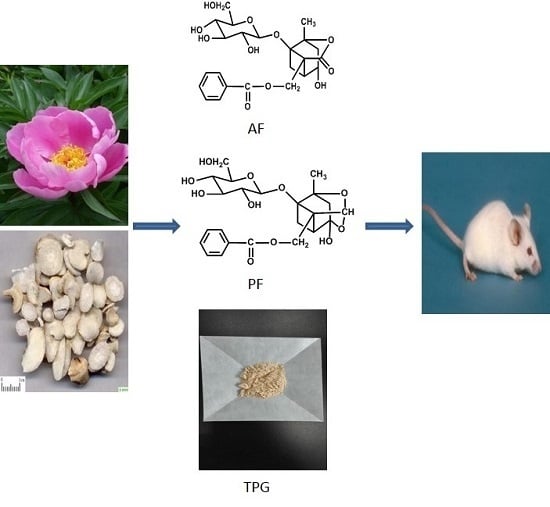
Enhancement of Exposure and Reduction of Elimination for Paeoniflorin or Albiflorin via Co-Administration with Total Peony Glucosides and Hypoxic Pharmacokinetics Comparison
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
2.2.1. Specificity and Linearity
2.2.2. Precision and Accuracy
2.2.3. Recovery and Matrix Effect
2.2.4. Stability
2.3. Pharmacokinetic Interactions
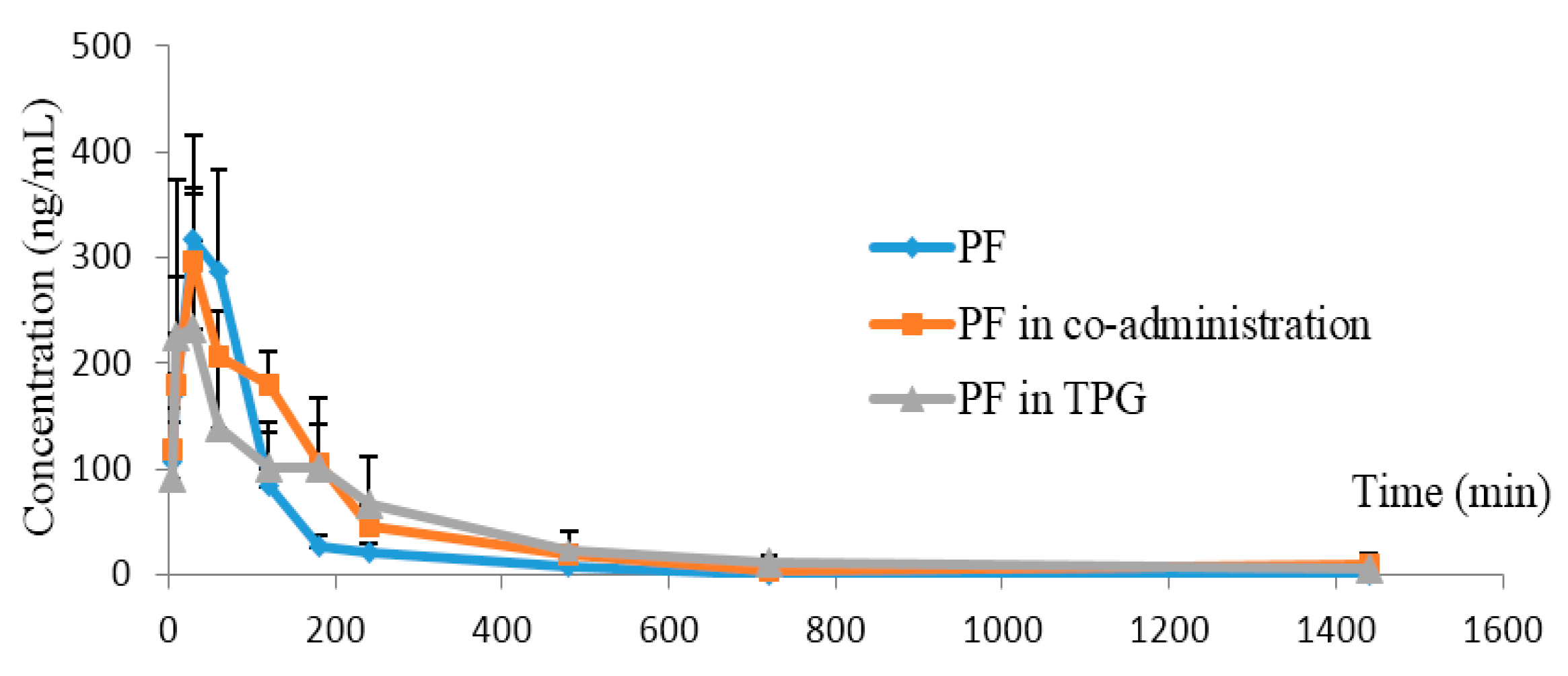
2.3.1. AF or Other Ingredients in TPG Enhanced Exposure and Reduced Elimination of PF in Rats
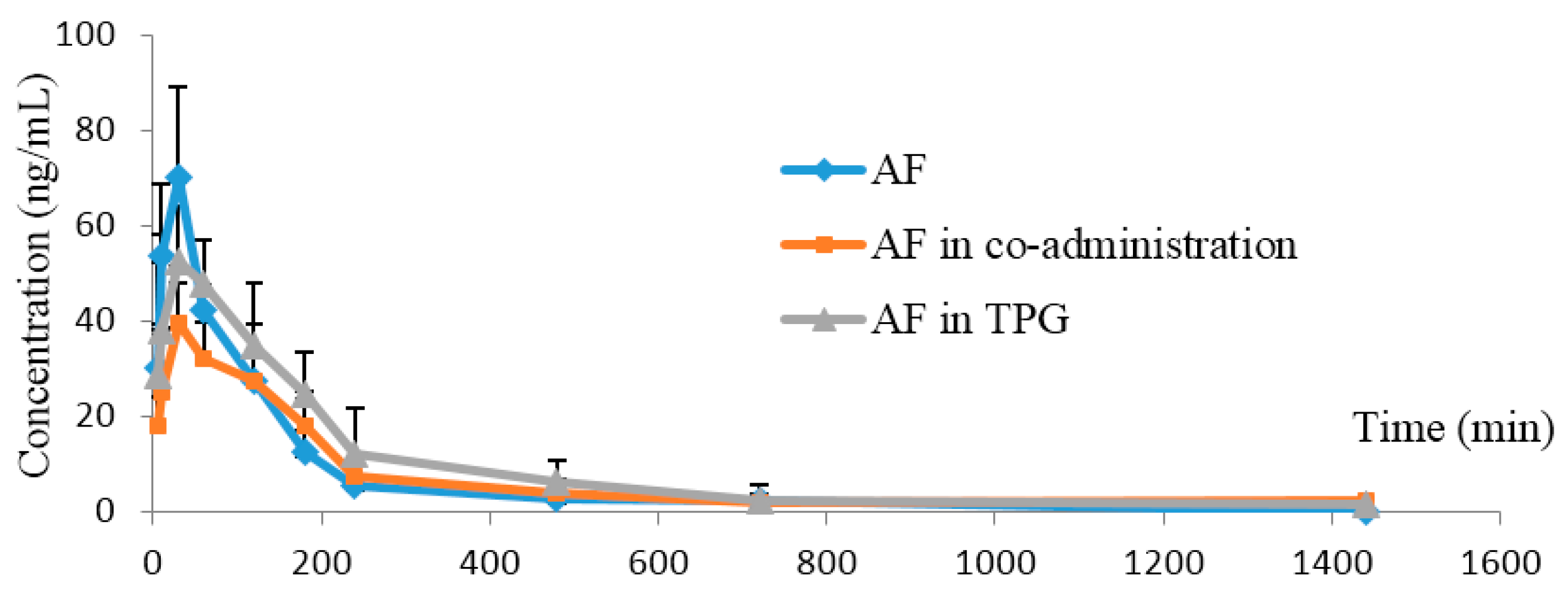
2.3.2. Other Ingredients in TPG Reduced Elimination of AF in Rats
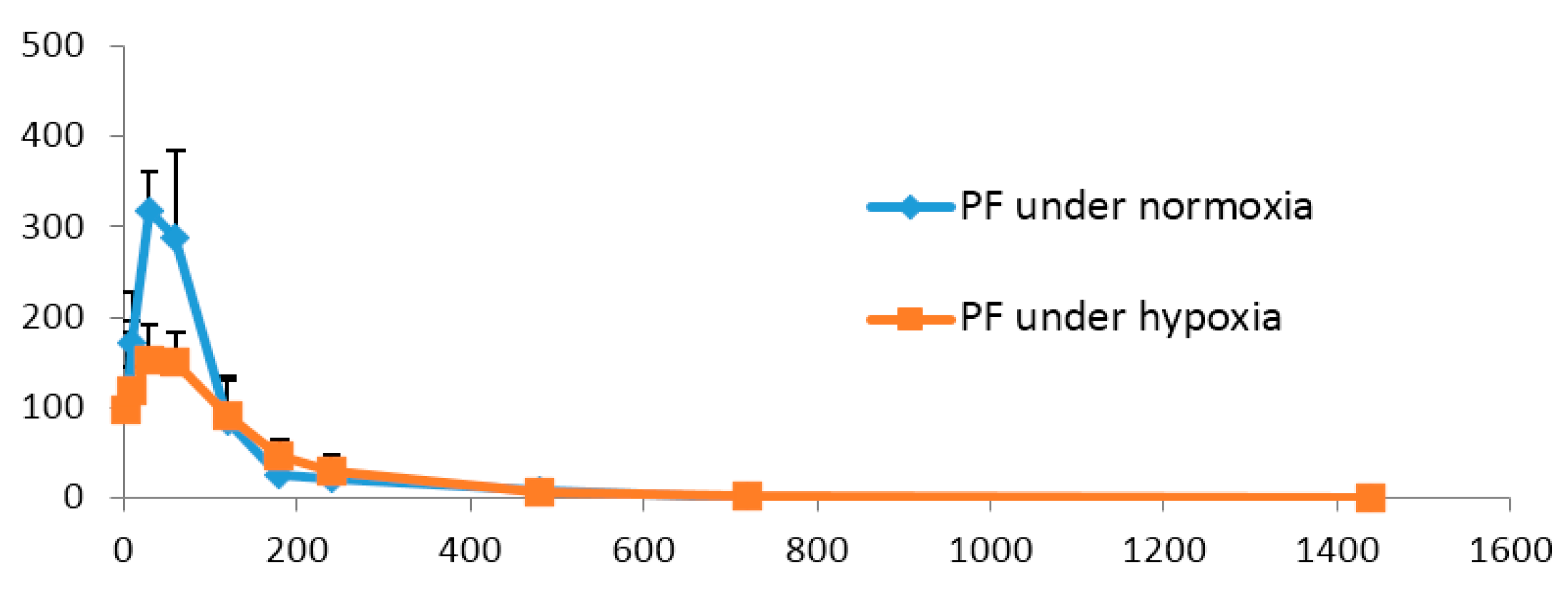
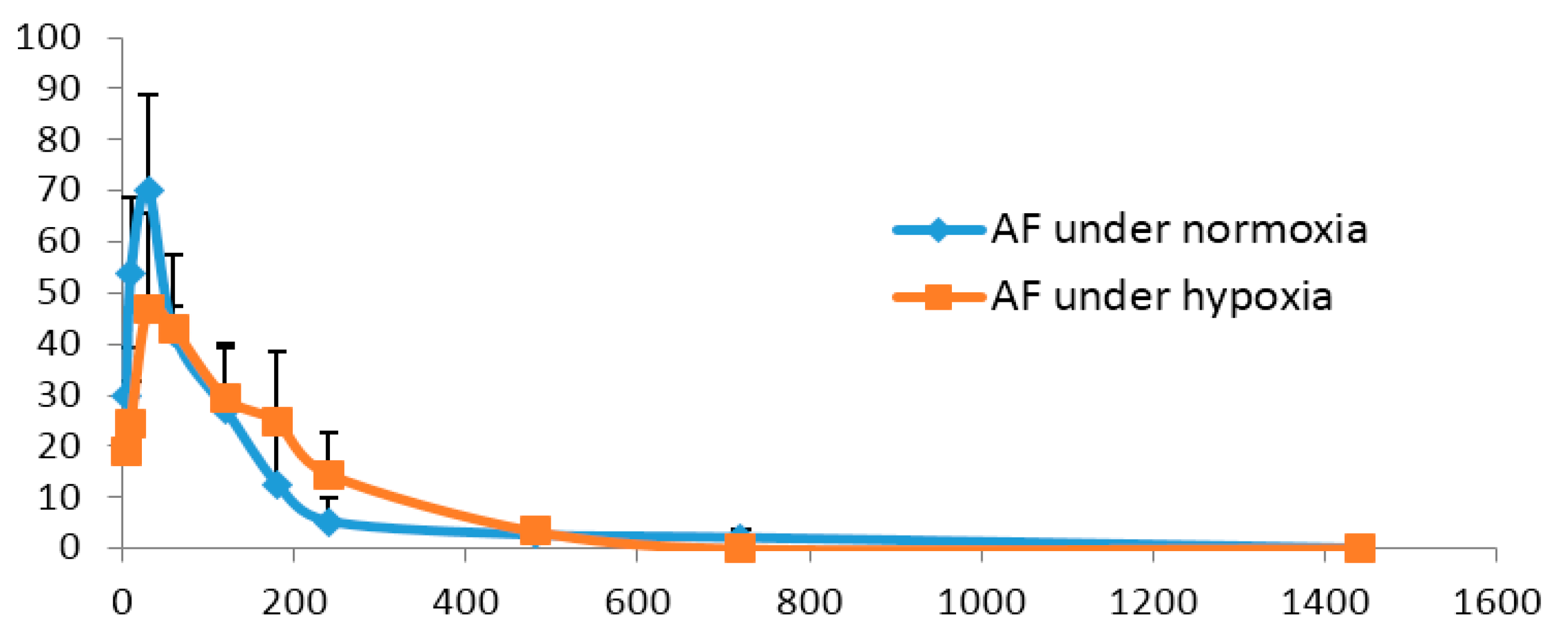
2.4. Pharmacokinetics Comparisonunder Normoxic and Hypoxic Conditions
3. Experimental Section
3.1. Materials and Reagents
3.2. Animals
3.3. UPLC-MS/MS Instruments and Conditions
3.4. Preparation of Standard Stock Solutions and Quality Control Samples
3.5. Plasma Sample Preparation
3.6. UPLC-MS/MS Method Validation
3.6.1. Specificity, Linearity and LLOQ
3.6.2. Accuracy and Precision
3.6.3. Extraction Recovery and Matrix Effect
3.6.4. Stability
3.7. in vivo Pharmacokinetic Experiment
3.8. Data Processing and Comparison
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, C.; Liu, M.; Shi, X.; Yang, W.; Kong, D.; Duan, K.; Wang, Q. Pharmacokinetic properties of paeoniflorin, albiflorin and oxypaeoniflorin after oral gavage of extracts of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. J. Ethnopharmacol. 