Treatment Modalities for Acne
Abstract
:1. Introduction
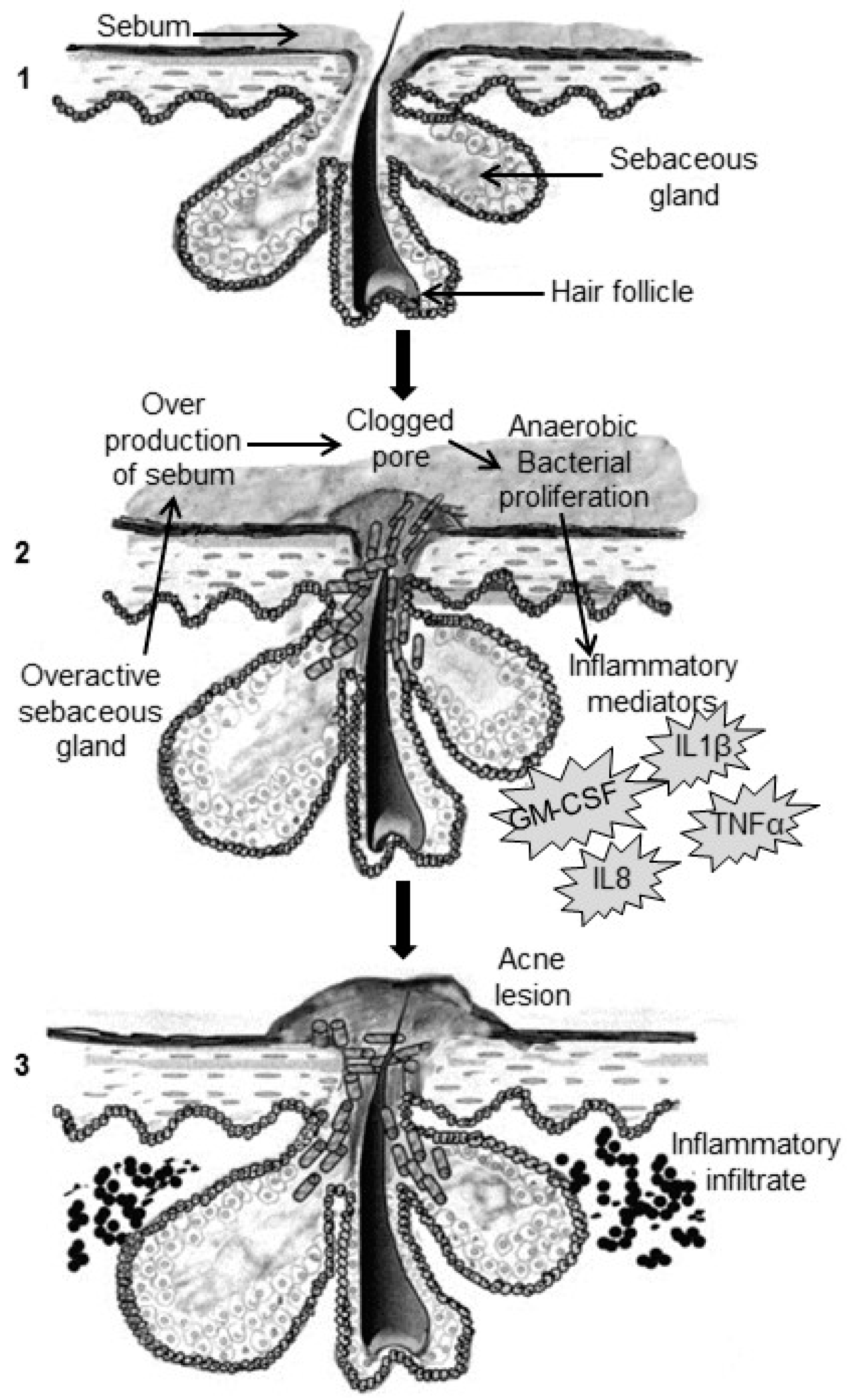
2. Pathogenesis of Acne
2.1. Excess Sebum Production
2.2. Epidermal Hyper-Proliferation and Formation of Comedones
2.3. Propionibacterium Acnes Infiltration
2.4. Inflammation Process
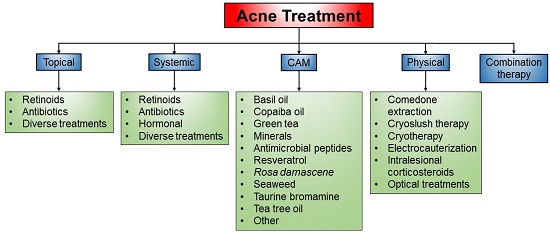
3. Current Treatment of Acne
3.1. Topical Treatment
3.1.1. Retinoids
Tretinoin
Adapalene
Tazarotene
Other Retinoids
3.1.2. Antibiotics
Erythromycin
Clindamycin
3.1.3. Diverse Treatments
Salicylic Acid
Chemical Peeling with Hydroxy Acids
Benzoyl Peroxide
Azelaic Acid
Sulfur
Hydrogen Peroxide
Niacinamide
Topical Corticosteroids
Triclosan
Sodium Sulfacetamide
Dapsone
3.2. Systemic Treatment
3.2.1. Retinoids
3.2.2. Antibiotics
3.2.3. Hormonal
3.2.4. Diverse Treatments
3.3. Complementary and Alternative Medicines (CAM)
3.3.1. Basil Oil
3.3.2. Copaiba Oil
3.3.3. Green Tea
3.3.4. Minerals
3.3.5. Antimicrobial Peptides
3.3.6. Resveratrol
3.3.7. Rosa Damascena
3.3.8. Seaweed
3.3.9. Taurine Bromamine (TauBr)
Tea Tree Oil
Other Complementary and Alternative Medicines
3.4. Physical Treatment
3.4.1. Comedone Extraction
3.4.2. Cryoslush Therapy
3.4.3. Cryotherapy
3.4.4. Electrocauterization
3.4.5. Intralesional Corticosteroids
3.4.6. Optical Treatments
3.5. Combination Therapy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bershad, S.V. The modern age of acne therapy: A review of current treatment options. Mt Sinai J. Med. 2001, 68, 279–285. [Google Scholar] [PubMed]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Krautheim, A.; Gollnick, H.P.M. Acne: Topical treatment. Clin. Dermatol. 2004, 22, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.S.; Krowchuk, D.P.; Leyden, J.J.; Lucky, A.W.; Shalita, A.R.; Siegfried, E.C.; Thiboutot, D.M.; van Voorhees, A.S.; Beutner, K.A.; Sieck, C.K.; et al. Guidelines of care for Acne vulgaris management. J. Am. Acad. Dermatol. 2007, 56, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Thibout, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New insights into the management of acne: An update from the global alliance to improve outcomes in acne group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef] [PubMed]
- Lavers, I. Diagnosis and management of Acne vulgaris. Nurse Prescr. 2014, 12, 330–336. [Google Scholar] [CrossRef]
- Adebamowo, C.A.; Spiegelman, D.; Berkey, C.S.; Danby, F.W.; Rockett, H.H.; Colditz, G.A.; Willett, W.C.; Holmes, M.D. Milk consumption and acne in teenage boys. J. Am. Acad. Dermatol. 2008, 58, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Bershad, S. Topical acne drugs. Am. J. Clin. Dermatol. 2003, 4, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.; Careccia, R.E.; Barham, K.L.; Hancox, J. Diagnosis and treatment of acne. Am. Fam Physician 2004, 69, 2123–2130. [Google Scholar] [PubMed]
- Olutunmbi, Y.; Paley, K.; English, J.C. Adolescent female acne: etiology and management. J. Pediatr. Adolesc. Gynecol. 2008, 21, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on Acne vulgaris: A randomized, double-blind controlled trial. Acta Derm Venereol. 2014, 94, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.F. Clinical review: Acne vulgaris. Br. Med. J. 2002, 325, 475–479. [Google Scholar] [CrossRef]
