The Complete Chloroplast Genome Sequence of the Medicinal Plant Swertia mussotii Using the PacBio RS II Platform
Abstract
:1. Introduction
2. Results and Discussion
2.1. Features of the S. mussotii Chloroplast Genome
2.2. Repeat Analysis
2.3. Comparative Chloroplast Genomic Analysis
2.4. IR Contraction and Expansion
3. Materials and Methods
3.1. DNA Sequencing, Genome Assembly, and Validation
3.2. Genome Annotation and Codon Usage
3.3. Genome Comparison and Repeat Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamahara, J.; Konoshima, T.; Sawada, T.; Fujimura, H. Biologically active principles of crude drugs: Pharmacological actions of Swertia japonica extracts, swertiamarin and gentianine (author′s transl). Yakugaku Zasshi: J. Pharm. Soc. Jpn. 1978, 98, 1446–1451. [Google Scholar]
- Kikuzaki, H.; Kawasaki, Y.; Kitamura, S.; Nakatani, N. Secoiridoid glucosides from Swertia mileensis. Planta Med. 1996, 62, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G.; Mondal, S.; Gangopadhyay, A.; Gorai, D.; Mukhopadhyay, B.; Saha, S.; Brahmachari, A.K. Swertia (Gentianaceae): Chemical and pharmacological aspects. Chem. Biodivers. 2004, 1, 1627–1651. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.N.; Tian, C.W.; Zhang, T.J.; Zhang, L.J.; Xu, X.H. Advances in study on iridoids in plants of Swertia L. and their pharmacological activity. Chin. Tradit. Herb. Drugs 2008, 39, 790–795. [Google Scholar]
- Kong, L.B.; Li, S.S.; Liao, Q.J.; Zhang, Y.N.; Sun, R.N.; Zhu, X.D.; Zhang, Q.H.; Wang, J.; Wu, X.Y.; Fang, X.N. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Cheng, Y.; Du, X.H.; Chen, S.; Feng, X.C.; Gao, Y.; Li, S.X.; Liu, L.; Yang, M.; Chen, L.; et al. Swertianlarin, an Herbal Agent Derived from Swertia mussotii Franch, Attenuates Liver Injury, Inflammation, and Cholestasis in Common Bile Duct-Ligated Rats. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef]
- Zhang, Y.M. The Effect of Gentiopicroside and Mangiferin, Two Major Ingredients of Tibet Capillary Artemisia, on Expression of Hepatocyte Membrane Transporters MRP2 and MRP3. Master′s Thesis, Third Military Medical University, Chongqing, China, 2011. [Google Scholar]
- Howe, C.J.; Barbrook, A.C.; Koumandou, V.L.; Nisbet, R.E.R.; Symington, H.A.; Wightman, T.F. Evolution of the chloroplast genome. Philos. Trans. R. Soc. Lond. B 2003, 358, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, H.E.; Emes, M.J. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Biol. 2000, 51, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ezpeleta, N.; Brinkmann, H.; Burey, S.C.; Roure, B.; Burger, G.; Löffelhardt, W.; Bohnert, H.J.; Philippe, H.; Lang, B.F. Monophyly of primary photosynthetic eukaryotes: Green plants, red algae, and glaucophytes. Curr. Biol. 2005, 15, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Kai, F.M.