2010, 130, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Kim, C.Y.; Kim, H.J.; Park, J.H.; Ahn, M.J. Differences in the chemical profiles and biological activities of Paeonialactiflora and Paeoniaobovata. J. Med. Food. 2014, 18, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, S.; Guo, Q.S.; Ye, Z.L.; Zhang, W.S. Determination of chemical components of Paeoniae Radix Alba decoction pieces and its quality evaluation. Zhongguo Zhong Yao Za Zhi 2015, 40, 484–489. [Google Scholar] [PubMed]
- Wang, H.; Wei, W.; Wang, N.P.; Wu, C.Y.; Yan, S.X.; Yue, L.; Zhang, L.L.; Xu, S.Y. Effects of Total peony glucosides on immunological hepatic fibrosis in rats. World J. Gastroenterol. 2005, 11, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonialactiflora Pall., a traditional Chinese herbal medicine. Front. Pharmacol. 2011, 2, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Matsumoto, K.; Murakami, Y.; Watanabe, H. Ameliorative effects of paeoniflorin, a major constituent of peony root, on adenosine A receptor-mediated impairment of passive avoidance performance and long-term potentiation in the hippocampus. Biol. Pharm. Bull. 2001, 24, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Xiao, L.; Cai, X.; Zeng, R.; Zhu, X.Z. Involvement of multitargets in paeoniflorin-induced preconditioning. J. Pharmacol. Exp. Ther. 2006, 319, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Ip, S.P.; Tsai, S.H.; Che, C.T. Antidepressant-like effect of peony glycosides in mice. J. Ethnopharmacol. 