- Gollnick, H.; Cunliffe, W.; Berson, D.; Dreno, B.; Finlay, A.; Leyden, J.J.; Shalita, A.R.; Thiboutot, D. Management of acne: A report from a global alliance to improve outcomes in acne. J. Am. Acad. Dermatol. 2003, 49, S1–S37. [Google Scholar] [CrossRef] [PubMed]
- Muizzuddin, N.; Giacomoni, P.; Maes, D. Acne—A multifaceted problem. Drug Discov. Today Dis. Mech. 2008, 5, e183–e188. [Google Scholar] [CrossRef]
- Gollnick, H. Current concepts of the pathogenesis of acne, Implications for Drug Treatment. Drugs 2003, 63, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Michniak, B.B. Sebum. In Cosmeceuticals; Elsner, P., Maibach, H.I., Eds.; Marcel Dekker: New York, NY, USA, 2000; Volume 23, pp. 45–56. [Google Scholar]
- Webster, G.F. Acne. Curr. Probl. Dermatol. 1996, 8, 237–268. [Google Scholar] [CrossRef]
- Habif, T.P. (Ed.) Clinical dermatology: A color guide to diagnosis and therapy. In Mosby’s Medical Dictionary, 4th ed.; Elsevier: St Louis, MO, USA, 2004.
- Burkhart, C.G.; Burkhart, C.N.; Lehmann, P.F. Acne: A review of immunologic and microbiologic factors. Postgrad. Med. J. 1999, 75, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Bialecka, A.; Mak, M.; Biedron, R.; Bobek, M.; Kasprowicz, A.; Marcinkiewicz, J. Different pro-inflammatory and immunogenic potentials of Propionibacterium acnes and Staphylococcus: Implications for chronic inflammatory acne. Arch. Immunol. Ther. Exp. 2005, 53, 79–85. [Google Scholar]
- Webster, G.F. Acne vulgaris and rosacea: Evaluation and management. Clin. Cornerstone 2001, 4, 15–22. [Google Scholar] [CrossRef]
- McInturff, J.E.; Kim, J. The role of toll-like receptors in the pathophysiology of acne. Semin Cutan Med. Surg. 2005, 24, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Akamutsu, H.; Horio, T.; Hattori, K. Increased hydrogen peroxide generation by neutrophils from patients with acne inflammation. Int. J. Dermatol. 2003, 42, 366–369. [Google Scholar] [CrossRef]
- Bowe, W.P.; Shalita, A.R. Effective over-the-counter acne treatments. Semin Cutan Med. Surg. 2008, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.P.M.; Krautheim, A. Topical treatment in acne: current status and future aspect. Dermatology 2003, 206, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L. Topical retinoids in the treatment of Acne vulgaris. Semin Cutan Med. Surg. 2008, 27, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Krautheim, A.; Gollnick, H. Transdermal penetration of topical drugs used in the treatment of acne. Clin. Pharmacokinet 2003, 42, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Kennedy, C. The treatment of acne. Paediatr. Child Health 2007, 17, 385–389. [Google Scholar] [CrossRef]
- Scheinfeld, N.S.; Tutrone, W.D.; Torres, O.; Weinberg, J.M. Macrolides in dermatology. Clin. Dermatol. 2003, 21, 40–49. [Google Scholar] [CrossRef]
- Kim, R.H.; Armstrong, A.W. Current state of acne treatment: Highlighting lasers, photodynamic therapy and chemical peels. Dermatol. Online J. 2011, 17. Available online: http://escholarship.org/uc/item/0t40h9px (accessed on 5 May 2015). [Google Scholar]
