Cardamonin, a Novel Antagonist of hTRPA1 Cation Channel, Reveals Therapeutic Mechanism of Pathological Pain
Abstract
:1. Introduction
2. Results
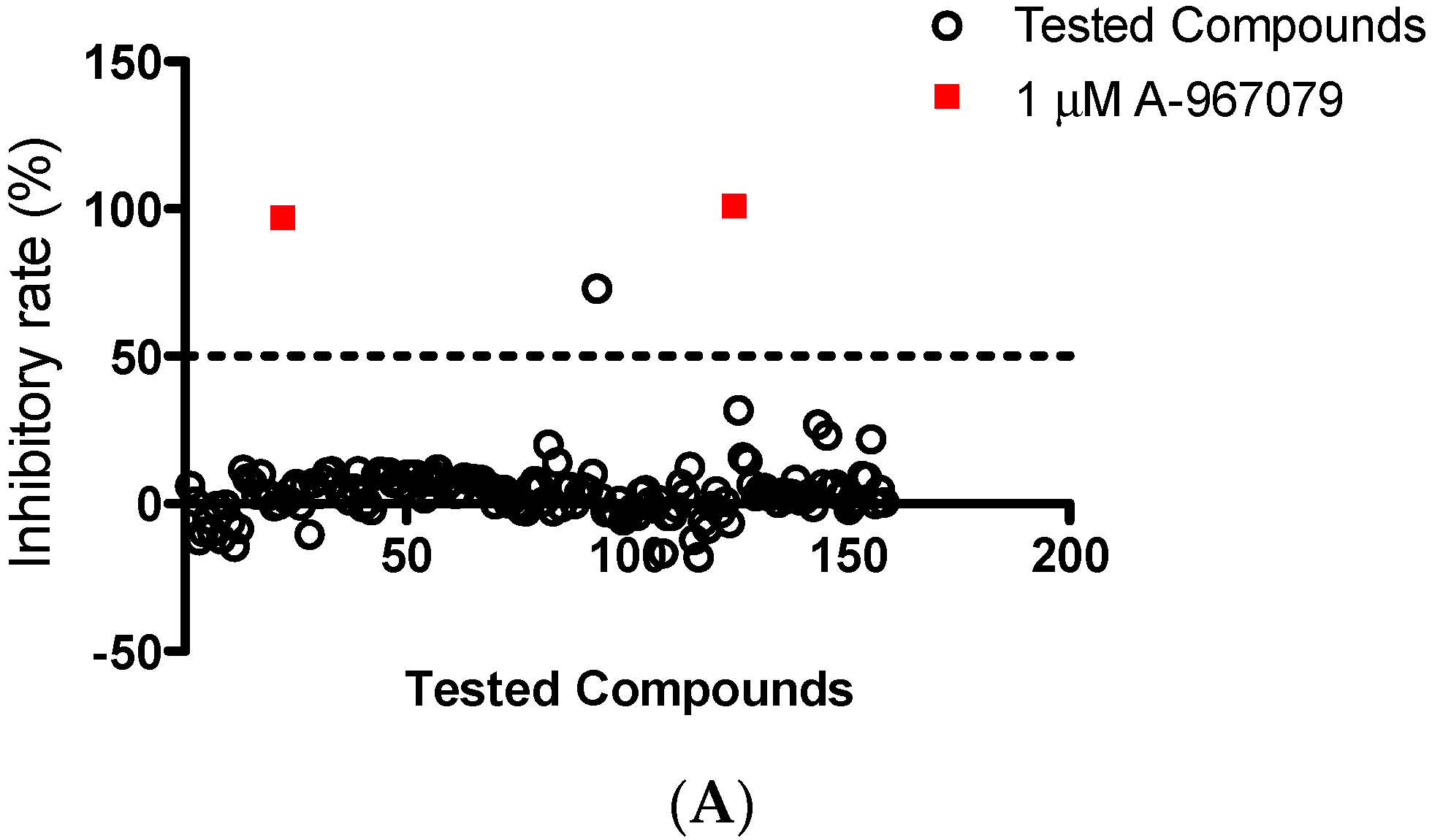
2.1. Identification of Cardamonin as a Novel TRPA1 Antagonist
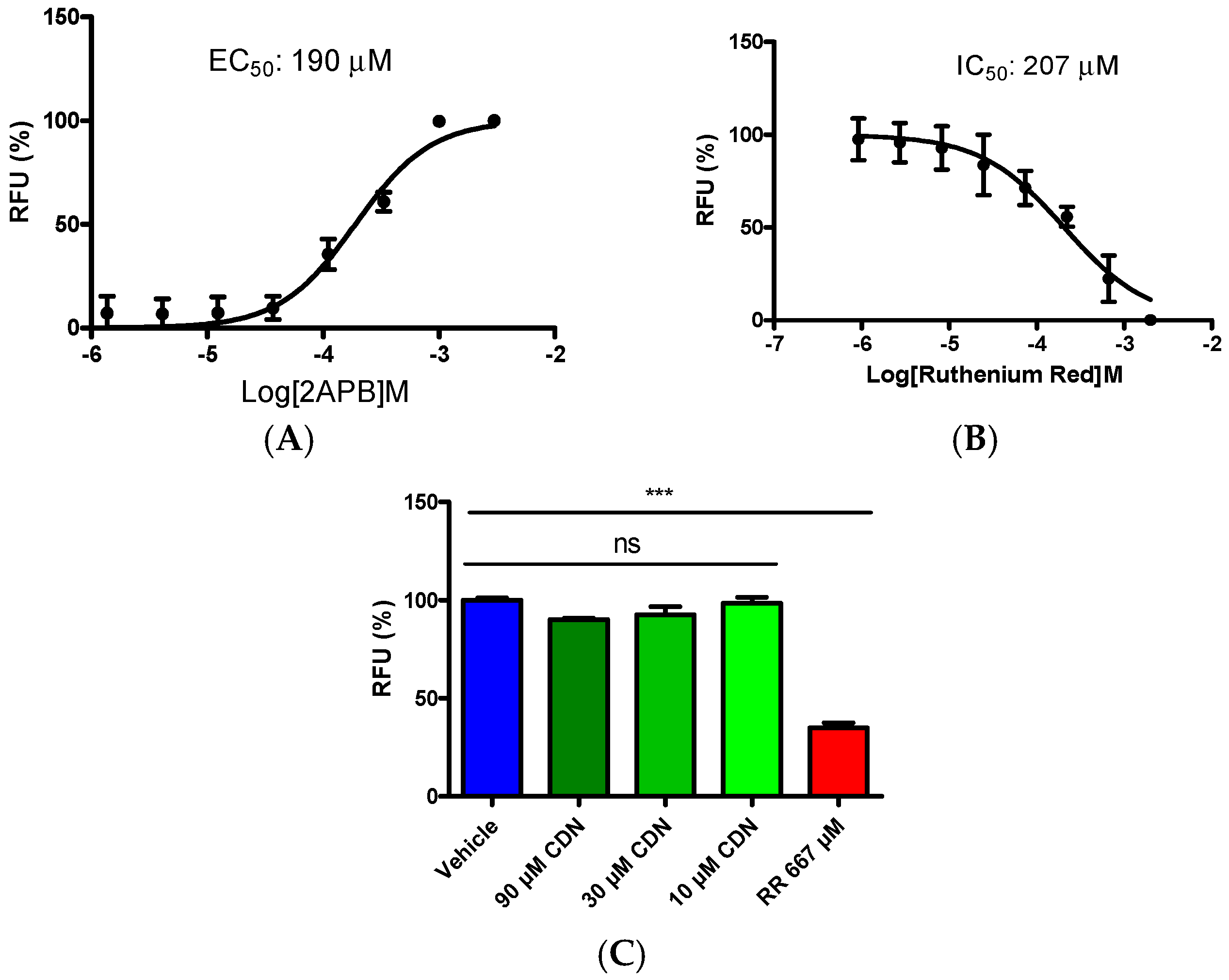
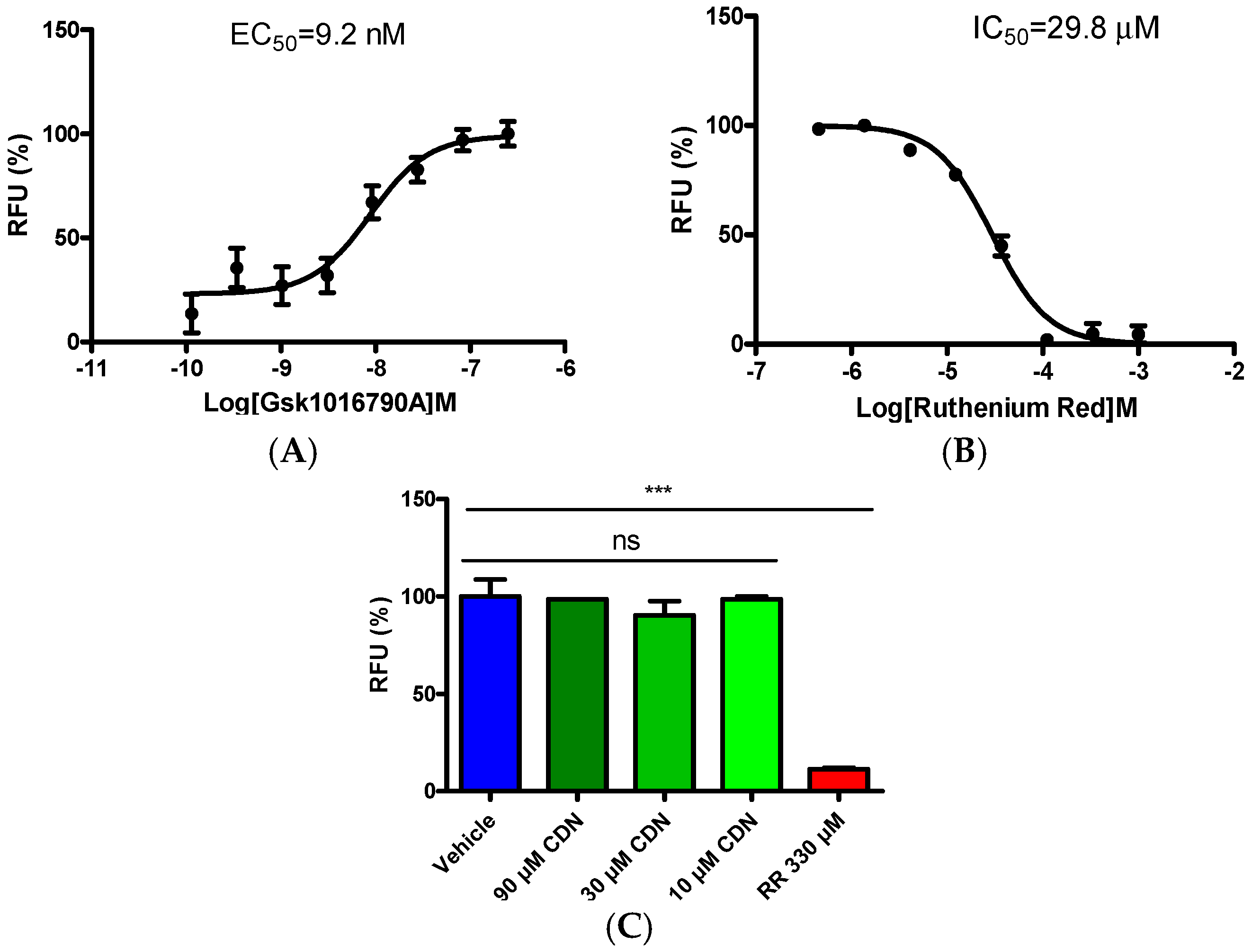
2.2. Selectivity Evaluation of Cardamonin on TRP Ion Channels
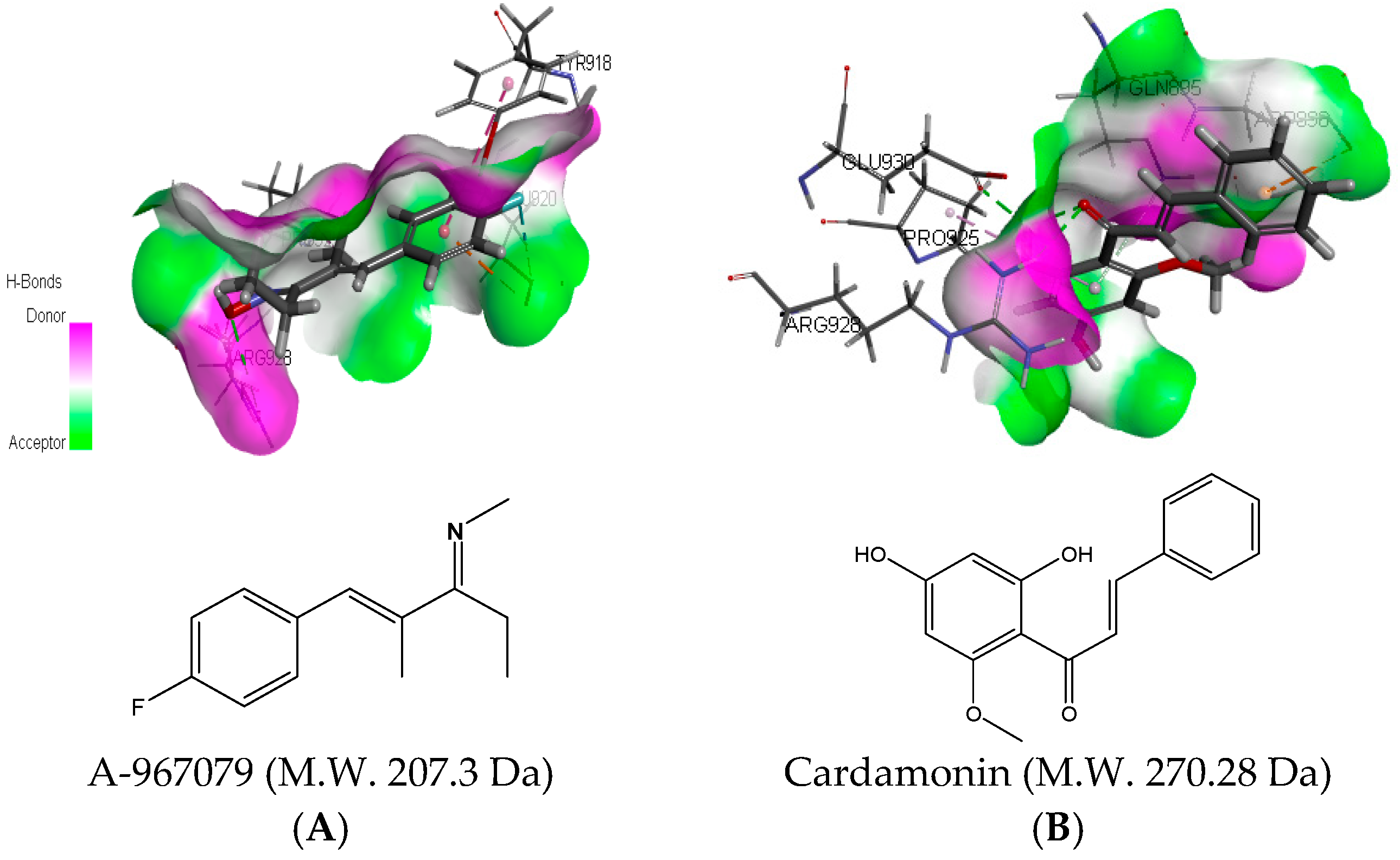
2.3. Molecular Docking Analysis of Cardamonin with TRPA1 Protein
2.4. Cardamonins Scarcely Influenced HEK293 Cell Viability
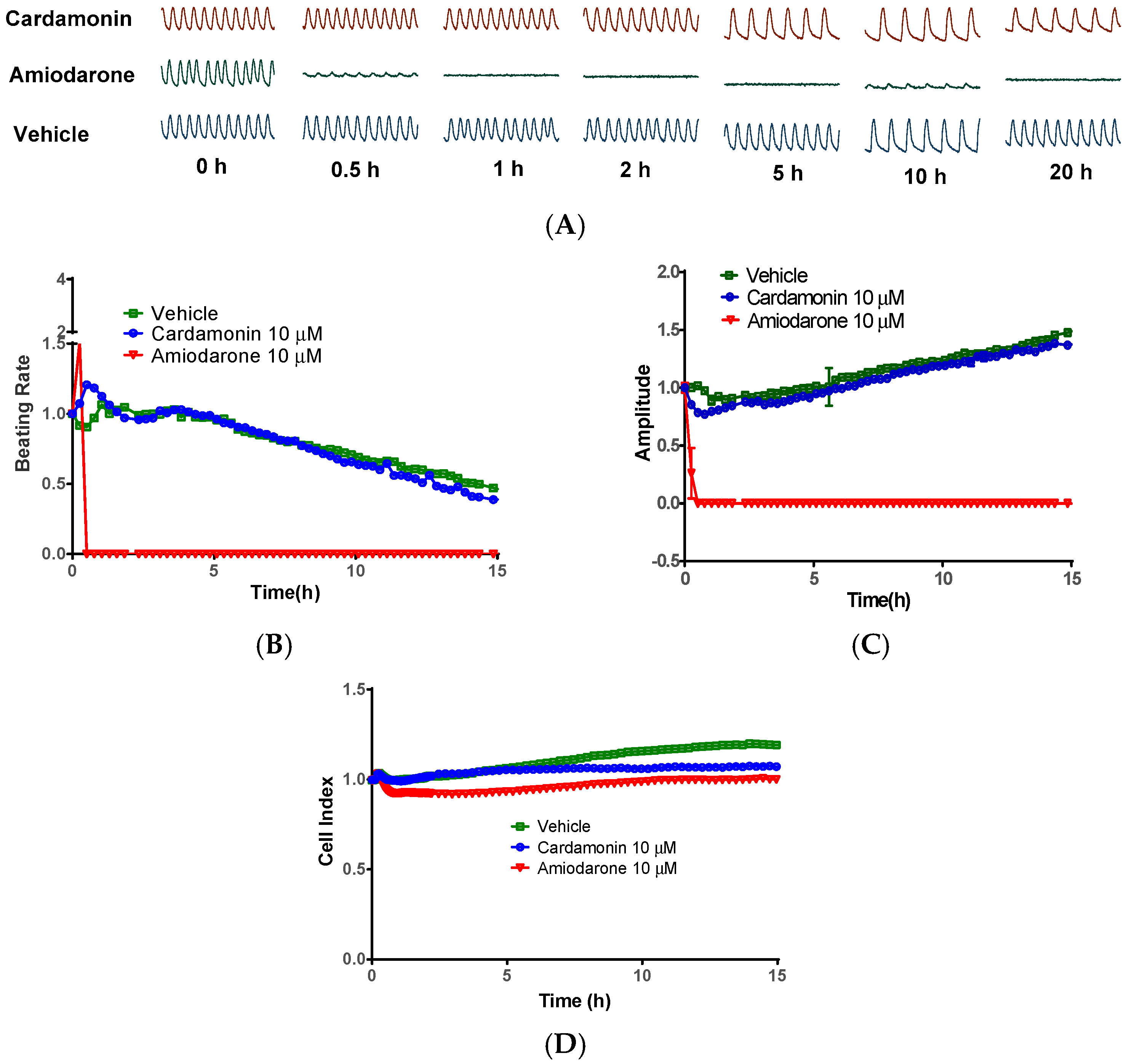
2.5. Cardamonin did not Inhibit Cardiomyocytes Contraction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Calcium Mobilization Assay
4.3. Molecular Docking Calculations
4.4. Cell Viability Analysis
4.5. Cardiotoxicity Evaluation
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, H.; Park, J.H.; Lee, J.C.; Yoo, K.Y.; Hwang, I.K.; Lee, C.H.; Choi, J.H.; Kim, J.D.; Kang, I.J.; Won, M.H. Neuroprotective effects of Alpinia katsumadai against experimental ischemic damage via control of oxidative stress. Pharm. Biol. 2013, 51, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yoo, K.Y.; Lee, C.H.; Choi, J.H.; Hwang, I.K.; Kim, J.D.; Kim, Y.M.; Kang, I.J.; Won, M.H. Neuroprotective effects of Alpinia katsumadai against neuronal damage in the gerbil hippocampus induced by transient cerebral ischemia. Int. J. Neurosci. 