Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies
Abstract
:1. Introduction
2. Results and Discussion
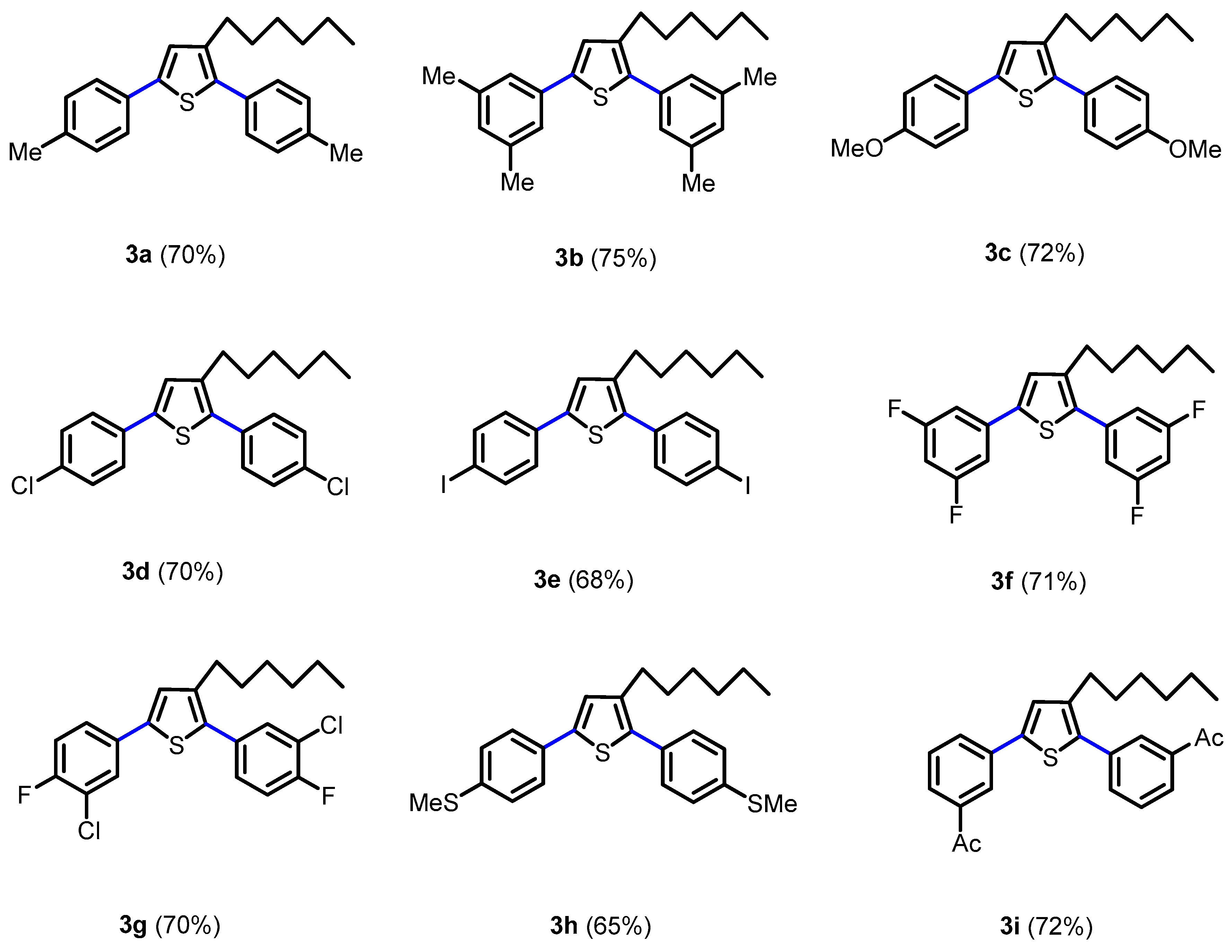
2.1. Synthesis of Novel Thiophene Derivatives
2.2. Biological Applications
2.2.1. Breast Cancer Cell Lines
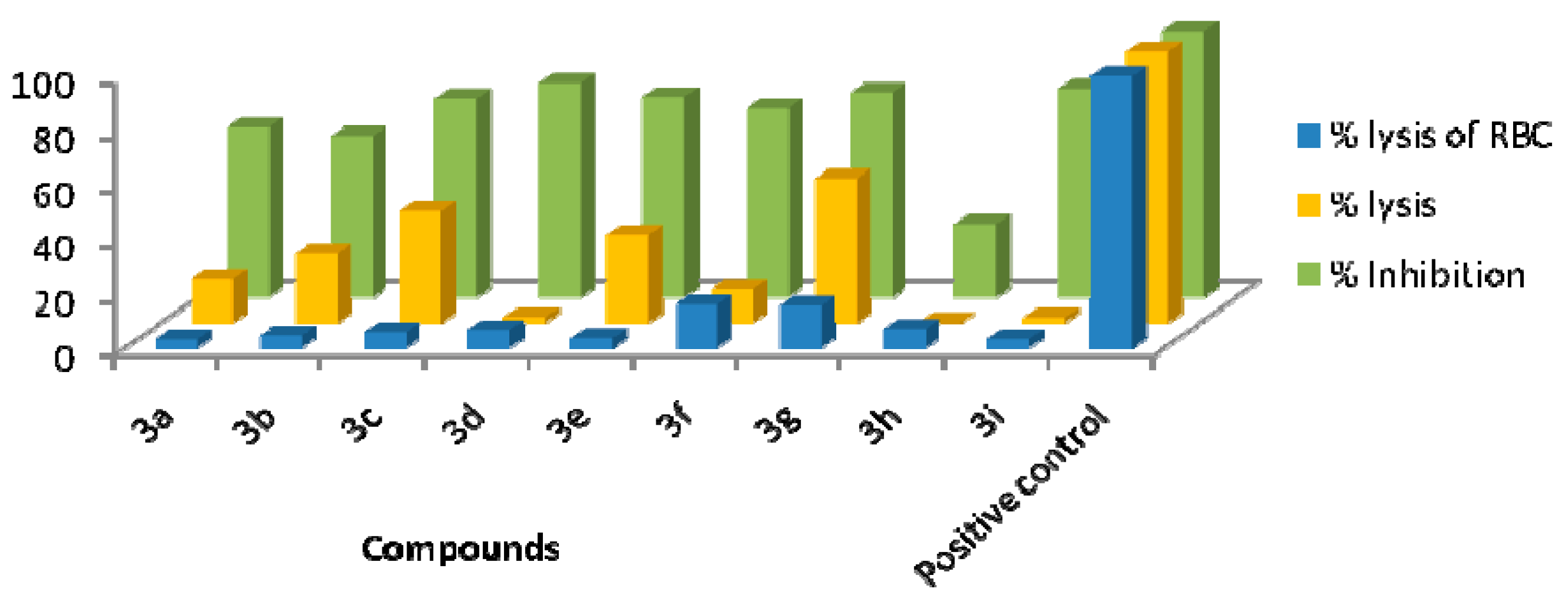
2.2.2. Anti-Thrombolytic Activity
2.2.3. Biofilm Inhibition Activity
2.2.4. Haemolytic Activity
3. Experimental Section
3.1. Characterization Techniques
3.2. General Procedure for the Synthesis of 2,5-Biaryl-3-hexylthiophene (3a–i)
3.3. Characterization Data
3.4. Anti-Thrombolytic Activity
3.5. Biofilm Inhibition Assay
3.6. Haemolytic Activity
3.7. MTT Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Nicolaou, K.; Boddy, C.N.; Brase, S.; Winssinger, N. Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew. Chem. Int. Ed. 1999, 38, 2096–2152. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Botella, L.; Najera, C. Cross-coupling reactions with boronic acids in water catalysed by oxime-derived palladacycles. J. Organomet. Chem. 2002, 663, 46–57. [Google Scholar] [CrossRef]
- Kumar, P.R.; Raju, S.; Satish, G.P.; Sailaja, M.; Sarma, M.; Om, R.G.; Prem, K.M.; Reddy, V.; Suresh, T.; Hegde, P. Synthesis and biological evaluation of thiophene [3,2-b] pyrrole derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. 2004, 12, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Wardakhan, W.; Abdel-Salam, O.; Elmegeed, G. Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharm. 2008, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bonini, C.; Chiummiento, L.; Bonis, M.D.; Funicello, M.; Lupattelli, P.; Suanno, G.; Berti, F.; Campaner, P. Synthesis, biological activity and modelling studies of two novel anti HIV PR inhibitors with a thiophene containing hydroxyethylamino core. Tetrahedron 2005, 61, 6580–6589. [Google Scholar] [CrossRef]
- Giordanetto, F.; Karlsson, O.; Lindberg, J.; Larsson, L.O.; Linusson, A.; Evertsson, E.; Morgan, D.G.; Inghardt, T. Discovery of cyclopentane and cyclohexane-trans-1,3-diamines as potent melanin-concentrating hormone receptor 1 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5231. [Google Scholar] [CrossRef] [PubMed]