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.H.; Mordent, C.W.; Ems, S.C.; Palmer, J.D. Rapid evolution of the plastid translational apparatus in a nonphotosynthetic plant: Loss or accelerated sequence evolution of tRNA and ribosomal protein genes. J. Mol. Evol. 1992, 35, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Jansen, R.K.; Chumley, T.W.; Kim, K.J. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Li, Y.; Song, J.Y.; Xu, H.B.; Xu, J.; Zhu, Y.J.; Li, X.W.; Gao, H.H.; Dong, L.L.; Qian, J.; et al. High-accuracy de novo assembly and SNP detection of chloroplast genomes using a SMRT circular consensus sequencing strategy. New Phytol. 2014, 204, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Carneiro, M.O.; Schatz, M.C. The advantages of SMRT sequencing. Genome Biol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- English, A.C.; Richards, S.; Han, Y.; Wang, M.; Vee, V.; Qu, J.; Qin, X.; Muzny, D.M.; Reid, J.G.; Worley, K.C. Mind the Gap: Upgrading Genomes with Pacific Biosciences RS Long-Read Sequencing Technology. PLoS ONE 2012, 7, e47768. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Schatz, M.C.; Walenz, B.P.; Martin, J.; Howard, J.T.; Ganapathy, G.; Wang, Z.; Rasko, D.A.; McCombie, W.R.; Jarvis, E.D. Phillippy Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nat. Biotechnol. 2012, 30, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, C.S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Vanburen, R.; Bryant, D.; Edger, P.P.; Tang, H.; Burgess, D.; Challabathula, D.; Spittle, K.; Hall, R.; Gu, J.; Lyons, E. Single-molecule sequencing of the desiccation-tolerant grass Oropetium thomaeum. Nature 2015, 527, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Huddleston, J.; Chaisson, M.J.; Hill, C.M.; Kronenberg, Z.N.; Munson, K.M.; Malig, M.; Raja, A.; Fiddes, I.; Hillier, L.W. Long-read sequence assembly of the gorilla genome. Science 2016, 352. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.X.; Peters, R.J.; Weirather, J.; Luo, H.M.; Liao, B.S.; Zhang, X.; Zhu, Y.J.; Ji, A.J.; Zhang, B.; Hu, S.N.; et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 2015, 82, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.E.; Hamilton, M.; Jacobi, J.L.; Ngam, P.; Devitt, N.; Schilkey, F.; Benhur, A.; Reddy, A.S.N. A survey of the sorghum transcriptome using single-molecule long reads. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Redwan, R.M.; Saidin, A.; Kumar, S.V. Complete chloroplast genome sequence of MD-2 pineapple and its comparative analysis among nine other plants from the subclass Commelinidae. BMC Plant Biol. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Li, Q.S.; Li, Y.; Qian, J.; Han, J.P. Chloroplast genome of Aconitum barbatum var. puberulum (Ranunculaceae) derived from CCS reads using the PacBio RS platform. Front. Plant Sci. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Stadermann, K.B.; Weisshaar, B.; Holtgräwe, D. SMRT sequencing only de novo assembly of the sugar beet (Beta vulgaris) chloroplast genome. BMC Bioinform. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.H.; Zhao, Z.L.; Xu, H.X.; Chen, S.L.; Dorje, G. The complete chloroplast genome of Gentiana straminea (Gentianaceae), an endemic species to the Sino-Himalayan subregion. Gene 2016, 577, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, M.; Moretto, M.; Ward, J.A.; Šurbanovski, N.; Stevanović, V.; Giongo, L.; Viola, R.; Cavalieri, D.; Velasco, R.; Cestaro, A. An evaluation of the PacBio RS platform for sequencing and de novo assembly of a chloroplast genome. BMC Genomics 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Gui, S.T.; Quan, Z.W.; Pan, L.; Wang, S.Z.; Ke, W.D.; Liang, D.Q.; Ding, Y. A precise chloroplast genome of Nelumbo nucifera (Nelumbonaceae) evaluated with Sanger, Illumina MiSeq, and PacBio RS II sequencing platforms: Insight into the plastid evolution of basal eudicots. BMC. Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Organelle Genome Esources. Available online: http://www.ncbi.nlm.nih.gov/genomes/ORGANELLES/organelles.html (access on 21 June 2016).
- Raveendar, S.; Na, Y.W.; Lee, J.R.; Shim, D.; Ma, K.H.; Lee, S.Y.; Chung, J.W. The complete chloroplast genome of Capsicum annuum var. glabriusculum using Illumina sequencing. Molecules 2015, 20, 13080–13088. [Google Scholar] [PubMed]
- Ibrahim, R.I.; Azuma, J.; Sakamoto, M. Complete nucleotide sequence of the cotton (Gossypium barbadense L.) chloroplast genome with a comparative analysis of sequences among 9 dicot plants. Genes Genetic Syst. 2006, 81, 311–321. [Google Scholar] [CrossRef]
- Wu, C.S.; Chaw, S.M. Highly rearranged and size-variable chloroplast genomes in conifers II clade (cupressophytes): Evolution towards shorter intergenic spacers. Plant Biotechnol. J. 2013, 12, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [PubMed]
- Sato, S.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Tabata, S. Complete structure of the chloroplast genome of Arabidopsis thaliana. Dna Res. Int. J. Rapid Publ. Rep. Genes Genomes 1999, 6, 283–290. [Google Scholar] [CrossRef]