2008, 119, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Du, P.; Wang, J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Mol. Med. Rep. 2015, 12, 3937–3943. [Google Scholar] [PubMed]
- Jiang, D.; Chen, Y.; Hou, X.; Xu, J.; Mu, X.; Chen, W. Influence of Paeonialactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol. 2011, 137, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tian, Y.P. Protective effects of total glucosides of paeony and the underlying mechanisms in carbon tetrachloride-induced experimental liver injury. Arch. Med. Sci. 2011, 7, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Su, H.C.; Qi, C.; Bo, L.; Gao, J.L.; Jie, S.; Li, G.Y. Antihypertensive effect of radix paeoniae alba in spontaneously hypertensive rats and excessive alcohol intake and high fat diet induced hypertensive rats. Evid. Based. Complement. Altern. Med. 2015, 2015, 731237. [Google Scholar]
- Su, J.; Wang, H.; Ma, C.; Lou, Z.; Liu, C.; Tanver, R.M.; Gao, C.; Nie, R. Anti-diabetic activity of peony seed oil, a new resource food in STZ-induced diabetic mice. Food Funct. 2015, 6, 2930–2938. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.J.; Teng, H.L.; Zhu, M.Y.; Li, C.; Huang, K.L.; Chen, B.; Dai, Y.S.; Wang, J. Paeoniflorin inhibits nucleus pulposus cell apoptosis by regulating the expression of Bcl-2 family proteins and caspase-9 in a rabbit model of intervertebral disc degeneration. Exp. Ther. Med. 2015, 10, 257–262. [Google Scholar] [PubMed]