- Ikram, H.M.; Rasool, N.; Ahmad, G.; Chotana, G.A.; Musharraf, S.G.; Zubair, M.; Rana, U.A.; Zia-Ul-Haq, M.; Jaafar, H.Z. Selective C-Arylation of 2, 5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects. Molecules 2015, 20, 5202–5214. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Martorana, A.; Dattolo, G.; Gia, O.; Dalla Via, L.; Cirrincione, G. Synthesis and antitumor properties of 2,5-bis (3′-indolyl) thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 2007, 17, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Parai, M.K.; Panda, G.; Chaturvedi, V.; Manju, Y.; Sinha, S. Thiophene containing triarylmethanes as antitubercular agents. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Puterova, Z.; Krutosikova, A.; Vegh, D. Gewald reaction: Synthesis, properties and applications of substituted 2-aminothiophenes. Arkivoc 2010, 1, 209–246. [Google Scholar]
- Cui, Y.; Zhang, X.; Jenekhe, S.A. Thiophene-linked polyphenylquinoxaline: a new electron transport conjugated polymer for electroluminescent devices. Macromolecules 1999, 32, 3824–3826. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Steybe, F.; Effenberger, F.; Beckmann, S.; Kramer, P.; Glania, C.; Wortmann, R. Enhanced nonlinear optical properties and thermal stability of donor-acceptor substituted oligothiophenes. Chem. Phy. 1997, 219, 317–331. [Google Scholar] [CrossRef]
- Handy, S.T.; Mayi, D. Regioselective double Suzuki couplings of 4,5-dibromothiophene-2-carboxaldehyde. Tetrahedron Lett. 2007, 48, 8108–8110. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rasool, N.; Ullah, A.; Nasim, F.H.; Yaqoob, A.; Zubair, M.; Rashid, U.; Riaz, M. Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation. Molecules 2013, 18, 14711–14725. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Rasool, N.; Noreen, M.; Gull, Y.; Zubair, M.; Ullah, A.; Rana, U.A. Palladium (0) catalyzed Suzuki cross-coupling reactions of 2,4-dibromothiophene: selectivity, characterization and biological applications. J. Sulfur Chem. 2015, 36, 240–250. [Google Scholar] [CrossRef]