2011, 121, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, K.M.; Yeom, M.H.; Cho, H.Y.; Lee, H.J.; Park, M.K.; Jeong, K.C.; Lee, B.I.; Noh, M.; Lee, C.H. Antinociceptive Effects of Alpinia katsumadai via Cyclooxygenase-2 Inhibition. Biomol. Ther. 2010, 18, 159–165. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Hu, Z. Separation and determination of alpinetin and cardamonin in Alpinia katsumadai Hayata by flow injection–micellar electrokinetic chromatography. Talanta 2007, 71, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Lee, H.J.; Choi, J.K.; Kim, H.J.; Kang, J.H.; Lee, E.J.; Kim, Y.R.; Kang, J.H.; Yoo, J.K.; Cho, H.Y.; et al. Novel anti-nociceptive effects of cardamonin via blocking expression of cyclooxygenase-2 and transglutaminase-2. Pharmacol. Biochem. Behav. 2014, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Salat, K.; Filipek, B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B 2015, 16, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The trp ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Survery, S.; Kreir, M.; Simonsen, C.; Kjellbom, P.; Hogestatt, E.D.; Johanson, U.; Zygmunt, P.M. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc. Natl. Acad. Sci. USA 2014, 111, 16901–16906. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Akopian, A.N.; Ruparel, N.B.; Jeske, N.A.; Hargreaves, K.M. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J. Physiol. 