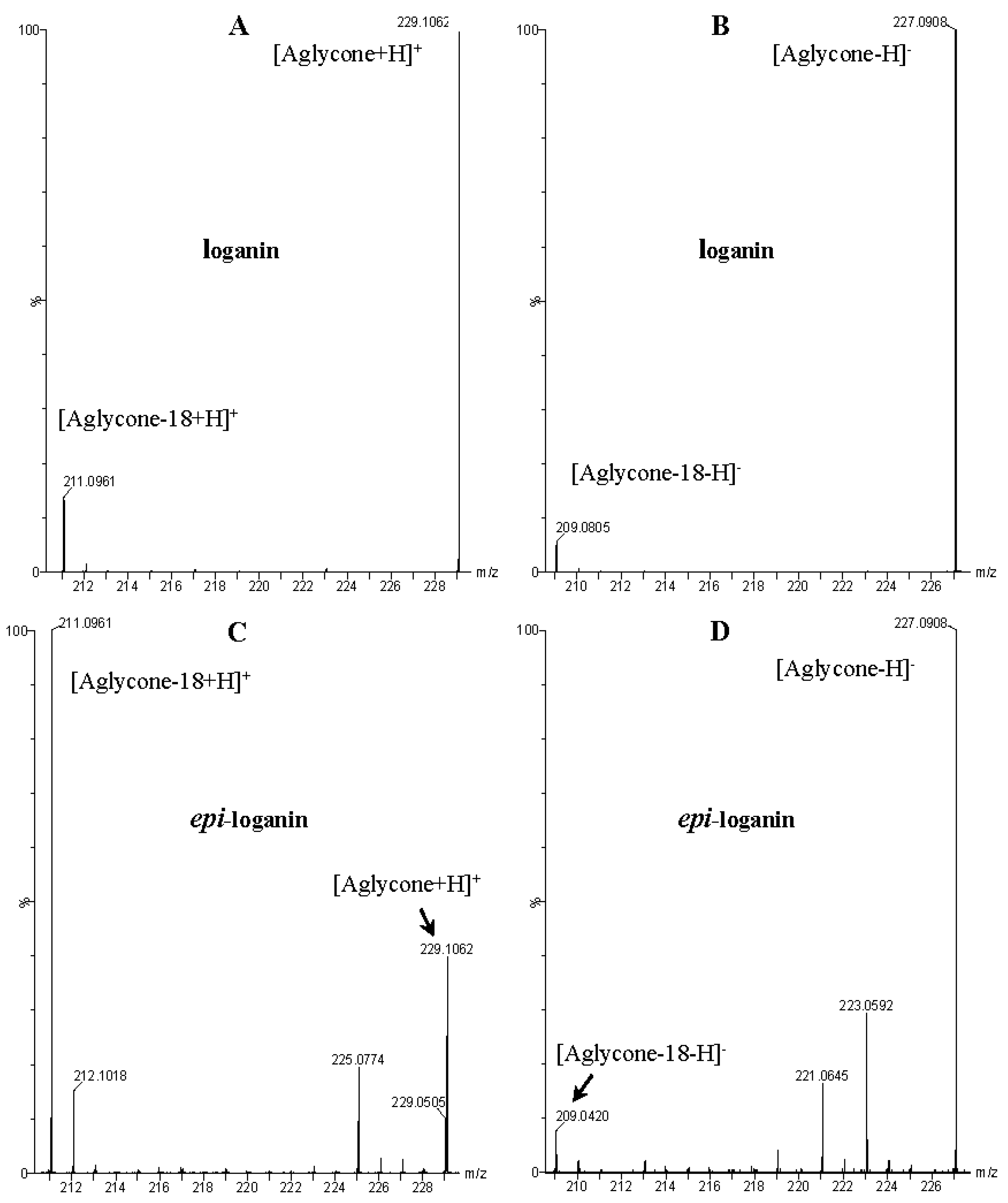
| 5 | 5.68 | − | 521.1854 [M − H]− 567.1918 [M – H + 46]− 1089.3577 [2M – H + 46]− | 389.1423 [M − 132 − H]−, 227.0908 [M − 132 − 162 − H]−, 209.0805 [M − 132 − 162 − 18 − H]−, 127.0748 [M − 132 − 162 − 100 − H]−, 101.0232 | pentosyl loganin |
| + | 523.2039 [M + H]+ | 391.1595 [M − 132 + H]+, 229.1062 [M − 132 − 162 + H]+, 211.0961 [M − 132 − 162 − 18 + H]+, 197.0802 [M − 132 − 162 − 32 + H]+, 179.0701 [M − 132 − 162 − 18 − 32 + H]+, 161.0598 [M − 132 − 162 − 18 − 32 − 18 + H]+, 151.0394 [M − 132 − 162 − 18 − 32 − 28 + H]+, 133.0659 [M − 132 − 162 − 18 − 32 − 18 − 28 + H]+, 109.0650 [M − 132 − 162 − 18 − 32 − 18 − 52 + H]+ | |||
| 6 | 5.73 | − | 357.1183 [M − H]− 403.1223 [M – H + 46]− 761.1247 [2M – H + 46]− | 195.0650 [M − 162 − H]−, 149.0438 [M − 162 − 18 − 28 − H]−, 125.0241 [M − 132 − 162 − 70 − H]−, 101.0232 | sweroside |
| + | 359.1347 [M + H]+ 717.2608 [2M + H]+ | 197.0802 [M − 162 + H]+, 179.0701 [M − 162 − 18 + H]+, 151.0771 [M − 162 − 18 − 28 + H]+, 127.0409 [M − 162 − 70+H]+, 109.0671 [M − 162 − 18 − 28 − 42 + H]+ | |||
| 7 | 5.73 | − | 389.1342 [M − H]− 435.1502 [M – H + 46]− 793.2800 [2M – H + 46]− | 227.0939 [M − 162 − H]−, 209.0805 [M − 162 − 18 − H]−, 197.0797 [M − 162 − 32 − H]−, 149 [M − 162 − 18 − 32 − 28 − H]−, 131.0356 [M − 162 − 18 − 32 − 28 − 18 − H]−, 101.0232 | loganin |
| + | 391.1554 [M + H]+ 749.2858 [2M + H]+ | 373.1504 [M − 18 + H]+, 229.1062 [M − 162 + H]+, 211.0961 [M − 162 − 18 + H]+, 179.0701 [M − 162 − 18 − 32 + H]+, 151.0771 [M − 162 − 18 − 32 − 28 + H]+, 133.0659 [M − 162 − 18 − 32 − 28 − 18 + H]+ | |||
| 8 | 5.93 | − | 489.1630 [M − H]− 535.1502 [M − H + 46]− 1025.3314 [2M – H + 46]− | 195.0650 [M − 132 − 162 − H]−, 125.0241 [M − 132 − 162 − 70 − H]−, 101.0232 | pentosyl sweroside |
| + | 491.1775 [M + H]+ | 359.1347 [M − 132 + H]+, 197.0802 [M − 132 − 162 + H]+, 179.0701 [M − 132 − 162 − 18 + H]+, 151.0746 [M − 132 − 162 − 18 − 28 + H]+, 127.0409 [M − 132 − 162 − 70+H]+, 109.0671 [M − 132 − 162 − 18 − 28 − 42 + H]+ | |||