- Inada, K.; Miyaura, N. The Cross-Coupling Reaction of Arylboronic Acids with Chloropyridines and Electron-Deficient Chloroarenes Catalyzed by a Polymer-Bound Palladium Complex. Tetrahedron 2000, 56, 8661–8664. [Google Scholar] [CrossRef]
- Patanaphan, V.; Salazar, O.M.; Risco, R. Breast cancer: metastatic patterns and their prognosis. South. Med. J. 1988, 81, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Allroggen, H.; Abbott, R.J. Cerebral venous sinus thrombosis. Postgrad. Med. J. 2000, 76, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Einhaupl, K.; Bousser, M.G.; de Bruijn, S.; Ferro, J.; Martinelli, I.; Masuhr, F.; Stam, J. EFNS guideline on the treatment of cerebral venous and sinus thrombosis. Eur. J. Neurol. 2006, 13, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.B.; Dash, R.N.; Chaudhari, M.; Kadam, S. Plasminogen activators: a comparison. Vascul. Pharmacol. 2006, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haines, S.T.; Bussey, H.I. Thrombosis and the pharmacology of antithrombotic agents. Ann. Pharmacother. 1995, 29, 892–905. [Google Scholar] [PubMed]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F.; Lee, I.; Waters, C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Shahid, M.; Jamil, A. Sajjad-Ur-Rehman Phytochemical Spectrum of Essential Oil of Paganum harmala by GC-MS and Antimicrobial Activity Using Sequential Solvents Fractions and Essential Oil. Asian J. Chem. 2014, 26, 574–578. [Google Scholar]
- Powell, W.; Catranis, C.; Maynard, C. Design of self-processing antimicrobial peptides for plant protection. Lett. Appl. Microbiol. 2000, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, Z.; Zhang, C.; Xin, T.; Wang, Y.; Song, H.; Jiang, Y.; Chen, Y.; Xu, Y.; Tan, C. Synthesis and Cytotoxic Activity of Some Novel N-Pyridinyl-2-(6-phenylimidazo [2,1-b] thiazol-3-yl) acetamide Derivatives. Molecules 2012, 17, 4703–4716. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.