- Do, H.D.K.; Kim, J.S.; Kim, J.H. Comparative genomics of four Liliales families inferred from the complete chloroplast genome sequence of Veratrum patulum O. Loes. (Melanthiaceae). Gene 2013, 530, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, X.; Liu, G.; Yin, Y.; Chen, K.; Yun, Q.; Zhao, D.; Al-Mssallem, I.S.; Yu, J. The complete chloroplast genome sequence of date palm (Phoenix dactylifera L.). PLoS ONE 2012, 5, e12762. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The Chloroplast Genome Sequence of Mungbean (Vigna radiata) Determined by High-throughput Pyrosequencing: Structural Organization and Phylogenetic Relationships. DNA. Res. 2010, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Rafalski, J.A. Polymorphic simple sequence repeat regions in chloroplast genomes: Applications to the population genetics of pines. Proc. Natl. Acad. Sci. USA 1995, 92, 7759–7763. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Jia, H.M.; Li, X.W.; Chai, M.L.; Jia, H.J.; Chen, Z.; Wang, G.Y.; Chai, C.Y.; Weg, E.V.D.; Gao, Z.S. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genomics 2012, 13, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Song, J.; Gao, H.; Zhu, Y.; Xu, J.; Pang, X.; Yao, H.; Sun, C.; Li, X.E.; Li, C. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PLoS ONE 2013, 8, e57607. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.Y.; Wu, H.; Wang, Y.L.; Gao, L.M.; Zhang, S.Z.; Lu, L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): Implication for DNA barcoding and population genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Huotari, T.; Korpelainen, H. Complete chloroplast genome sequence of Elodea canadensis and comparative analyses with other monocot plastid genomes. Gene 2012, 508, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.J.; Lv, S.Z.; Zhang, Y.X.; Du, X.H.; Wang, L.; Biradar, S.S.; Tan, X.F.; Wan, F.H.; Song, W.N. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora). PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Okada, H. Phylogenetic relationships among subgenera, species, and varieties of Japanese Salvia L. (Lamiaceae). J. Plant Res. 2011, 124, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Schäferhoff, B.; Fleischmann, A.; Fischer, E.; Albach, D.C.; Borsch, T.; Heubl, G.; Kai, F.M. Towards resolving Lamiales relationships: Insights from rapidly evolving chloroplast sequences. BMC Evol. Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, B.; Zhu, W.; Sun, L.; Tian, J.; Wang, X. The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms. Gene 2013, 528, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Chaisson, M.J.; Tesler, G. Mapping single molecule sequencing reads using basic local alignment with successive refinement (BLASR): Application and theory. BMC Bioinform. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Schattner, P.; Brooks, A.N.; Lowe, T.M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2007, 33, W686–W689. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Biol. 2004, 5. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The Manifold Applications of Repeat Analysis on a Genomic Scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Haider, N.; Kumar, H.; Singh, N.B. Plant Plastid Engineering. Curr. Genomics 2010, 11, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. Engineering Plastid Genomes: Methods, Tools, and Applications in Basic Research and Biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar] [CrossRef] [PubMed]
- Apel, W.; Bock, R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol. 2009, 151, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.; Lenzi, P.; Scotti, N.; Palma, M.D.; Saggese, P.; Carbone, V.; Curran, M.G.; Magee, A.M.; Medgyesy, P.; Kavanagh, T.A. Transplastomic tobacco plants expressing a fatty acid desaturase gene exhibit altered fatty acid profiles and improved cold tolerance. Transgenic Res. 2008, 17, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Madoka, Y.; Tomizawa, K.; Mizoi, J.; Nishida, I.; Nagano, Y.; Sasaki, Y. Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol. 2002, 43, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Cai, Y.; Zhang, F.; Xia, G.; Xiang, F. Cloning and functional analysis of geraniol 10-hydroxylase, a cytochrome P450 from Swertia mussotii Franch. Biosci. Biotechnol. Biochem. 2010, 74, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available.
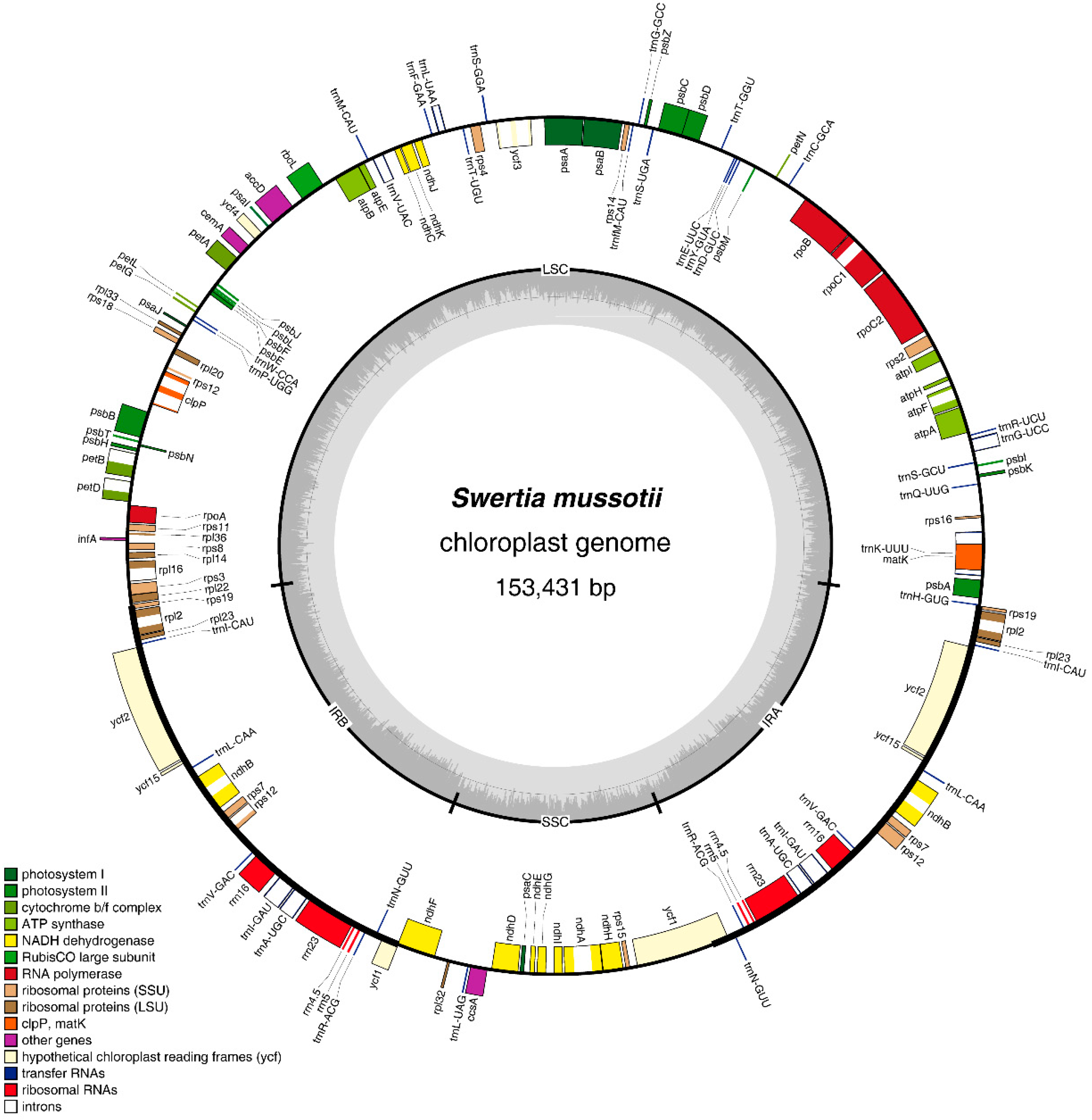

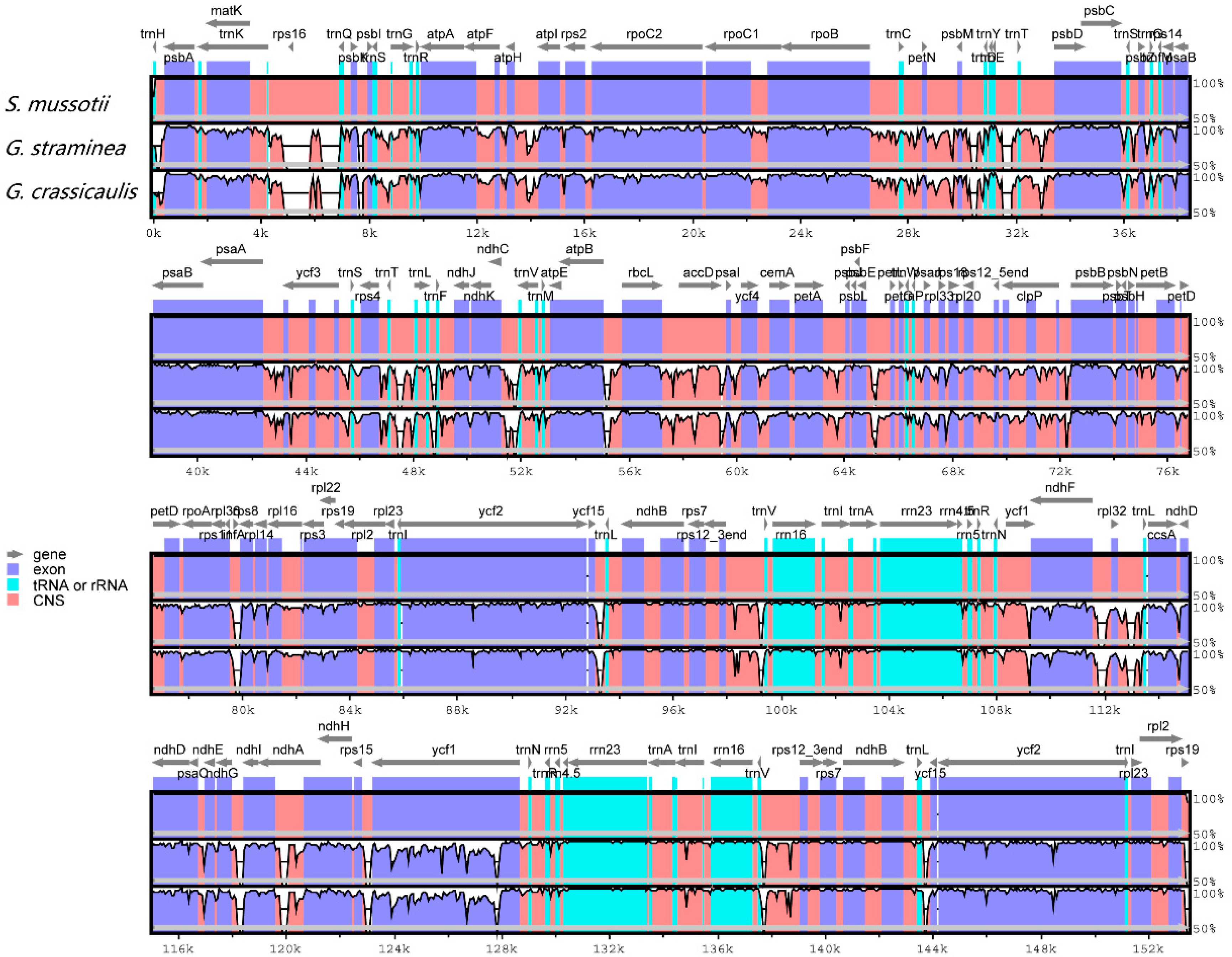
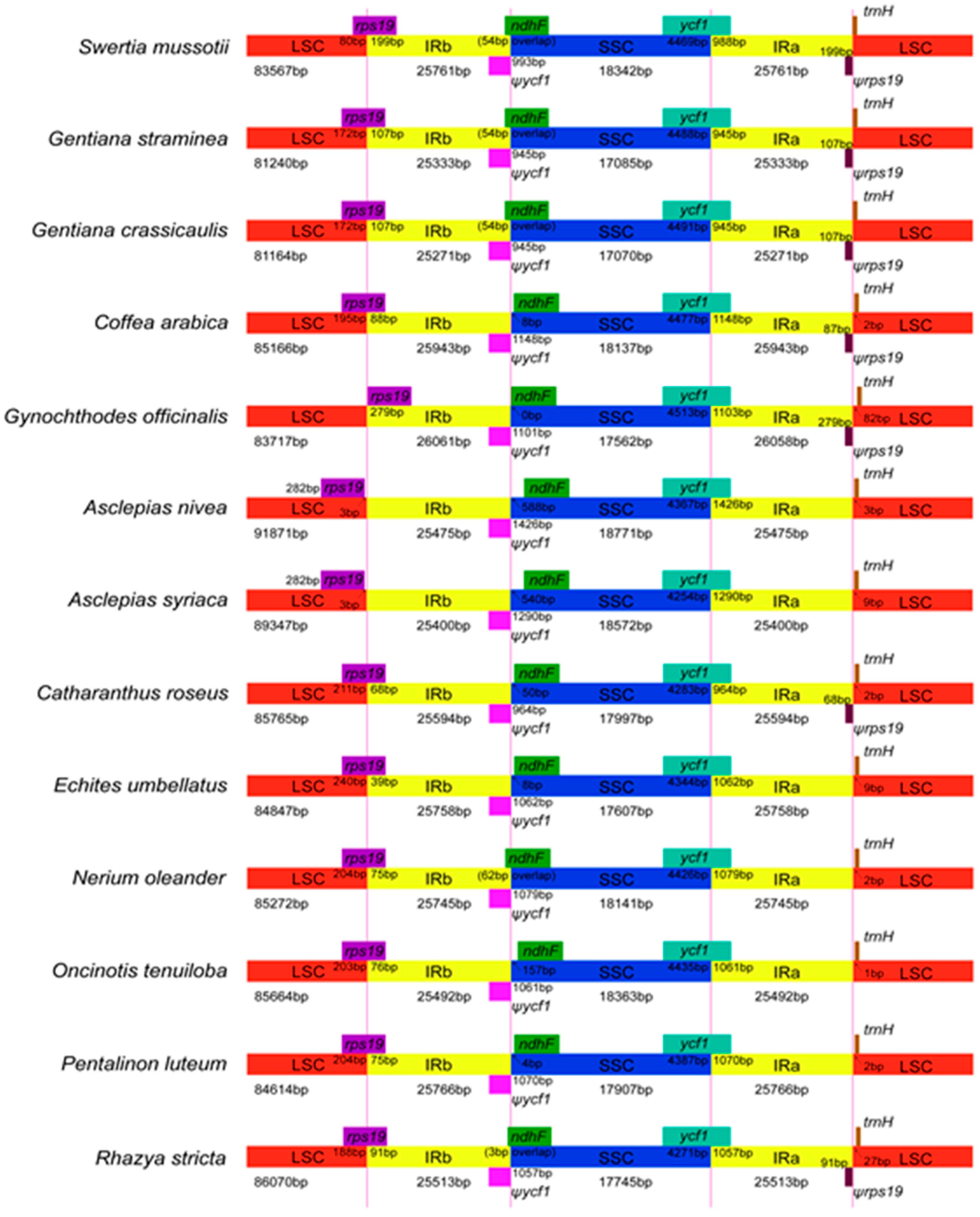
| Region | T (U) (%) | C (%) | A (%) | G (%) | Length (bp) | |
|---|---|---|---|---|---|---|
| LSC | 32.6 | 18.5 | 31.2 | 17.7 | 83,567 | |
| SSC | 34.1 | 16.3 | 34.0 | 15.6 | 18,342 | |
| IRa | 28.3 | 22.5 | 28.2 | 21.0 | 25,761 | |
| IRb | 28.2 | 21.0 | 28.3 | 22.5 | 25,761 | |
| Total | 31.3 | 19.3 | 30.5 | 18.8 | 153,431 | |
| CDS | 31.3 | 18.1 | 30.2 | 20.4 | 77,193 | |
| 1st position | 23.9 | 19.2 | 30.4 | 26.5 | 25731 | |
| 2nd position | 32.6 | 20.6 | 28.7 | 18.1 | 25731 | |
| 3rd position | 37.2 | 14.6 | 31.6 | 16.6 | 25731 |
| No. | Group of Genes | Gene Names |
|---|---|---|
| 1 | Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| 2 | Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| 3 | Cytochrome b/f complex | petA, petB *, petD *, petG, petL, petN |
| 4 | ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI |
| 5 | NADH dehydrogenase | ndhA *, ndhB * (×2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| 6 | RuBisCO large subunit | rbcL |
| 7 | RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 |
| 8 | Ribosomal proteins (SSU) | rps2, rps3, rps4, rps7 (×2), rps8, rps11, rps12 ** (×2), rps14, rps15, rps18, rps19 |