- Li, P.; Li, Z.H. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Exp. Ther. Med. 2015, 9, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, C.X.; Wang, Y.S.; Li, H.Y.; Luo, J.D.; Zhou, Y. Paeoniflorin inhibits growth of human colorectal carcinoma HT 29 cells in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Poon, C.Y.; Lin, C.Y.; Kwong, D.W.J.; Yung, K.K.L.; Wong, M.S.; Bian, Z.X.; Li, H.W. Inhibition of beta-amyloid aggregation by albiflorin, aloe emodin and neohesperidin and their neuroprotective effect on primary hippocampal cells against beta-amyloid induced toxicity. Curr. Alzheimer Res. 2015, 12, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.M.; Lee, Y.S.; Kim, Y.S. Protective effect of albiflorin against oxidative-stress-mediated toxicity in osteoblast-like MC3T3-E1 cells. Fitoterapia 2013, 89, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.; Wang, P.; Lin, X.; Yang, Y.; Li, D.; Li, H.; Wu, X.; Liu, H. Pharmacokinetic comparisons of different combinations of Shaoyao-Gancao Decoction in rats: simultaneous determination of ten active constituents by HPLC-MS/MS. J. Chromatogr. B 2013, 932, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Li, Z.; Qian, D.; Qian, Y.; Guo, J.; Duan, J.A.; Zhu, M. Simultaneous determination of bioactive components of Radix Angelicae Sinensis-Radix Paeoniae Alba herb couple in rat plasma and tissues by UPLC-MS/MS and its