In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums
Abstract
:1. Introduction
2. Results
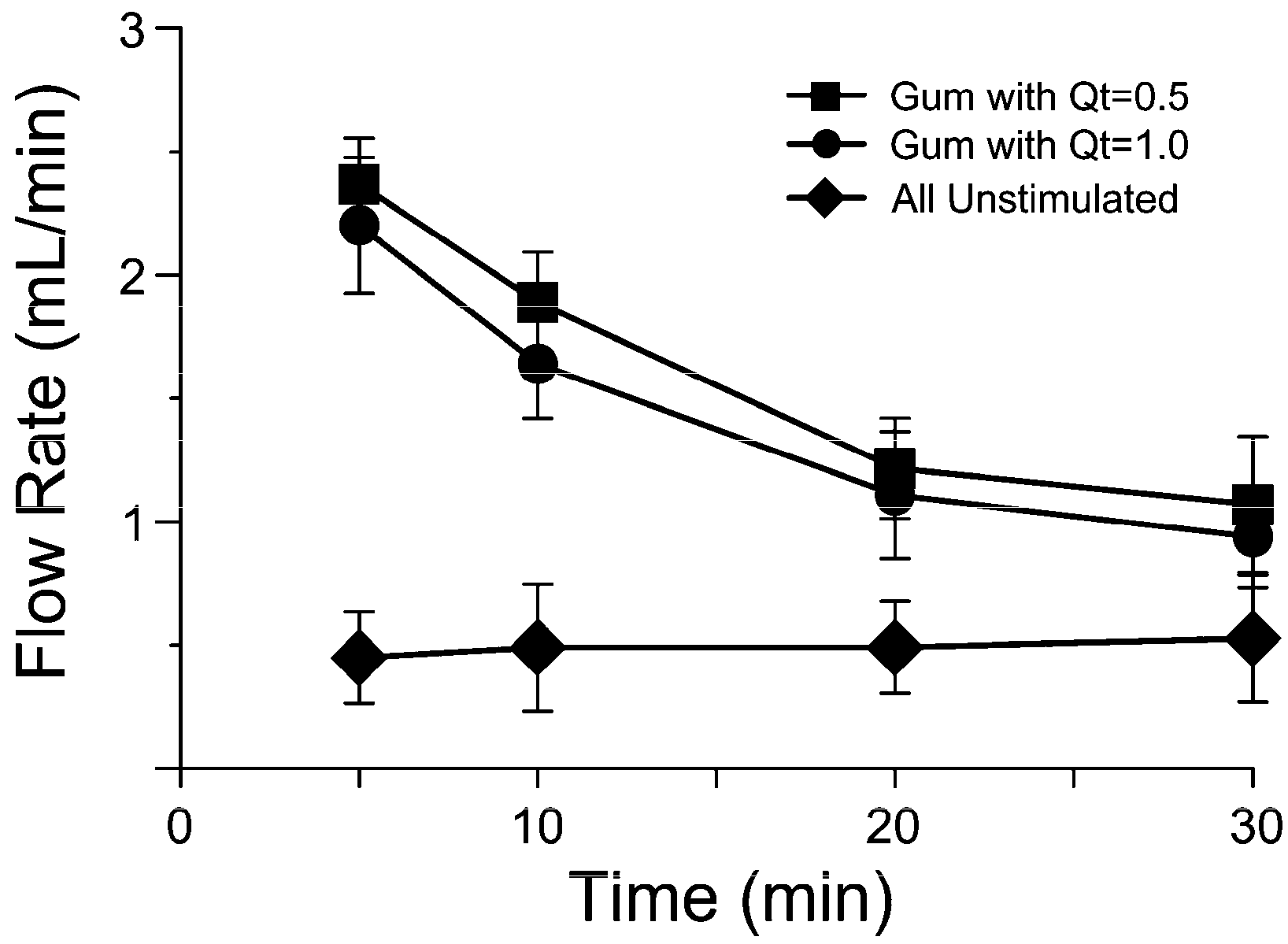
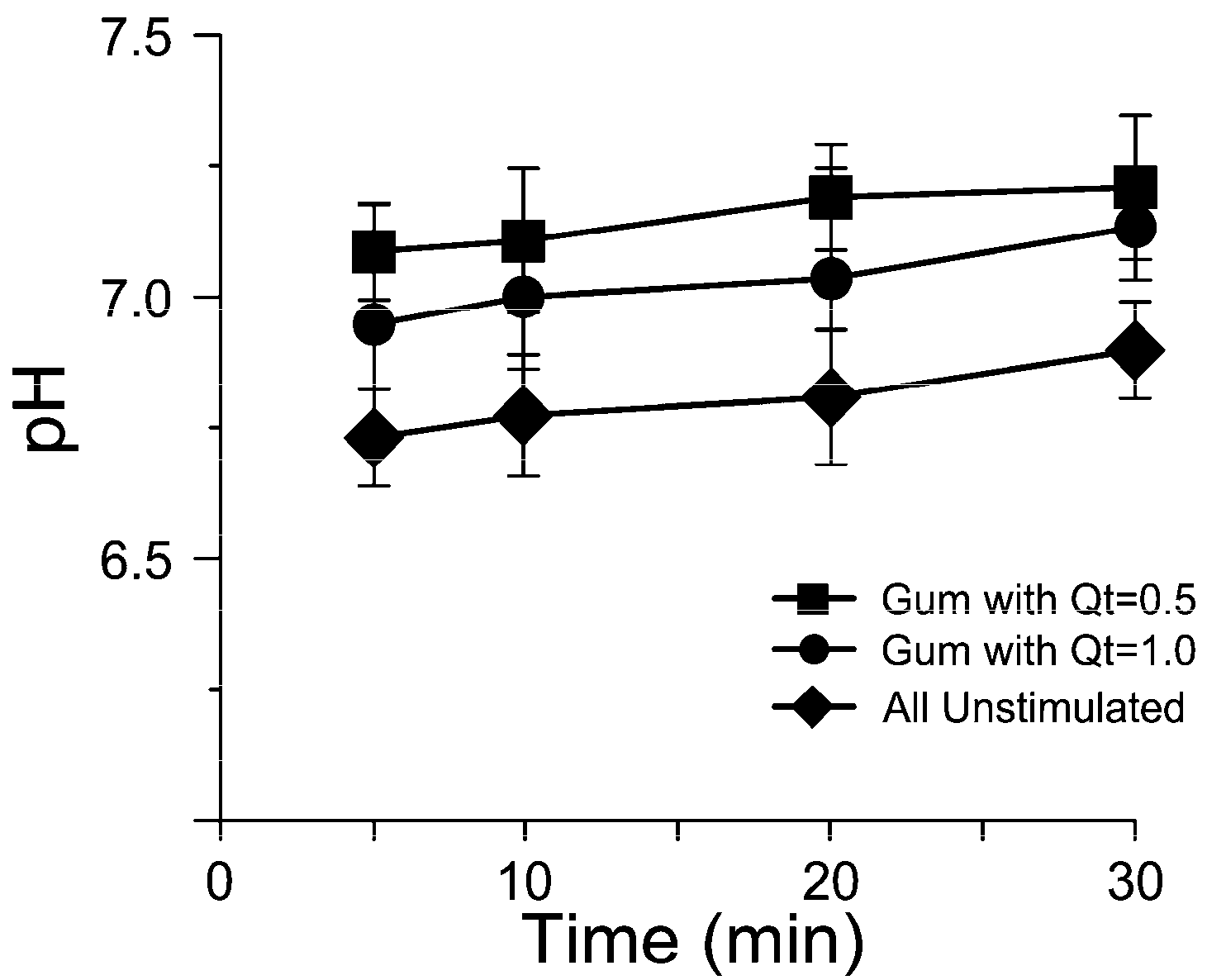
2.1. Salivary Flow Rate and pH Analysis
2.2. Quercetin Content
2.3. In Vivo Assessment of Antimicrobial Activity
3. Discussion
3.1. In Vivo Release Kinetics Evaluation
3.2. In Vivo Antimicrobial Activity of Experimental Gums
4. Materials and Methods
4.1. Chewing Gum Formulation
4.2. First in Vivo Trial: Release Capability of Enriched Chewing Gums
4.3. Determination of Quercetin Content in Saliva
4.4. Second in Vivo Trial: Antimicrobial Activity of Enriched Chewing Gums
- (1)
- Experimental (Group A): An experimental chewing gum containing 10 mg of quercetin was used. This concentration of Qt was preferred for its highest release in oral saliva during the first minutes of chewing in the first in vivo trial.
