Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses
Abstract
:1. Introduction
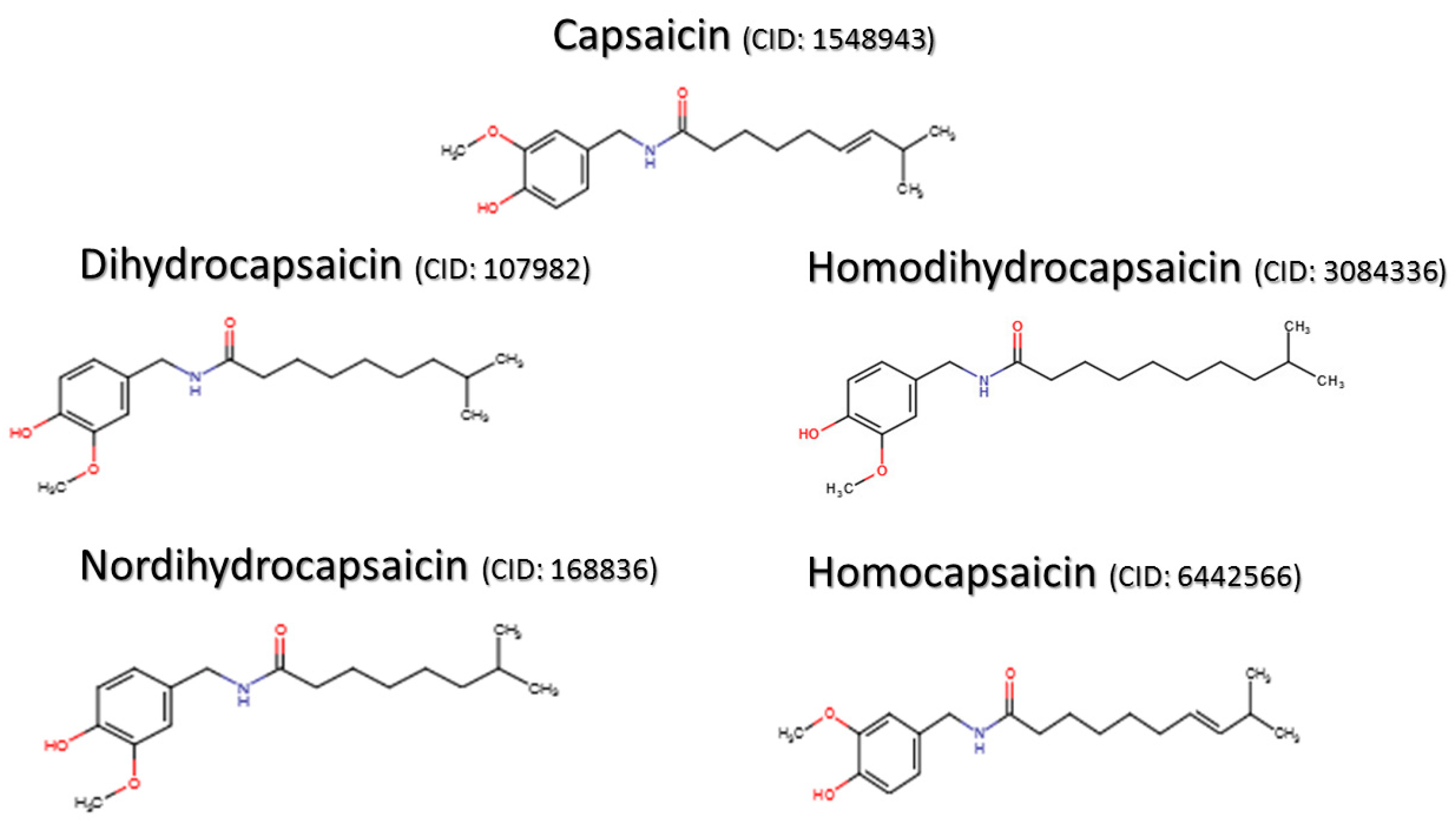
1.1. Discovery, Natural Sources, Role in Plants, Isolation, and Structure of Capsaicin
1.2. Capsaicin-Derived Molecules and Analogs
2. Capsaicin and Pain
2.1. Importance of Capsaicin in Pain Research
2.2. Mechanisms of Capsaicin-Induced Pain
2.3. Targeting TRPV1 as a Pharmacological Approach
3. Mechanisms of Capsaicin-Induced Analgesia
4. Pre-Clinical and Clinical Uses, and Pharmacological Actions of Capsaicin in Conditions Other than Pain
4.1. Capsaicin in Weight Reduction and Obesity
4.2. Capsaicin in Glucose Homeostasis and Diabetes
4.3. Capsaicin in Cardiovascular Conditions
4.4. Capsaicin in Cancer
4.5. Capsaicin in Airway Diseases
4.6. Capsaicin in Itch
4.7. Capsaicin in Gastric Disorders
4.8. Capsaicin in Urological Disorders
5. Clinically Available Capsaicin Pharmaceutical Formulations
6. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Wolkerstorfer, A.; Handler, N.; Buschmann, H. New approaches to treating pain. Bioorg. Med. Chem. Lett. 2016, 26, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Pryzbylkowski, P. Opioid Induced Hyperalgesia. Pain. Med. 2015, 16 (Suppl. S1), S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Kaga, H.; Miura, M.; Orito, K. A facile procedure for synthesis of capsaicin. J. Org. Chem. 1989, 54, 3477–3478. [Google Scholar] [CrossRef]
- Nelson, E.K.; Dawson, L.E. The constitution of capsaicin, the pungent principle of Capsicum III. J. Am. Chem. Soc. 1923, 45, 2179–2181. [Google Scholar] [CrossRef]
- Thiele, R.; Mueller-Seitz, E.; Petz, M. Chili pepper fruits: presumed precursors of fatty acids characteristic for capsaicinoids. J. Agric. Food. Chem. 2008, 56, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Reagan, K.M.; Machnicki, N.J.; Carlo, T.A.; Haak, D.C.; Penaloza, A.L.; Levey, D.J. Evolutionary ecology of pungency in wild chilies. Proc. Natl. Acad. Sci. USA 2008, 105, 11808–11811. [Google Scholar] [CrossRef] [PubMed]
- Veloso, J.; Prego, C.; Varela, M.M.; Carballeira, R.; Bernal, A.; Merino, F.; Diaz, J. Properties of capsaicinoids for the control of fungi and oomycetes pathogenic to pepper. Plant Biol. 2014, 16, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ziglio, A.C.; Gonçalves, D. On the use of capsaicin as a natural preservative against fungal attack on Pinus sp. and Hymenaea sp. woods. Mater. Res. 2014, 17, 271–274. [Google Scholar] [CrossRef]
- Thresh, J.C. Isolation of capsaicin. Pharm. J. Trans. 1876, 6, 941–947. [Google Scholar]
- Nelson, E.K. The constitution of capsaicin, the pungent principle of capsicum. J. Am. Chem. Soc. 1919, 41, 1115–1121. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Database; CID=1548943. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1548943 (accessed on 17 April 2016).