| 9 | Ribosomal proteins (LSU) | rpl2 * (×2), rpl14, rpl16 *, rpl20, rpl22, rpl23 (×2), rpl32, rpl33, rpl36 |
| 10 | Other genes | clpP *, matK, ccsA, cemA |
| 11 | Proteins of unknown function | ycf1, ycf2 (×2), ycf3 **, ycf4, ycf15 (×2) |
| 12 | Transfer RNAs | 37 tRNAs (6 contain one intron each, 7 in the IRs) |
| 13 | Ribosomal RNAs | rrn4.5 (×2), rrn5 (×2), rrn16 (×2), rrn23 (×2) |
| Gene | Location | Exon I (bp) | Intron I (bp) | Exon II (bp) | Intron II (bp) | Exon III (bp) |
|---|---|---|---|---|---|---|
| atpF | LSC | 161 | 700 | 403 | ||
| clpP | LSC | 71 | 784 | 292 | 680 | 228 |
| ndhA | SSC | 561 | 1117 | 540 | ||
| ndhB | IR | 777 | 683 | 756 | ||
| petB | LSC | 6 | 727 | 642 | ||
| petD | LSC | 8 | 678 | 475 | ||
| rpl16 | LSC | 9 | 764 | 399 | ||
| rpl2 | IR | 393 | 657 | 435 | ||
| rpoC1 | LSC | 435 | 734 | 1623 | ||
| rps12 * | LSC | 114 | - | 232 | 535 | 26 |
| trnA-UGC | IR | 38 | 824 | 35 | ||
| trnG-UCC | LSC | 23 | 689 | 48 | ||
| trnI-GAU | IR | 37 | 950 | 35 | ||
| trnK-UUU | LSC | 37 | 2496 | 35 | ||
| trnL-UAA | LSC | 37 | 374 | 50 | ||
| trnV-UAC | LSC | 38 | 601 | 37 | ||
| ycf3 | LSC | 126 | 745 | 228 | 770 | 153 |
| Amino Acid | Codon | No. | RSCU | tRNA | Amino Acid | Codon | No. | RSCU | tRNA |
|---|---|---|---|---|---|---|---|---|---|
| Phe | UUU | 981 | 1.32 | Tyr | UAU | 738 | 1.59 | ||
| Phe | UUC | 507 | 0.68 | trnF-GAA | Tyr | UAC | 189 | 0.41 | trnY-GUA |
| Leu | UUA | 847 | 1.84 | trnL-UAA | Stop | UAA | 49 | 1.75 | |
| Leu | UUG | 551 | 1.19 | trnL-CAA | Stop | UAG | 21 | 0.75 | |
| Leu | CUU | 610 | 1.32 | His | CAU | 467 | 1.5 | ||
| Leu | CUC | 187 | 0.41 | His | CAC | 157 | 0.5 | trnH-GUG | |
| Leu | CUA | 392 | 0.85 | trnL-UAG | Gln | CAA | 698 | 1.54 | trnQ-UUG |
| Leu | CUG | 182 | 0.39 | Gln | CAG | 207 | 0.46 | ||
| Ile | AUU | 1047 | 1.47 | Asn | AAU | 920 | 1.5 | ||
| Ile | AUC | 435 | 0.61 | trnI-GAU | Asn | AAC | 303 | 0.5 | trnN-GUU |
| Ile | AUA | 660 | 0.92 | trnI-CAU | Lys | AAA | 988 | 1.45 | trnK-UUU |
| Met | AUG | 582 | 1 | trn(f)M-CAU | Lys | AAG | 377 | 0.55 | |
| Val | GUU | 510 | 1.45 | Asp | GAU | 802 | 1.61 | ||
| Val | GUC | 187 | 0.53 | trnV-GAC | Asp | GAC | 194 | 0.39 | trnD-GUC |
| Val | GUA | 528 | 1.5 | trnV-UAC | Glu | GAA | 923 | 1.45 | trnE-UUC |
| Val | GUG | 185 | 0.52 | Glu | GAG | 350 | 0.55 | ||
| Ser | UCU | 540 | 1.6 | Cys | UGU | 221 | 1.52 | ||
| Ser | UCC | 352 | 1.04 | trnS-GGA | Cys | UGC | 70 | 0.48 | trnC-GCA |
| Ser | UCA | 382 | 1.13 | trnS-UGA | Stop | UGA | 14 | 0.5 | |
| Ser | UCG | 221 | 0.66 | Trp | UGG | 461 | 1 | trnW-CCA | |
| Pro | CCU | 395 | 1.42 | Arg | CGU | 339 | 1.28 | trnR-ACG | |
| Pro | CCC | 234 | 0.84 | Arg | CGC | 102 | 0.39 | ||
| Pro | CCA | 318 | 1.14 | trnP-UGG | Arg | CGA | 356 | 1.35 | |