| 9 | 6.15 | − | 521.1854 [M − H]− 567.1918 [M – H + 46]− 1043.3804 [2M − H]− 1089.3577 [2M – H + 46]− | 389.1342 [M − 132 − H]−, 371.0636 [M − 132 − 18 − H]−, 227.0939 [M − 132 − 162 − H]−, 209.0805 [M − 132 − 162 − 18 − H]−, 127.0748 [M − 132 − 162 − 100 − H]−, 101.0232 | loganin 7-O-pentoside |
| + | 523.2039 [M + H]+ 1045.4009 [2M + H]+ | 505.1936 [M − 18 + H]+, 391.1595 [M − 132 + H]+, 373.1504 [M − 18 − 132 + H]+, 361.1494 [M − 162 + H]+, 343.1367 [M − 18 − 162 + H]+, 229.1062 [M − 132 − 162 + H]+, 211.0961 [M − 132 − 162 − 18 + H]+, 197.0802 [M − 132 − 162 − 32 + H]+, 179.0701 [M − 132 − 162 − 32 − 18 + H]+, 151.0394 [M − 132 − 162 − 32 − 18 − 28 + H]+, 161.0598 [M − 132 − 162 − 32 − 18 − 18 + H]+, 133.0659 [M − 132 − 162 − 32 − 18 − 18 − 28 + H]+, 109.0650 [M − 132 − 162 − 32 − 18 − 18 − 52 + H]+ | |||
| 10 | 6.54 | − | 435.1502 [M – H + 46]− 793.2800 [2M – H + 46]− | 227.0939 [M − 162 − H]−, 209.0805 [M − 162 − 18 − H]−, 197.0797 [M − 162 − 32 − H]−, 149 [M − 162 − 18 − 32 − 28 − H]−, 131.0356 [M − 162 − 18 − 32 − 28 − 18 − H]−, 101.0232 | 7-epi-loganin |
| + | 391.1554 [M + H]+ 749.2858 [2M + H]+ | 373.1504 [M − 18 + H]+, 229.1062 [M − 162 + H]+, 211.0961 [M − 162 − 18 + H]+, 179.0701 [M − 162 − 18 − 32 + H]+, 151.0771 [M − 162 − 18 − 32 − 28 + H]+, 133.0659 [M − 162 − 18 − 32 − 28 − 18 + H]+ | |||
| 11 | 6.58 | − | 403.1263 [M − H]− 807.2567 [2M − H]− | 371.0952[M − 32 − H]−, 333.0848 [M − 42 − 28 − H]−, 223.0622 [M − 162 − 18 − H]−, 191.0354 [M − 162 − 18 − 32 − H]−, 165.0565 [M − 162 − 18 − 32 − 28 − H]−, 121.0288 [M − 162 − 18 − 32 − 28 − H]−, 101.0232 | secoxyloganin |
| + | 405.1383 [M + H]+ | 373.1504 [M − 32 + H]+, 345.0262 [M − 32 − 28 + H]+, 243.0849 [M − 162 + H]+, 225.0774 [M − 162 − 18 + H]+, 211.0961 [M − 162 − 32 + H]+, 193.0862 [M − 162 − 18 − 32 + H]+, 167.0700 [M − 162 − 18 − 32 − 26 + H]+, 165.0562 [M − 162 − 18 − 32 − 28 + H]+, 127.0380 [M − 32 − 28 − 28 − 28 + H]+, 123.0450 [M − 162 − 18 − 32 − 26 − 44 + H]+ | |||