- National Center for Biotechnology Information. PubChem Compound Database; CID=107982. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/107982 (accessed on 17 April 2016).
- National Center for Biotechnology Information. PubChem Compound Database; CID=3084336. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3084336 (accessed on 17 April 2016).
- National Center for Biotechnology Information. PubChem Compound Database; CID=168836. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/168836 (accessed on 17 April 2016).
- National Center for Biotechnology Information. PubChem Compound Database; CID=6442566. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6442566 (accessed on 17 April 2016).
- Blum, E.; Mazourek, M.; O’Connell, M.; Curry, J.; Thorup, T.; Liu, K.; Jahn, M.; Paran, I. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Yahia, E.M. Changes in Capsaicinoids during Development, Maturation, and Senescence of Chile Peppers and Relation with Peroxidase Activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Walpole, C.S.; Bevan, S.; Bloomfield, G.; Breckenridge, R.; James, I.F.; Ritchie, T.; Szallasi, A.; Winter, J.; Wrigglesworth, R. Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J. Med. Chem. 1996, 39, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.K.; Simone, D.A.; Shain, C.N.; LaMotte, R.H. Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J. Neurophysiol. 1991, 66, 212–227. [Google Scholar] [PubMed]
- Sluka, K.A.; Willis, W.D. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997, 71, 165–178. [Google Scholar] [CrossRef]
- Wood, J.N.; Winter, J.; James, I.F.; Rang, H.P.; Yeats, J.; Bevan, S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [PubMed]
- Oh, U.; Hwang, S.W.; Kim, D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 1996, 16, 1659–1667. [Google Scholar] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Wang, S.; Joseph, J.; Diatchenko, L.; Ro, J.Y.; Chung, M.K. Agonist-dependence of functional properties for common nonsynonymous variants of human transient receptor potential vanilloid 1. Pain 2016, 157, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Szolcsanyi, J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J. Physiol. 1977, 73, 251–259. [Google Scholar]
- Zou, X.; Lin, Q.; Willis, W.D. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience 2002, 115, 775–786. [Google Scholar] [CrossRef]