- (2)
- Placebo (Group B): The placebo chewing gums were prepared as described above, but without adding quercetin.
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Larsen, M.J.; Pearce, E.I. Saturation of human saliva with respect to calcium salts. Arch. Oral Biol. 2003, 48, 317–322. [Google Scholar] [CrossRef]
- Aiuchi, H.; Kitasako, Y.; Fukuda, Y.; Nakashima, S.; Burrow, M.F.; Tagami, J. Relationship between quantitative assessments of salivary buffering capacity and ion activity product for hydroxyapatite in relation to cariogenic potential. Aust. Dent. J. 2008, 53, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Flink, H. Studies on the prevalence of reduced salivary flow rate in relation to general health and dental caries, and effect of iron supplementation. Swed. Dent. J. Suppl. 2007, 192, 3–50. [Google Scholar] [PubMed]
- Mostafavi, S.A.; Varshosaz, J.; Arabian, S. Formulation development and evaluation of metformin chewing gum with bitter taste masking. Adv. Biomed. Res. 2014, 3, 92. [Google Scholar] [CrossRef] [PubMed]
- Faraj, J.A.; Dorati, R.; Schoubben, A.; Worthen, D.; Selmin, F.; Capan, Y.; Leung, K.; De Luca, P.P. Development of a peptide-containing chewing gum as a sustained release antiplaque antimicrobial delivery system. AAPS Pharm. Sci. Technol. 2007, 8, E177–E185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Do not put too much value on conventional medicines. J. Ethnopharmacol. 2005, 100, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; de Natale, A.; Pollio, A. Anti-Cariogenic effects of polyphenols from plant stimulant beverages (cocoa, coffee, tea). Fitoterapia 2009, 80, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Balz, F.; Jane, V.H. Antioxidant of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar]
- Luczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Maru, G.B. Inhibitory effect(s) of polymeric black tea polyphenol fractions on the formation of [3H]-B(a)P-derived DNA adducts. J. Agric. Food Chem. 2004, 52, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Tian, S.; Hong, J.; Hou, Z.; Ryu, J.H.; Stark, R.E.; Rosen, R.T.; Huang, M.T.; Yang, C.S.; Ho, C.T. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Otake, S.; Makimura, M.; Kuroki, T.; Nishihara, Y.; Hirasawa, M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991, 25, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. Biomed. Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.; Young, D. The use of topical fluoride to prevent or reverse dental caries. Spec. Care Dent. 2003, 23, 177–179. [Google Scholar] [CrossRef]
- Simonsen, R.J. Pit and fissure sealant: Review of the literature. Pediatr. Dent. 2002, 24, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Cantile, T.; Sangianantoni, G.; Ingenito, A. Effectiveness of a motivation method on the oral hygiene of children. Eur. J. Paediatr. Dent. 2008, 9, 183–187. [Google Scholar] [PubMed]
- Bourgeois, D.M.; Llodra, J.C. Global burden of dental condition among children in nine countries participating in an international oral health promotion programme, 2012–2013. Int. Dent. J. 2014, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Scaravilli, M.S.; Ingenito, A. Dental and periodontal health status in Campanian children and relation between caries experience and socio-economic behavioural factors. Eur. J. Paediatr. Dent. 2006, 7, 174–178. [Google Scholar] [PubMed]