| 12 | 6.78 | − | 521.1854 [M − H]− 567.1918 [M – H + 46]− 1043.3804 [2M − H]− 1089.3577 [2M – H + 46]− | 389.1342 [M − 132 − H]−, 371.0636 [M − 132 − 18 − H]−, 227.0939 [M − 132 − 162 − H]−, 209.0805 [M − 132 − 162 − 18 − H]−, 101.0232 | 7-epi-loganin 7-O-pentoside |
| + | 523.2039 [M + H]+ 1045.4009 [2M + H]+ | 505.1936 [M − 18 + H]+, 391.1595 [M − 132 + H]+, 373.1504 [M − 18 − 132 + H]+, 361.1494 [M − 162 + H]+, 343.1367 [M − 18 − 162 + H]+, 229.1062 [M − 132 − 162 + H]+, 211.0961 [M − 132 − 162 − 18 + H]+, 197.0802 [M − 132 − 162 − 32 + H]+, 179.0701 [M − 132 − 162 − 18 − 32 + H]+, 151.0394 [M − 132 − 162 − 18 − 32 − 28 + H]+, 161.0598 [M − 132 − 162 − 18 − 32 − 18 + H]+, 133.0659 [M − 132 − 162 − 18 − 32 − 18 − 28 + H]+, 109.0650 [M − 132 − 162 − 18 − 32 − 18 − 52 + H]+ | |||
| 13 | 6.89 | − | 387.1302 [M − H]− 433.1331 [M – H + 46]− | 225.0781 [M − 162 − H]−, 179.0540 [M − 162 − 18 − 28 − H]−, 155.0347 [M − 162 − 18 − 28 − 24 − H]−, 123.0456 [M − 162 − 18 − 28 − 24 − 32 − H]−, 101.0232 | secologanin |
| + | 389.1433 [M + H]+ | 227.0920 [M − 162 + H]+, 209.0799 [M − 162 − 18 + H]+, 195.0666 [M − 162 − 32 + H]+, 177.0557 [M − 162 − 18 − 32 + H]+, 165.0562 [M − 162 − 18 − 44 + H]+, 151.0394 [M − 162 − 18 − 32 − 26 + H]+, 149.0599 [M − 162 − 18 − 32 − 28 + H]+, 139.0399 [M − 162 − 32 − 28 − 28 + H]+, 109.0308 [M − 162 − 18 − 32 − 26 − 42 + H]+, 107.0513 [M − 162 − 18 − 32 − 28 − 42 + H]+ |
| Monoisotopic Ion [M − H]− m/z | Plausible Structure | |||
|---|---|---|---|---|
| Found Calculated Error | Formula | |||
| 507.1746 507.17137 −0.00323 | C21H31O14 |  | ||
| 375.1276 375.12912 0.00152 | C16H23O10 |  | ||
| 357.1144 357.11855 0.00415 | C16H21O9 |  | ||
| 345.1122 345.11855 0.00635 | C15H21O9 |  | ||
| 327.1092 327.10799 −0.00121 | C15H19O8 |  | ||