application to pharmacokinetics and tissue distribution. J. Chromatogr. B 2014, 963, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.D.; Liu, Y.E.; Zhang, X.X.; Zhou, Y.W. Effects of paeoniflorin on the production of NO, eNOS and CAMs in cultured human umbilical vein endothelial cells under hypoxia. Chin. J. Pharm. Anal. 2007, 27, 555–557. [Google Scholar]
- Ji, Q.; Yang, L.; Zhou, J.; Lin, R.; Zhang, J.; Lin, Q.; Wang, W.; Zhang, K. Protective effects of paeoniflorin against cobalt chloride-induced apoptosis of endothelial cells via HIF-1alpha pathway. Toxicol. In Vitro 2012, 26, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.M.; Gao, J.W.; Shi, Z.; Huang, P.; Lu, Y.S.; Yao, M.C.; Huang, M. Herb-drug pharmacokinetic interaction between radix astragali and pioglitazone in rats. J. Ethnopharmacol. 2012, 144, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Li, X.R.; Li, Y.H.; Wang, L.J.; Xue, M. Pharmacokinetic interaction between puerarin and edaravone, and effect of borneol on the brain distribution kinetics of puerarin in rats. J. Pharm. Pharmacol. 2010, 62, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.R.; Li, Y.H.; Wang, L.J.; Xue, M. Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic-mass spectrometry and the application to pharmacokinetics in rats. J. Pharm. Biomed. Anal. 2010, 53, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Koh, H.L.; Gao, Y.H.; Gong, Z.Y.; Lee, E.J.D. Herbal bioactivation: The good, the bad and the ugly. Life Sci. 2004, 74, 935–968. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wang, P.; Wang, Y.; Yang, Y.; Li, D.H.; Li, H.F.; Sun, S.Q.; Wu, X.Z. Pharmacokinetic comparisons of two different combinations of Shaoyao-Gancao Decoction in rats: Competing mechanisms between paeoniflorin and glycyrrhetinic acid. J. Ethnopharmacol. 2013, 149, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Zhou, R.; Zhou, F.; Cheng, H.; Xia, B. Total peony glucosides attenuates 2,4,6-trinitrobenzene sulfonic acid/ethanol-induced colitis in rats through adjustment of th1/th2 cytokines polarization. Cell Biochem. Biophys. 2014, 68, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.W.; Liu, S.; Xu, P.X.; Fan, M.; Xue, M. Simultaneous quantification of diazepam and dexamethasone in plasma by high-performance liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetic comparison between normoxic and hypoxic rats. Molecules 2015, 20, 6901–6912. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Li, J.; Wang, R.; Xie, H.; Jia, Z.P. MDR1 will play a key role in pharmacokinetic changes under hypoxia at high altitude and its potential regulatory networks. Drug Metab. Rev. 2015, 47, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
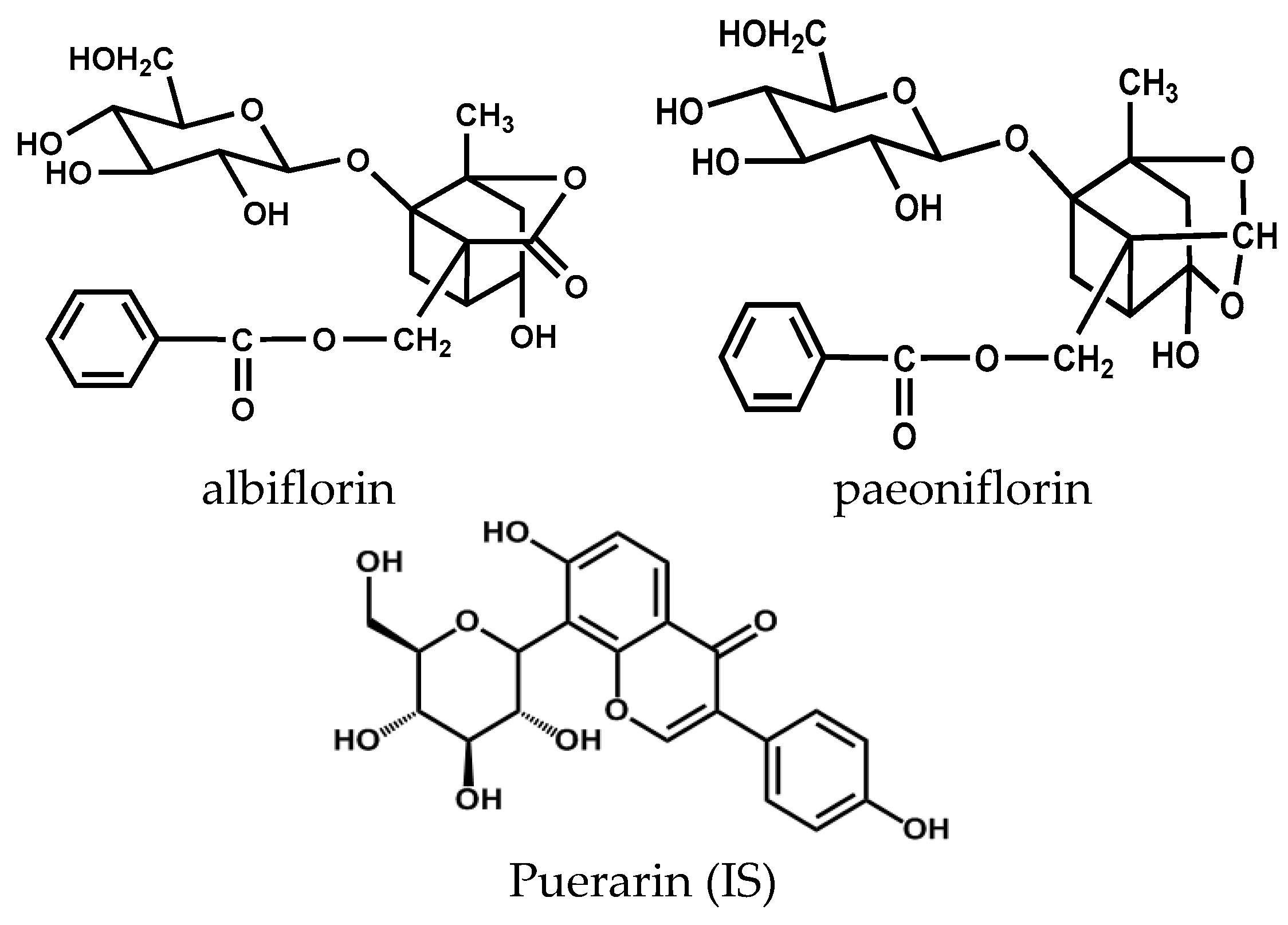
| Compounds | Added (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 15) | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD%) | Accuracy (%) | Precision (RSD%) | ||
| AF | 1.0 | 96.9 | 13.0 | 108.4 | 14.6 |