2007, 583 Pt 1, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Nassini, R.; Andre, E.; Campi, B.; Amadesi, S.; Trevisani, M.; Bunnett, N.W.; Patacchini, R.; Geppetti, P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2008, 105, 12045–12050. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Liu, Q.; Zhang, Y.; Wang, S.; Zhang, Y.; Li, S.; Qiao, Y. Identification of natural compound carnosol as a novel TRPA1 receptor agonist. Molecules 2014, 19, 18733–18746. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Chung, G.; Im, S.T.; Kim, Y.H.; Jung, S.J.; Rhyu, M.R.; Oh, S.B. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience 2014, 261, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Michael Edwardson, J. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflug. Arch. Eur. J. Physiol. 2014, 466, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef] [PubMed]
- Todaka, H.; Taniguchi, J.; Satoh, J.; Mizuno, A.; Suzuki, M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J. Biol. Chem. 2004, 279, 35133–35138. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004, 24, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Tribbensee, S.M.; Karna, M.; Khalil, M.; Neurath, M.F.; Reeh, P.W.; Engel, M.A. Differential Contribution of TRPA1, TRPV4 and TRPM8 to Colonic Nociception in Mice. PLoS ONE 2015, 10, e0128242. [Google Scholar] [CrossRef] [PubMed]