| Pro | CCG | 166 | 0.6 | Arg | CGG | 139 | 0.53 | ||
| Thr | ACU | 485 | 1.46 | Arg | AGA | 385 | 1.14 | trnR-UCU | |
| Thr | ACC | 272 | 0.82 | trnT-GGU | Arg | AGG | 143 | 0.42 | |
| Thr | ACA | 413 | 1.24 | trnT-UGU | Ser | AGU | 477 | 1.81 | |
| Thr | ACG | 157 | 0.47 | Ser | AGC | 171 | 0.65 | trnS-GCU | |
| Ala | GCU | 614 | 1.8 | Gly | GGU | 534 | 1.2 | ||
| Ala | GCC | 225 | 0.66 | Gly | GGC | 198 | 0.45 | trnG-GCC | |
| Ala | GCA | 378 | 1.11 | trnA-UGC | Gly | GGA | 705 | 1.59 | trnG-UCC |
| Ala | GCG | 148 | 0.43 | Gly | GGG | 342 | 0.77 |
| No. | Size (bp) | Type | Repeat 1 Start | Repeat 1 Location | Repeat 2 Start | Repeat 2 Location | Region |
|---|---|---|---|---|---|---|---|
| 1 | 39 | F | 97971 | IGS (rps12, trnV-GAC) | 119586 | ndhA (intron) | IRb, SSC |
| 2 | 38 | F | 44377 | ycf3 (intron 1) | 97971 | IGS (rps12, trnV-GAC) | LSC, IRb |
| 3 | 38 | F | 44377 | ycf3 (intron 1) | 119586 | ndhA (intron) | LSC, SSC |
| 4 | 37 | F | 216 | IGS (trnH-GUG, psbA) | 244 | IGS (trnH-GUG, psbA) | LSC |
| 5 | 38 | F | 39302 | psaB (CDS) | 41526 | psaA (CDS) | LSC |
| 6 | 32 | F | 8154 | trnS-GCU | 36099 | trnS-UGA | LSC |
| 7 | 30 | F | 7704 | IGS (psbK, psbI) | 28958 | IGS (petN, psbM) | LSC |
| 8 | 30 | F | 9536 | trnG-UCC | 37013 | trnG-GCC | LSC |
| 9 | 30 | F | 38751 | psaB (CDS) | 40966 | psaA (CDS) | LSC |
| 10 | 30 | F | 58479 | ΨaccD | 58512 | ΨaccD | LSC |
| 11 | 30 | F | 75545 | petB (intron) | 138996 | IGS (trnV-GAC, rps12) | LSC, IRa |
| 12 | 51 | P | 114672 | IGS (ccsA, ndhD) | 114675 | IGS (ccsA, ndhD) | SSC |
| 13 | 39 | P | 119586 | ndhA (intron) | 138988 | IGS (trnV-GAC, rps12) | SSC, IRa |
| 14 | 38 | P | 44377 | ycf3 (intron 1) | 138989 | IGS (trnV-GAC, rps12) | LSC, IRa |
| 15 | 32 | P | 8154 | trnS-GCU | 45722 | trnS-GGA | LSC |
| 16 | 32 | P | 36096 | trnS-UGA | 45725 | trnS-GGA | LSC |
| 17 | 30 | P | 44378 | ycf3 (intron 1) | 75545 | petB (intron) | LSC |
| 18 | 30 | P | 75545 | petB (intron) | 119587 | ndhA (intron) | LSC, SSC |
| 19 | 30 | P | 75545 | petB (intron) | 97972 | IGS (rps12, trnV-GAC) | LSC, IRb |
| 20 | 31 | R | 42871 | IGS (psaA, ycf3) | 42875 | IGS (psaA, ycf3) | LSC |
| Unit | Length | No. | SSR Start | Region |
|---|---|---|---|---|
| A | 16 | 1 | 68265 | LSC |
| 13 | 3 | 45315 | LSC | |
| 80949 | LSC | |||
| 114240 | SSC | |||
| 11 | 1 | 22183 | LSC | |
| 10 | 7 | 8410 | LSC | |
| 12227 | LSC | |||
| 57572 | LSC | |||
| 63341 | LSC | |||
| 71135 | LSC | |||
| 77632 | LSC | |||
| 122496 | SSC | |||
| C | 11 | 1 | 60812 | LSC |
| T | 14 | 1 | 60823 | LSC |
| 13 | 2 | 118296 | SSC | |
| 118428 | SSC | |||
| 12 | 4 | 5757 | LSC | |
| 32886 | LSC | |||
| 35984 | LSC | |||
| 112141 | SSC | |||
| 11 | 3 | 1828 | LSC | |
| 124064 | SSC | |||