| 213.0769 213.07629 −0.00069 | Aglycone C10H13O5 |  |  |  |
| 195.0650 195.06573 0.00073 | C10H11O4 |  |  | |
| 177.0549 177.05517 0.00027 | C10H9O3 |  |  | |
| 169.0855 169.08646 0.00096 | C9H13O3 |  |  | |
| 151.0771 151.0759 −0.0012 | C9H11O2 |  |  |  |
| 133.0654 133.06534 −0.00006 | C9H9O |  |  | |
| 125.0608 125.06025 −0.00055 | C7H9O2 |  |  |  |
 |  |  | ||
| 107.0494 107.04969 0.00029 | C7H7O |  | ||
| 179.0522 161.0444 143.0344 149.0438 131.0356 119.0341 113.0238 101.0232 | Glucose/pentose fragments: 179 (−18) → 161 (−18) → 143 (−18) → 125 149 (−18) → 131 (−18) → 113 119 (−18) → 101 | |||
| Monoisotopic Ion [M + H]+ m/z | Plausible Structure | |||
|---|---|---|---|---|
| Found Calculated Error | Formula | |||
| 509.1876 509.18702 −0.00058 | C21H33O14 |  |  |  |
| 491.1775 491.17646 −0.00104 | C21H31O13 |  |  |  |
| 377.1493 377.14477 −0.00453 | C16H25O10 |  |  |  |
| 359.1348 359.1342 −0.0006 | C16H23O9 |  |  |  |
 |  |  | ||
| 347.1344 347.1342 −0.0002 | C15H23O9 |  |  |  |
| 329.1228 329.12364 0.00084 | C15H21O8 |  |  |  |
 |  |  | ||
| 215.0919 215.09194 0.00004 | Aglycone C10H15O5 |  |  |  |
| 197.0816 197.08138 −0.00022 | C10H13O4 |  |  |  |
 |  |  | ||
 |  |  | ||
 |  | |||
| 179.0721 179.07082 −0.00128 | C10H11O3 |  |  |  |
 |  |  | ||
 |  |  | ||
 |  | |||
| 161.0598 161.06025 0.00045 | C10H9O2 |  |  |  |
 | ||||
| 151.0771 151.0759 −0.0012 | C9H11O2 |  |  | |
 |  | |||
| 133.0659 133.06534 −0.00056 | C9H9O |  |  |  |
 | ||||
| 109.0671 109.06534 −0.00176 | C7H9O |  | ||
| Monoisotopic Ion [M − H]− m/z | Plausible Structure | |||
|---|---|---|---|---|
| Found Calculated Error | Formula | |||
| 567.1918 [M + FA − H]− 567.1925 0.0007 521.1854 521.18702 0.00162 | C22H33O14 CH2O2 |  |  | |
| 435.1502 [M + FA − H]− 435.15024 0.00004 389.1423 389.14477 0.00247 | C17H25O10 CH2O2 |  | ||
| 359.1379 359.1342 −0.0037 | C16H23O9 |  | ||