- Kanju, P.; Chen, Y.; Lee, W.; Yeo, M.; Lee, S.H.; Romac, J.; Shahid, R.; Fan, P.; Gooden, D.M.; Simon, S.A.; et al. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci. Rep. 2016, 6, 26894. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, Z.; Chen, L.; Jaimes, J.; Collins, D.; Walters, E.T.; O’Neil, R.G. Determinants of TRPV4 Activity following Selective Activation by Small Molecule Agonist GSK1016790A. PLoS ONE 2011, 6, e16713. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, O.; Crittenden, C.; Callamaras, N.; Hesley, J.; Chen, Y.W.; Funes, C.; Rusyn, I.; Anson, B.; Cromwell, E.F. Multiparameter in vitro assessment of compound effects on cardiomyocyte physiology using iPSC cells. J. Biomol. Screen. 2013, 18, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, V.; Simmons, C.; Gong, T.; Sharma, A.; Burridge, P.W.; Patlolla, B.; Lee, A.S.; et al. Screening Adverse Drug-Induced Arrhythmia Events Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Low-Impedance Microelectrode Arrays. Circulation 2013, 128, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Pazienza, V.; Pomara, C.; Cappello, F.; Calogero, R.; Carrara, M.; Mazzoccoli, G.; Vinciguerra, M. The TRPA1 channel is a cardiac target of mIGF-1/SIRT1 signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Colenso, C.K.; El Harchi, A.; Cheng, H.; Witchel, H.J.; Dempsey, C.E.; Hancox, J.C. Interactions between amiodarone and the hERG potassium channel pore determined with mutagenesis and in silico docking. Biochem. Pharmacol. 