| 125507 | SSC | |||
| 10 | 7 | 92 | LSC | |
| 7909 | LSC | |||
| 54930 | LSC | |||
| 66001 | LSC | |||
| 120007 | LSC | |||
| 125752 | LSC | |||
| 127189 | SSC | |||
| AT | 10 | 1 | 47791 | LSC |
| TA | 10 | 1 | 47617 | LSC |
| ATT | 15 | 1 | 119656 | LSC |
| TTA | 12 | 1 | 127046 | LSC |
| TTC | 12 | 1 | 35761 | LSC |
| TTG | 12 | 1 | 111418 | SSC |
| AATT | 16 | 1 | 29843 | LSC |
| ATTT | 12 | 1 | 116917 | SSC |
| CATA | 12 | 1 | 151279 | IRa |
| TATG | 12 | 1 | 85709 | IRb |
| TATT | 12 | 1 | 116932 | SSC |
| TGTC | 12 | 1 | 30554 | LSC |
| TAATA | 15 | 1 | 116944 | SSC |
| TATTG | 15 | 1 | 62151 | LSC |
| CCTTTA | 18 | 1 | 37196 | LSC |
| Taxon | Genome Size (bp) | AT (%) | SSR Type | CDS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Di | Tri | Tetra | Penta | Hexa | Total | % a | No. b | % c | |||
| Swertia mussotii | 153,431 | 62 | 30 | 2 | 4 | 6 | 2 | 1 | 45 | 58 | 10 | 22 |
| Gentiana straminea | 148,991 | 62 | 27 | 3 | 2 | 7 | 0 | 0 | 39 | 61 | 10 | 26 |
| Gentiana crassicaulis | 148,776 | 62 | 27 | 4 | 2 | 7 | 0 | 1 | 41 | 61 | 10 | 24 |
| Coffea arabica | 155,189 | 63 | 31 | 5 | 3 | 4 | 0 | 0 | 43 | 59 | 8 | 19 |
| Catharanthus roseus | 154,950 | 62 | 33 | 6 | 7 | 9 | 1 | 0 | 56 | 59 | 5 | 9 |
| Asclepias nivea | 161,592 | 62 | 47 | 15 | 6 | 23 | 3 | 4 | 98 | 56 | 17 | 17 |
| Asclepias syriaca | 158,719 | 62 | 56 | 13 | 7 | 16 | 2 | 7 | 101 | 55 | 17 | 17 |
| Rhazya stricta | 154,841 | 62 | 33 | 5 | 9 | 12 | 3 | 0 | 62 | 58 | 6 | 10 |
| Echites umbellatus | 153,970 | 62 | 47 | 9 | 7 | 7 | 1 | 1 | 72 | 59 | 7 | 10 |
| Nerium oleander | 154,903 | 62 | 42 | 6 | 3 | 8 | 2 | 0 | 61 | 59 | 10 | 16 |
| Oncinotis tenuiloba | 155,011 | 62 | 41 | 7 | 4 | 9 | 2 | 0 | 63 | 58 | 5 | 8 |
| Pentalinon luteum | 154,053 | 62 | 34 | 5 | 2 | 5 | 3 | 0 | 49 | 57 | 6 | 12 |
| Gynochthodes officinalis | 153,398 | 62 | 26 | 4 | 7 | 3 | 4 | 1 | 45 | 60 | 7 | 16 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, B.; Li, X.; Qian, J.; Wang, L.; Ma, L.; Tian, X.; Wang, Y. The Complete Chloroplast Genome Sequence of the Medicinal Plant Swertia mussotii Using the PacBio RS II Platform. Molecules 2016, 21, 1029. https://doi.org/10.3390/molecules21081029
Xiang B, Li X, Qian J, Wang L, Ma L, Tian X, Wang Y. The Complete Chloroplast Genome Sequence of the Medicinal Plant Swertia mussotii Using the PacBio RS II Platform. Molecules. 2016; 21(8):1029. https://doi.org/10.3390/molecules21081029
Chicago/Turabian StyleXiang, Beibei, Xiaoxue Li, Jun Qian, Lizhi Wang, Lin Ma, Xiaoxuan Tian, and Yong Wang. 2016. "The Complete Chloroplast Genome Sequence of the Medicinal Plant Swertia mussotii Using the PacBio RS II Platform" Molecules 21, no. 8: 1029. https://doi.org/10.3390/molecules21081029