| 227.0908 227.09194 0.00114 | Aglycone C11H15O5 |  |  |  |
| C10H13O3 CH2O2 |  |  |  | |
| 209.0805 209.08138 0.00088 | C11H13O4 |  |  |  |
| 127.0748 127.0759 0.0011 | C7H11O2 |  |  |  |
| 101.0252 101.02387 −0.00133 | C4H5O3 |  | ||
| 179.0540 161.0449 143.0368 149.0448 131.0333 119.0354 113.0238 101.0268 | Glucose/pentose fragments: 179 (−18) → 161 (−18) → 143 (−18) → 125 149 (−18) → 131 (−18) → 113 119 (−18) → 101 | |||
| Monoisotopic Ion [M + H]+ m/z | Plausible Structure | |||
|---|---|---|---|---|
| Found Calculated Error | Formula | |||
| 523.2039 523.20267 −0.00123 | C22H35O14 |  |  |  |
 |  |  | ||
| 505.1936 505.19211 −0.00149 | C22H33O13 |  |  |  |
| 391.1595 391.16041 0.00091 | C17H27O10 |  |  |  |
| 373.1504 373.14985 −0.00055 | C17H25O9 |  |  |  |
| 361.1494 361.14985 −0.00045 | C16H25O9 |  |  |  |
| 343.1367 343.13929 0.00299 | C16H23O8 |  |  |  |
| 229.1062 229.10759 0.00259 | Aglycone C11H17O5 |  |  |  |
| 211.0961 197.09703 −0.00093 | C11H15O4 |  |  |  |
 |  |  | ||
| 197.0802 197.08138 0.000118 | C10H13O4 |  |  |  |
 |  | |||
| 179.0701 179.07082 0.00072 | C10H11O3 | See Table 3 | ||
| 161.0598 161.06025 0.00045 | C10H9O2 | |||
| 151.0771 151.0759 −0.0012 | C9H11O2 | |||
| 133.0659 133.06534 −0.00056 | C9H9O | |||
| 109.0650 109.06534 0.00034 | C7H9O | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, A.Z.; Fecka, I. Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Sevast.) by UPLC-ESI-qTOF-MS/MS. Molecules 2016, 21, 1157. https://doi.org/10.3390/molecules21091157
Kucharska AZ, Fecka I. Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Sevast.) by UPLC-ESI-qTOF-MS/MS. Molecules. 2016; 21(9):1157. https://doi.org/10.3390/molecules21091157
Chicago/Turabian StyleKucharska, Alicja Z., and Izabela Fecka. 2016. "Identification of Iridoids in Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Sevast.) by UPLC-ESI-qTOF-MS/MS" Molecules 21, no. 9: 1157. https://doi.org/10.3390/molecules21091157