- Kaminsky, A. Less common methods to treat acne. Dermatology 2003, 206, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Bhate, K.; Williams, H.C. What’s new in acne? An analysis of systematic reviews published in 2011–2012. Clin. Exp. Dermatol. 2014, 39, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.F.; Graber, E.M. Antibiotic treatment for Acne vulgaris. Semin Cutan Med. Surg. 2008, 27, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Weiss, G.; Amichai, B.; Kaplan, B.; Trau, H. Azelaic acid (20%) cream in the treatment of Acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; Thieroff-ekerdt, R.; Graupe, K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: Results from two vehicle-controlled, randomized phase III studies. J. Am. Acad. Dermatol. 2003, 48, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.; Kayne, A. Implications of azelaic acid’s multiple mechanisms of action: Therapeutic versatility. J. Am. Acad. Dermatol. 2008, 58, AB40. [Google Scholar]
- Webster, G. Combination azelaic acid therapy for Acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Berardesca, E.; Dall’Oglio, F.; Micali, G.; Sinagra, J.; Nodzenski, M.; Veraldi, S. Novel over-the-counter hydrogen peroxide based acne kit in treating acne: Randomized, controlled, multicenter study of a hydrogen peroxide-based acne system versus the benzoyl peroxide-based acne system in the treatment of mild to moderate Acne vulgaris. J. Am. Acad. Dermatol. 2014, 70, AB9. [Google Scholar]
- Barai, N.D. Effect of Hydration on Skin Permeability. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2002. [Google Scholar]
- Namazi, M. Nicotinamide in dermatology: A capsule summary. Int. J. Dermatol. 2007, 46, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The effect of 2% niacinamide on facial sebum production. J. Cosmet Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z. Novel topical therapies in cosmetic dermatology. Curr. Probl. Dermatol. 2000, 12, 235–239. [Google Scholar] [CrossRef]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Kim, J.C.; Hwang, S.J. Hydrogel patches containing triclosan for acne treatment. Eur. J. Pharm. Biopharm. 2003, 56, 407–412. [Google Scholar] [CrossRef]
- Jones, R.D.; Jampani, H.B.; Newman, J.L.; Lee, A.S. State of the science. Triclosan: A review of effectiveness and safety in health care settings. Am. J. Infect. Control. 2000, 28, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef] [PubMed]
- Couthinho, B. Dapsone (Aczone) 5% Gel for the Treatment of Acne. Am. Fam Physician 2010, 81, 451–452. [Google Scholar]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Simonart, T. Newer approaches to the treatment of Acne vulgaris. Am. J. Clin. Dermatol. 2012, 13, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Katsambas, A.; Papakonstantinou, A. Acne: Systemic treatment. Clin. Dermatol. 2004, 22, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Martin, J.P. Update and future of systemic acne treatment. Dermatology 2003, 206, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; McGinley, K.J.; Foglia, A.N. Qualitative and quantitative changes in cutaneous bacteria associated with systemic isotretinoin therapy for acne conglobata. J. Investig. Dermatol. 1986, 86, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, W.; Jordaan, H.F. Acne guideline 2005 update. S. Afr. Med. J. 2005, 95, 883–892. [Google Scholar]