| Entry | Compounds | IC50 (μM) | ||
|---|---|---|---|---|
| 4T1 | MDA-MB-231 | MCF-7 | ||
| 1 | 3a | 30.5 ± 0.08 | 36.8 ± 0.21 | 26.2 ± 0.70 |
| 2 | 3b | 30.0 ± 0.81 | 32.2 ± 2.73 | 33.8 ± 0.29 |
| 3 | 3c | 29.5 ± 4.30 | 40.0 ± 0.41 | 35.7 ± 0.09 |
| 4 | 3d | 22.8 ± 0.04 | 24.8 ± 0.56 | 27.5 ± 0.67 |
| 5 | 3e | 21.5 ± 3.71 | 22.9 ± 3.80 | 91.4 ± 0.59 |
| 6 | 3f | 31.5 ± 1.10 | 28.1 ± 0.72 | 42.8 ± 2.87 |
| 7 | 3g | 22.0 ± 0.57 | 26.2 ± 0.28 | 44.0 ± 0.17 |
| 8 | 3h | 16.1 ± 0.09 | 29.2 ± 0.04 | 35.1 ± 3.8 |
| 9 | 3i | 23.2 ± 0.08 | 36.8 ± 0.82 | 36.8 ± 0.03 |
| 10 | Cisplatin | 21.5 | 29.1 | 48.3 |
| Entry | Compounds | % Lysis |
|---|---|---|
| 1 | 3a | 16.25 ± 0.028 |
| 2 | 3b | 25.32 ± 0.092 |
| 3 | 3c | 41.12 ± 0.035 |
| 4 | 3d | 2.04 ± 0.091 |
| 5 | 3e | 32.42 ± 0.046 |
| 6 | 3f | 12.34 ± 0.073 |
| 7 | 3g | 53.12 ± 0.063 |
| 8 | 3h | 0.27 ± 0.021 |
| 9 | 3i | 1.84 ± 0.083 |
| 10 | Positive control | 100 |
| Entry | Compounds | % Inhibition |
|---|---|---|
| 1 | 3a | 62.50 ± 0.038 |
| 2 | 3b | 59.48 ± 0.073 |
| 3 | 3c | 73.27 ± 0.023 |
| 4 | 3d | 78.85 ± 0.057 |
| 5 | 3e | 73.80 ± 0.032 |
| 6 | 3f | 69.68 ± 0.049 |
| 7 | 3g | 75.53 ± 0.041 |
| 8 | 3h | 27.12 ± 0.039 |
| 9 | 3i | 76.86 ± 0.083 |
| 10 | Positive control | 97.43 |
| Entry | Compounds | % Lysis of RBC |
|---|---|---|
| 1 | 3a | 3.10 ± 0.040 |
| 2 | 3b | 4.54 ± 0.014 |
| 3 | 3c | 5.80 ± 0.045 |
| 4 | 3d | 6.66 ± 0.057 |
| 5 | 3e | 3.62 ± 0.024 |
| 6 | 3f | 15.91 ± 0.095 |
| 7 | 3g | 15.57 ± 0.034 |
| 8 | 3h | 7.01 ± 0.054 |
| 9 | 3i | 3.27± 0.046 |
| 10 | Positive control | 100 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, H.M.; Rasool, N.; Zubair, M.; Khan, K.M.; Abbas Chotana, G.; Akhtar, M.N.; Abu, N.; Alitheen, N.B.; Elgorban, A.M.; Rana, U.A. Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies. Molecules 2016, 21, 977. https://doi.org/10.3390/molecules21080977
Ikram HM, Rasool N, Zubair M, Khan KM, Abbas Chotana G, Akhtar MN, Abu N, Alitheen NB, Elgorban AM, Rana UA. Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies. Molecules. 2016; 21(8):977. https://doi.org/10.3390/molecules21080977
Chicago/Turabian StyleIkram, Hafiz Mansoor, Nasir Rasool, Muhammad Zubair, Khalid Mohammed Khan, Ghayoor Abbas Chotana, Muhammad Nadeem Akhtar, Nadiah Abu, Noorjahan Banu Alitheen, Abdallah Mohamed Elgorban, and Usman Ali Rana. 2016. "Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies" Molecules 21, no. 8: 977. https://doi.org/10.3390/molecules21080977