| 100 | 97.3 | 1.7 | 99.8 | 6.7 | |
| 1000 | 105.9 | 1.3 | 95.8 | 9.3 | |
| PF | 1.0 | 98.8 | 12.3 | 97.8 | 14.2 |
| 100 | 108.2 | 5.3 | 111.3 | 5.2 | |
| 1000 | 105.4 | 6.6 | 104.8 | 5.2 | |
| Parameters (Unit) | PF Alone | PF Combined with AF | PF in TPG |
|---|---|---|---|
| AUC(0→24 h) (ng/mL·min ) | 35,702 ± 6355.8 | 51,907 ± 13,552 * | 54,128 ± 18,416 * |
| MRT(0→24 h) (min) | 109.6 ± 25.3 | 199.9 ± 97.4 ** | 288.8 ± 44.7 *** |
| t1/2 (min) | 108.6 ± 37.8 | 97.9 ± 39.9 | 234.6 ± 96.2 ** |
| tmax (min) | 35.0 ± 12.2 | 41.7 ± 39.2 | 23.3 ± 10.3 |
| Cmax (ng/mL) | 332.7 ± 63.6 | 319.8 ± 120.9 | 290.9 ± 141.1 |
| CL (L·kg/min) | 2.3 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.6 |
| Parameters (Unit) | AF Alone | AF Combined with PF | AF in TPG |
|---|---|---|---|
| AUC(0→24 h) (ng/mL·min) | 8527.2 ± 2194.3 | 7754.7 ± 1897.5 | 11,777 ± 3419.4 |
| MRT(0→24 h) (min) | 135.0 ± 29.3 | 178.2 ± 93.2 | 225.8 ± 32.1 * |
| t1/2 (min) | 119.6 ± 29.7 | 130.1 ± 58.7 | 215.7 ± 91.9 * |
| tmax (min) | 26.7 ± 8.2 | 41.7 ± 39.2 | 31.7 ± 16.0 |
| Cmax (ng/mL) | 70.3 ± 19.1 | 42.4 ± 18.7 ** | 56.9 ± 12.9 |
| CL (L·kg/min) | 1.7 ± 0.5 | 1.8 ± 0.6 | 1.2 ± 0.4 |
| Parameters (Unit) | PF under Normoxia | PF under Hypoxia |
|---|---|---|
| AUC(0→24 h) (ng/mL·min) | 35,702± 6355 | 26,600 ± 4729 * |
| MRT(0→24 h) (min) | 109.6 ± 25.3 | 127.2 ± 35.3 |
| t1/2 (min) | 108.6 ± 37.8 | 111.6 ± 28.5 |
| tmax (min) | 35.0 ± 12.2 | 50.8 ± 39.8 |
| Cmax (ng/mL) | 332.7 ± 63.6 | 176.9 ± 55.1 ** |