- Ebede, T.L.; Arch, E.L.; Berson, D. Hormonal treatment of acne in women. J. Clin. Aesthet Dermatol. 2009, 2, 16–22. [Google Scholar] [PubMed]
- Gollnick, H.P.; Zouboulis, C.C. Not all acne is Acne vulgaris. Dtsch. Arztebl. Int. 2014, 111, 301–312. [Google Scholar] [PubMed]
- Agnew, T.; Leach, M.; Segal, L. The clinical impact of cost-effectiveness of essential oils and aromatherapy for the treatment of Acne vulgaris: A protocol for a randomized controlled trial. J. Altern. Complement. Med. 2014, 20, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Magin, P.J.; Adams, J.; Pond, C.D.; Smith, W. Topical and oral CAM in Acne: A review of the empirical evidence and a consideration of its context. Complement Ther. Med. 2006, 14, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy for acne: A systematic review. Phytother. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.M.; Chandrasekar, M.J.N.; Nanajan, M.J.; Suresh, B. Herbal remedies for acne. Nat. Prod. Radiance 2005, 4, 328–334. [Google Scholar]
- Viyoch, J.; Pisutthanan, N.; Faikreua, A.; Nupangta, K.; Wangtorpol, K.; Ngokkuen, J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Int. J. Cosmet Sci. 2006, 28, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Orafidiya, L.O.; Agbani, E.O.; Oyedele, A.O.; Babalola, O.O.; Onayemi, O. Preliminary clinical tests on topical preparations of Ocimum gratissimum Linn leaf essential oil for the treatment of Acne vulgaris. Clin. Drug Investig. 2002, 22, 313–319. [Google Scholar] [CrossRef]
- Da Silva, A.G.; De Freitas Puziol, P.; Leitao, R.N.; Gomes, T.R.; Scherer, R.; Martins, M.L.L.; Cavalcanti, S.S.; Cavalcanti, A.S.S.; Cavalcanti, L.C. Application of the essential oil from copaiba (Copaifera langsdorffii Desf.) for Acne vulgaris: A double-blind, placebo controlled clinical trial. Altern. Med. Rev. 2012, 17, 69–75. [Google Scholar] [PubMed]
- Gaur, S.; Agnihotri, R. Green tea: A novel functional food for the oral health of older adults. Geriatr. Gerontol. Int. 2014, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer application. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Sigma. Polyphenon 60 from Green Tea. Available online: http://www.sigmaaldrich.com/catalog/product/sigma/p1204?lang=en®ion=ZA (accessed on 5 August 2015).
- Jung, M.K.; Ha, S.; Son, J.; Song, J.H.; Houh, Y.; Cho, E.; Chun, J.H.; Yoon, S.R.; Yang, Y.; Bang, S.I.; et al. Polyphenon-60 displays a therapeutic effect on acne by suppression of TLR2 and IL-8 expression via down-regulating the ERK1/2 pathway. Arch. Dermatol. Res. 2012, 304, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Forest, J.M.; Rafikhah, N. Oral aqueous green tea extract and Acne vulgaris: A placebo-controlled study. Asian J. Clin. Nutr. 2014, 6, 41–46. [Google Scholar]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Khan, H.M.S.; Saeed, T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn J. Basic Med. Sci. 2010, 10, 260–264. [Google Scholar] [PubMed]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, C.W.; Lee, M.Y. Antibacterial effects of minerals from ores indigenous to Korea. J. Environ. Biol. 2009, 30, 151–154. [Google Scholar] [PubMed]