2016, 113, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.D.; Wolf, A.; Schramm, U. Effect of PSC 833, verapamil and amiodarone on adriamycin toxicity in cultured rat cardiomyocytes. Toxicol. In Vitro 2000, 14, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Koronowski, K.B.; Li, L.; Freeman, B.A.; Woodcock, S.; de Groat, W.C. Nitro-oleic acid desensitizes TRPA1 and TRPV1 agonist responses in adult rat DRG neurons. Exp. Neurol. 2014, 251, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, L.J.; Hämäläinen, M.; Nummenmaa, E.; Ilmarinen, P.; Vuolteenaho, K.; Nieminen, R.M.; Lehtimäki, L.; Moilanen, E. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice—potential role of TRPA1 in osteoarthritis. Osteoarthr. Cartilage. 2015, 23, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Beeg, M.; Toropova, M.A.; Toropov, A.A.; Salmona, M. Monte Carlo method for predicting of cardiac toxicity: hERG blocker compounds. Toxicol. Lett. 2016, 250–251, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Gualdani, R.; Dragoni, E.; di Cesare Mannelli, L.; Sostegni, S.; Norcini, M.; Gabrielli, G.; la Marca, G.; Richichi, B.; Francesconi, O.; et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci. Rep. 2013, 3, 2005. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Hajna, Z.; Bagoly, T.; Boros, M.; Kemény, Á.; Materazzi, S.; Nassini, R.; Helyes, Z.; Szolcsányi, J.; Pintér, E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur. J. Pharmacol. 2012, 689, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y.; Gong, N.; Wang, Y. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism 2016, 65, 463–474. [Google Scholar] [CrossRef] [PubMed]