| CL (L·kg/min) | 2.3 ± 0.4 | 3.05 ± 0.54 |
| Parameters (Unit) | AF under Normoxia | AF under Hypoxia |
|---|---|---|
| AUC(0→24 h) (ng/mL·min) | 8527.2 ± 2194 | 8299.0 ± 2202 |
| MRT(0→24 h) (min) | 135.0 ± 29.3 | 129.5 ± 24.9 |
| t1/2 (min) | 119.6 ± 29.7 | 131.5 ± 57.5 |
| tmax (min) | 26.7 ± 8.2 | 35.0 ± 12.3 |
| Cmax (ng/mL) | 70.3 ± 19.1 | 49.1 ± 16.2 |
| CL (L·kg/min) | 1.7 ± 0.5 | 1.6 ± 0.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhao, Y.; Qin, Y.; Ge, B.; Gong, W.; Wu, Y.; Li, X.; Zhao, Y.; Xu, P.; Xue, M. Enhancement of Exposure and Reduction of Elimination for Paeoniflorin or Albiflorin via Co-Administration with Total Peony Glucosides and Hypoxic Pharmacokinetics Comparison. Molecules 2016, 21, 874. https://doi.org/10.3390/molecules21070874
Xu W, Zhao Y, Qin Y, Ge B, Gong W, Wu Y, Li X, Zhao Y, Xu P, Xue M. Enhancement of Exposure and Reduction of Elimination for Paeoniflorin or Albiflorin via Co-Administration with Total Peony Glucosides and Hypoxic Pharmacokinetics Comparison. Molecules. 2016; 21(7):874. https://doi.org/10.3390/molecules21070874
Chicago/Turabian StyleXu, Weizhe, Yan Zhao, Yi Qin, Beikang Ge, Wenwen Gong, Yingting Wu, Xiaorong Li, Yuming Zhao, Pingxiang Xu, and Ming Xue. 2016. "Enhancement of Exposure and Reduction of Elimination for Paeoniflorin or Albiflorin via Co-Administration with Total Peony Glucosides and Hypoxic Pharmacokinetics Comparison" Molecules 21, no. 7: 874. https://doi.org/10.3390/molecules21070874