- Ma’or, Z.; Henis, Y.; Alon, Y.; Orlov, E.; Sorensen, K.B.; Oren, A. Antimicrobial properties of Dead Sea black mineral mud. Int. J. Dermatol. 2006, 45, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Chen, L.; Guang, H.; Li, Z.; Yang, H.; Li, J.; You, D.; Yu, H.; Lai, R. Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS ONE 2011, 6, e22120. [Google Scholar] [CrossRef] [PubMed]
- Urban, E.; Nagy, E.; Pal, T.; Sonnevend, A.; Conlon, J.M. Activities of four frog skin-derived antimicrobial peptides (temporin-1 DRa, temporin-1Va and the melittin-related peptides AR-23 and RV-23) against anaerobic bacteria. Int. J. Antimicrob. Agents 2007, 29, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chen, J.Y.; Lin, T.L.; Lin, C.H. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides 2009, 30, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; McEwen, H.A.; Sweet, T.J.; Bailey, E.; Booth, T.D. Resveratrol inhibition of Propionibacterium acnes. J. Antimicrob. Chemother. 2007, 59, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung. Cell Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Gennaro, I.; La Rotonda, M.I.; Longobardi, A.; Mazzella, M.; Siano, M.; et al. Resveratrol-containing gel for the treatment of Acne vulgaris: A single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Doaguie, A.R.; Ghazanfari, A.; Tabil, L.G. Mesophilic anaerobic digestion of damask rose bagasse with different proportions of cattle manure. Can. Biosyst. Eng. 2012, 54, 8.1–8.6. [Google Scholar] [CrossRef]
- Tofighi, Z.; Molazem, M.; Doostdar, B.; Taban, P.; Shahverdi, A.R.; Samadi, N.; Yassa, N. Antimicrobial activities of three medicinal plants and investigation of flavonoids of Tripleurospermum disciforme. Iran J. Pharm. Res. 2015, 14, 225–231. [Google Scholar] [PubMed]
- Jain, M. Ayurvedic textiles: A wonderful approach to handle health disorders. Colourage 2010, 57, 45–52. [Google Scholar]
- Shahriari, S.; Yasa, N.; Mohammadirad, A.; Khorasani, R.; Abdollahi, M. In vivo antioxidant potentials of Rosa damascena petal extract from Guilan, Iran, comparable to α-tocopherol. Int. J. Pharmacol. 2007, 3, 187–190. [Google Scholar]
- Hajhashemi, V.; Ghannadi, A.; Hajiloo, M. Analgesic and anti-inflammatory effects of Rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J. Pharm. Res. 2010, 9, 163–168. [Google Scholar] [PubMed]
- Tsai, T.H.; Tsai, T.H.; Wu, W.H.; Tseng, J.T.P.; Tsai, P.J. In vitro antimicrobial and anti-inflammatory effects of herbs against Propionibacterium acnes. Food Chem. 2010, 119, 964–968. [Google Scholar] [CrossRef]
- Capitanio, B.; Sinagra, J.L.; Weller, R.B.; Brown, C.; Berardesca, E. Randomized controlled study of a cosmetic treatment for mild acne. Clin. Exp. Dermatol. 2012, 37, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bae, H.J.; Kim, S.J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar] [PubMed]