- de Pinheiro, F.V.; Villarinho, J.G.; Silva, C.R.; Oliveira, S.M.; de Pinheiro, K.V.; Petri, D.; Rossato, M.F.; Guerra, G.P.; Trevisan, G.; Rubin, M.A.; et al. The involvement of the TRPA1 receptor in a mouse model of sympathetically maintained neuropathic pain. Eur. J. Pharmacol. 2015, 747, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yang, X.; Lim, S.; Cao, Z.; Honek, J.; Lu, H.; Zhang, C.; Seki, T.; Hosaka, K.; Wahlberg, E.; et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell. Metab. 2013, 18, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Bodkin, J.V.; Graepel, R.; Bevan, S.; Andersson, D.A.; Brain, S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc. Res. 2010, 87, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zimmermann, K.; Vetter, I.; Lewis, R.J. Therapeutic opportunities for targeting cold pain pathways. Biochem. Pharmacol. 2015, 93, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Deng, Y.; Zhang, B.; Bai, Y.; Cao, J.; Li, S.; Liu, J.F. Pinacidil, a Katp channel opener, identified as a novel agonist for TRPA1. Chin. Sci. Bull. 2012, 57, 1810–1817. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Guo, H.; Sun, S.; Wang, S.; Zhang, Y.; Li, S.; Qiao, Y. [6]-gingerol: a novel AT(1) antagonist for the treatment of cardiovascular disease. Planta Med. 2013, 79, 322–326. [Google Scholar] [CrossRef] [PubMed]