- Zou, X.; Lin, Q.; Willis, W.D. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain. Res. 2004, 1020, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.M.; Peters, M.C.; Ma, J.Y.; Kerr, I.; Mangadu, R.; Chakravarty, S.; Dugar, S.; Medicherla, S.; Protter, A.A.; Yeomans, D.C. Peripheral and central p38 MAPK mediates capsaicin-induced hyperalgesia. Pain 2004, 111, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Lawand, N.B.; Willis, W.D. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain 2003, 104, 201–208. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Casagrande, R.; Verri, W.A. Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-kappaB activation. Inflamm. Res. 2015, 64, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000, 279, L1005–1028. [Google Scholar] [PubMed]
- Lee, I.; Kim, H.K.; Kim, J.H.; Chung, K.; Chung, J.M. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 2007, 133, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Sakamoto, T.; Tiwari, V.; Kim, Y.S.; Yang, F.; Dong, X.; Guler, A.D.; Guan, Y.; Caterina, M.J. Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain 2015, 156, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, T.K.; Basso, L.; Iftinca, M.C.; Flynn, R.; Chapman, K.; Dietrich, G.; Vergnolle, N.; Altier, C. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G87–G99. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S. Capsaicin receptor as target of calcitonin gene-related peptide in the gut. Prog. Drug. Res. 2014, 68, 259–276. [Google Scholar] [PubMed]
- Yee, J.R.; Kenkel, W.; Caccaviello, J.C.; Gamber, K.; Simmons, P.; Nedelman, M.; Kulkarni, P.; Ferris, C.F. Identifying the integrated neural networks involved in capsaicin-induced pain using fMRI in awake TRPV1 knockout and wild-type rats. Front. Syst. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Jurik, A.; Ressle, A.; Schmid, R.M.; Wotjak, C.T.; Thoeringer, C.K. Supraspinal TRPV1 modulates the emotional expression of abdominal pain. Pain 2014, 155, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; Mapp, P.I.; Kelly, S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br. J. Clin. Pharmacol. 2015, 80, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Bullock, C.M.; Wookey, P.; Bennett, A.; Mobasheri, A.; Dickerson, I.; Kelly, S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014, 66, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, A.M.; Bean, B.P.; Woolf, C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 2007, 449, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Guler, A.D.; Caterina, M.J. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 2008, 11, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Hyakkoku, K.; Ogawa, K.; Takasu, K.; Imai, S.; Sakurai, Y.; Fujita, M.; Ono, H.; Yamamoto, M.; Fukuda, I.; et al. Sensitization of TRPV1 by protein kinase C in rats with mono-iodoacetate-induced joint pain. Osteoarthr. Cartil. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Elokely, K.; Velisetty, P.; Delemotte, L.; Palovcak, E.; Klein, M.L.; Rohacs, T.; Carnevale, V. Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc. Natl. Acad. Sci. USA 2016, 113, E137–E145. [Google Scholar] [CrossRef] [PubMed]