- Marcinikiewicz, J. Taurine bromamine (TauBr)—Its role in immunity and new perspectives for clinical use. J. Biomed. Sci. 2010, 17, S3. [Google Scholar] [CrossRef] [PubMed]
- Marcinikiewicz, J.; Biedron, R.; Bialecka, A.; Kasprowicz, A.; Mak, M.; Targosz, M. Susceptibility of Propionibacterium acnes and Staphylococcus epidermidis to killing by MPO-halide system products. Implication for taurine bromamine as a new candidate for topical therapy in treating Acne vulgaris. Arch. Immunol. Ther. Exp. 2006, 54, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Marcinikiewicz, J.; Wojas-Pelc, A.; Walczewska, M.; Lipko-Godlewska, S.; Jachowicz, R.; Maciejewska, A.; Bialecka, A.; Kasprowicz, A. Topical taurine bromamine, a new candidate in the treatment of moderate inflammatory Acne vulgaris: A pilot study. Eur. J. Dermatol. 2008, 18, 433–439. [Google Scholar]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The efficacy of 5% topical tea tree oil gel in mild to moderate Acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [PubMed]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Turfi, I.A.; Al-Shimary, W.M. Treatment of Acne vulgaris with 2% topical tea lotion. Saudi Med. J. 2006, 27, 83–85. [Google Scholar] [PubMed]
- Panichayupaaranant, P.; Tewtrakul, S.; Yuenyongsawad, S. Antibacterial, anti-inflammatory and anti-allergic activities of standardized pomegranate rind extract. Food Chem. 2010, 123, 400–403. [Google Scholar] [CrossRef]
- Saising, J.; Voravuthikunchai, S.P. Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe 2012, 18, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Selvan, K.; Sentila, R.; Michael, A. Generation and characterization of chicken egg yolk antibodies against Propionibacterium acnes for the prevention of Acne vulgaris. Indian J. Dermatol. 2012, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Revathy, J.; Karthika, S.; Sentila, R.; Michael, A. In vitro evaluation of the efficacy of chicken egg yolk antibodies (IgY) generated against Propionibacterium acnes. Int. J. Cosmet Sci. 2014, 36, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.-M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in Acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.M.; Graber, E.M. Clinical pearl: Comedone extraction for persistent macrocomedones while on insotretinoin therapy. J. Clin. Aesthet Dermatol. 2011, 4, 20–21. [Google Scholar] [PubMed]
- Steventon, K. Expert opinion and review article: the timing of comedone extraction in the treatment of premenstrual acne—A proposed therapeutic approach. Int. J. Cosmet Sci. 2011, 33, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Khandpur, S. Guidelines for cryotherapy. Indian J. Dermatol. Venereol. Leprol. 2009, 75, S90–S100. [Google Scholar]