- World Wide Protein Data Bank. Available online: http://www.rcsb.org/pdb/home/home.do (accessed on 20 August 2016).
- Peters, M.F.; Scott, C.W.; Ochalski, R.; Dragan, Y.P. Evaluation of Cellular Impedance Measures of Cardiomyocyte Cultures for Drug Screening Applications. Assay Drug Dev. Technol. 2012, 10, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Sun, S.N.; Wang, S.F.; Zhang, Q.; Li, M.; Lan, F.; Li, S.; Liu, C. Liensinine- and Neferine-Induced Cardiotoxicity in Primary Neonatal Rat Cardiomyocytes and Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Int. J. Mol. Sci. 2016, 17, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhai, C.; Zhang, Y.; Yu, Y.; Zhang, Y.; Ma, L.; Li, S.; Qiao, Y. Cardamonin, a Novel Antagonist of hTRPA1 Cation Channel, Reveals Therapeutic Mechanism of Pathological Pain. Molecules 2016, 21, 1145. https://doi.org/10.3390/molecules21091145
Wang S, Zhai C, Zhang Y, Yu Y, Zhang Y, Ma L, Li S, Qiao Y. Cardamonin, a Novel Antagonist of hTRPA1 Cation Channel, Reveals Therapeutic Mechanism of Pathological Pain. Molecules. 2016; 21(9):1145. https://doi.org/10.3390/molecules21091145
Chicago/Turabian StyleWang, Shifeng, Chenxi Zhai, Yanling Zhang, Yangyang Yu, Yuxin Zhang, Lianghui Ma, Shiyou Li, and Yanjiang Qiao. 2016. "Cardamonin, a Novel Antagonist of hTRPA1 Cation Channel, Reveals Therapeutic Mechanism of Pathological Pain" Molecules 21, no. 9: 1145. https://doi.org/10.3390/molecules21091145