- Ferrazzano, G.F.; Orlando, S.; Sangianantoni, G.; Cantile, T.; Ingenito, A. Dental and periodontal health status in children affected by cystic fibrosis in a southern Italian region. Eur. J. Paediatr. Dent. 2009, 10, 65–68. [Google Scholar] [PubMed]
- Seethalakshmi, C.; Reddy, R.C.; Asifa, N.; Prabhu, S. Correlation of Salivary pH, Incidence of Dental Caries and Periodontal Status in Diabetes Mellitus Patients: A Cross-Sectional Study. J. Clin. Diagn. Res. 2016, 10, ZC12–ZC14. [Google Scholar] [CrossRef] [PubMed]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- González-Segovia, R.; Quintanar, J.L.; Salinas, E.; Ceballos-Salazar, R.; Aviles-Jiménez, F.; Torres-López, J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J. Gastroenterol. 2008, 43, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharm. Res. 2008, 31, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and mode of action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, M.; Nazzaro, M.; Volpe, M.G. Release kinetics of calcium and quercetin from chewing gum as a novel antiplaque and antimicrobial device. Curr. Drug Deliv. 2013, 10, 261–267. [Google Scholar] [PubMed]
- Emamieh, S.; Khaterizadeh, Y.; Goudarzi, H.; Ghasemi, A.; Baghban, A.A.; Torabzadeh, H. The effect of two types chewing gum containing casein phosphopeptide-amorphous calcium phosphate and xylitol on salivary Streptococcus mutans. J. Conserv. Dent. 2015, 18, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Roberto, L.; Amato, I.; Cantile, T.; Sangianantoni, G.; Ingenito, A. Antimicrobial properties of green tea extract against cariogenic microflora: An in vivo study. J. Med. Food 2011, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and scoring of antimicrobial and biological activities of italian vulnerary plants against major oral pathogenic bacteria. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, P.; Cai, F.; Nowicki, A.; Vincent, J.; Reynolds, E.C. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2001, 80, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Rhythms in salivary flow rate and composition. Int. J. Chronobiol. 1974, 2, 253–279. [Google Scholar] [PubMed]
- Cross, K.J.; Huq, N.L.; Palamara, J.E.; Perich, J.W.; Reynolds, E.C. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J. Biol. Chem. 2005, 280, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Woisky, R.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.



| Time | Percentage of Samples Containing High S. mutans Density | |
|---|---|---|
| Group A (Test) | Group B (Control) | |
| T0 | 80.6% | 80.6% |
| T1 | 50.1% | 86.1% |
| T2 | 36.1% | 77.8% |
| Ingredients | Qt0.5 (%) | Qt1.0 (%) |
|---|---|---|
| Gum-base | 78.5 | 78.0 |
| Quercetin | 0.5 | 1.0 |
| Flavoring agents and sweeteners | 20.0 | 20.0 |
| Co-adjuvant excipients | 1.0 | 1.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Di Stasio, M.; Volpe, M.G. In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums. Molecules 2016, 21, 1008. https://doi.org/10.3390/molecules21081008
Ferrazzano GF, Cantile T, Coda M, Alcidi B, Sangianantoni G, Ingenito A, Di Stasio M, Volpe MG. In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums. Molecules. 2016; 21(8):1008. https://doi.org/10.3390/molecules21081008
Chicago/Turabian StyleFerrazzano, Gianmaria Fabrizio, Tiziana Cantile, Marco Coda, Brunella Alcidi, Giancarla Sangianantoni, Aniello Ingenito, Michele Di Stasio, and Maria Grazia Volpe. 2016. "In Vivo Release Kinetics and Antibacterial Activity of Novel Polyphenols-Enriched Chewing Gums" Molecules 21, no. 8: 1008. https://doi.org/10.3390/molecules21081008