- Thomson, K.F.; Goulden, V.; Sheehan-Dare, R.; Cunliffe, W.J. Light cautery of macrocomedones under general anaesthesia. Br. J. Dermatol. 1999, 141, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.L.; Zeichner, J.A. Management of acne scarring, Part II. A comparative review of non-laser-based, minimally invasive approaches. Am. J. Clin. Dermatol. 2012, 13, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.D.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. Therapeutic considerations for severe nodular acne. Am. J. Clin. Dermatol. 2011, 12, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.; Lebzelter, J. Light therapy in the treatment of Acne vulgaris. Dermatol. Surg. 2004, 30, 139–146. [Google Scholar] [PubMed]
- Krakowski, A.C.; Stendardo, S.; Eichenfield, L.F. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr. Dermatol. 2008, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seidler, E.M.; Kimball, A.B. Meta-analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in ace. J. Am. Acad. Dermatol. 2010, 63, 52–62. [Google Scholar] [CrossRef] [PubMed]
| Treatment Methods | Examples |
|---|---|
| Topical | Retinoids: adapalene, isotretinoin, motretinide, retinoyl-β-glucuronide, tazarotene, tretinoin |
| Antibiotics: clindamycin, erythromycin | |
| Diverse: azelaic acid, benzoyl peroxide, chemical peels, corticosteroids, dapsone, hydrogen peroxide, niacinamide, salicylic acid, sodium sulfacetamide, sulfur, triclosan | |
| Systemic | Retinoids: isotretinoin |
| Antibiotics: azithromycin, clindamycin, co-trimoxazole, doxycycline, erythromycin, levofloxacin, lymecycline, minocycline, roxithromycin | |
| Hormonal: contraceptives | |
| Diverse: clofazimine, corticosteroids, ibuprofen, zinc sulfate | |
| Complementary and Alternative Medicines (CAM) | Achillea millefolium, amaranth, antimicrobial peptides, arnica, asparagus, basil oil, bay, benzoin, birch, bittersweet nightshade, black cumin, black walnut, borage, Brewer’s yeast, burdock root, calendula, celandine, chamomile, chaste tree, Commiphora mukul, copaiba oil, coriander, cucumber, duckweed, Du Zhong extract, English walnut, Eucalyptus dives, fresh lemon, garlic, geranium, grapefruit seeds, green tea, jojoba oil, juniper twig, labrador tea, lemon grass, lemon, minerals, neem, oak bark, onion, orange peel, orange, Oregon grape root, patchouli, pea, petitgrain, pine, pomegranate rind extract, poplar, probiotics, pumpkin, resveratrol, rose myrtle, rhubarb, Rosa damascena, rosemary, rue, safflower oil, sandalwood, seaweed, soapwort, Sophora flavescens, specific antibodies, stinging nettle, sunflower oil, Taraxacum officinale, taurine bromamine, tea tree oil, thyme, turmeric, vinegar, vitex, witch hazel, Withania somnifera and yerba mate extract |
| Physical Treatment | Comedone extraction, cryoslush therapy, cryotherapy, electrocauterization, intralesional corticosteroids and optical treatments |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fox, L.; Csongradi, C.; Aucamp, M.; Du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. https://doi.org/10.3390/molecules21081063
Fox L, Csongradi C, Aucamp M, Du Plessis J, Gerber M. Treatment Modalities for Acne. Molecules. 2016; 21(8):1063. https://doi.org/10.3390/molecules21081063
Chicago/Turabian StyleFox, Lizelle, Candice Csongradi, Marique Aucamp, Jeanetta Du Plessis, and Minja Gerber. 2016. "Treatment Modalities for Acne" Molecules 21, no. 8: 1063. https://doi.org/10.3390/molecules21081063