- Wrigglesworth, R.; Walpole, C.S.; Bevan, S.; Campbell, E.A.; Dray, A.; Hughes, G.A.; James, I.; Masdin, K.J.; Winter, J. Analogues of capsaicin with agonist activity as novel analgesic agents: Structure-activity studies. 4. Potent, orally active analgesics. J. Med. Chem. 1996, 39, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
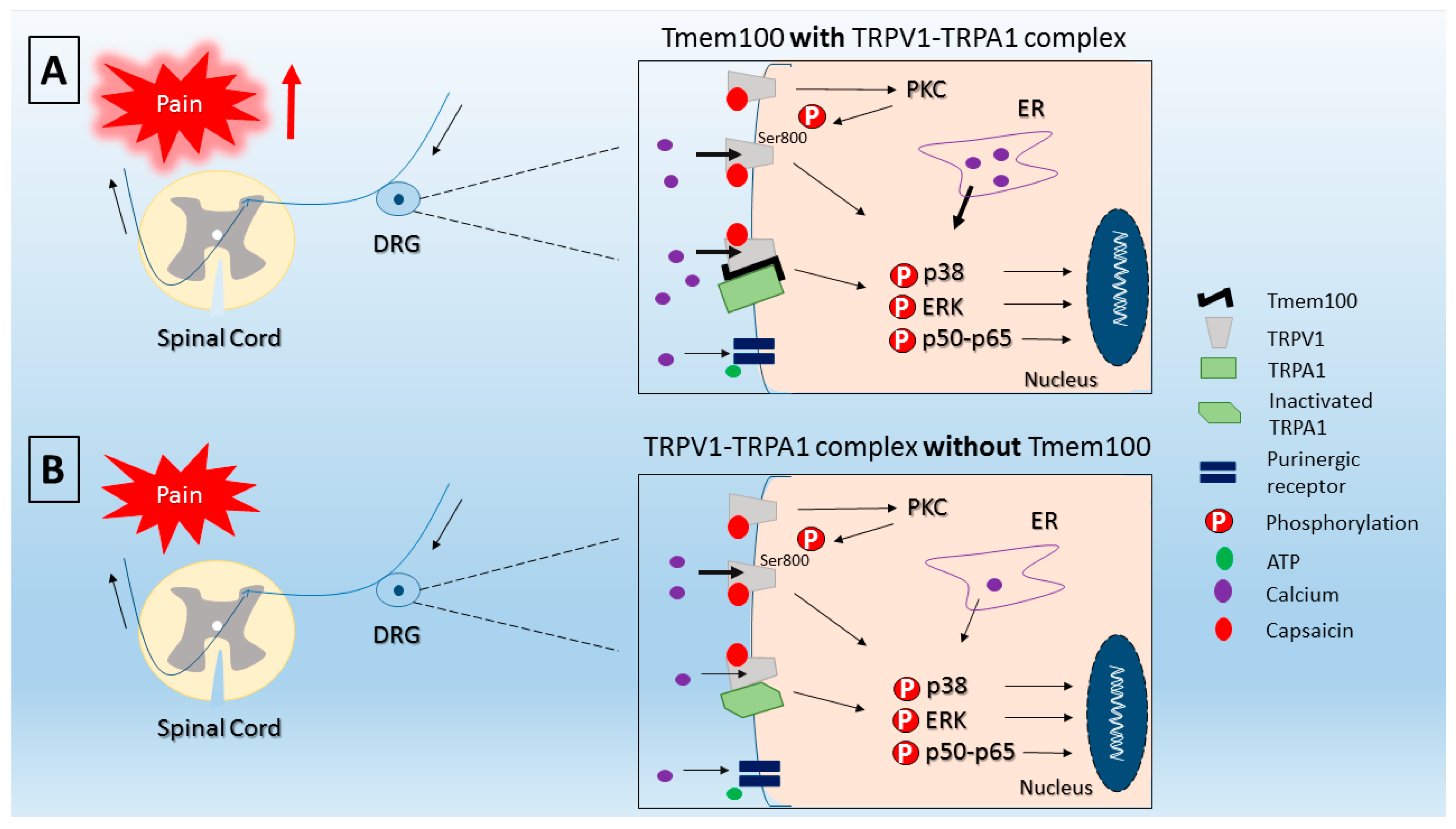
- Akopian, A.N. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr. Pharm. Biotechnol. 2011, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 2014, 466, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Saghy, E.; Szoke, E.; Payrits, M.; Helyes, Z.; Borzsei, R.; Erostyak, J.; Janosi, T.Z.; Setalo, G.; Szolcsanyi, J. Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of Transient Receptor Potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 2015, 100, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.; Wang, Y.; et al. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Spahn, V.; Stein, C.; Zollner, C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol. Pharmacol. 2014, 85, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.; Manchope, M.F.; Casagrande, R.; Verri, W.A. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-kappaB. Chem. Biol. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.F.; Trevisan, G.; Walker, C.I.; Klafke, J.Z.; de Oliveira, A.P.; Villarinho, J.G.; Zanon, R.B.; Royes, L.F.; Athayde, M.L.; Gomez, M.V.; et al. Eriodictyol: A flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem. Pharmacol. 2011, 81, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.L.; Gonzalez-Trujano, M.E.; Chavez, M.; Pellicer, F.; Moreno, J.; Lopez-Munoz, F.J. Hesperidin produces antinociceptive response and synergistic interaction with ketorolac in an arthritic gout-type pain in rats. Pharmacol. Biochem. Behav. 2011, 97, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, C.W.; Oh, S.J.; Yun, J.; Lee, K.; Park, S.K.; Kim, H.M.; Han, S.B.; Kim, Y.; Kim, H.C.; et al. Protective effect of silymarin against ethanol-induced gastritis in rats: role of sulfhydryls, nitric oxide and gastric sensory afferents. Food Chem. Toxicol. 2013, 55, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Rossato, M.F.; Walker, C.I.; Klafke, J.Z.; Rosa, F.; Oliveira, S.M.; Tonello, R.; Guerra, G.P.; Boligon, A.A.; Zanon, R.B.; et al. Identification of the plant steroid alpha-spinasterol as a novel transient receptor potential vanilloid 1 antagonist with antinociceptive properties. J. Pharmacol. Exp. Ther. 2012, 343, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Dong, L.; Kong, D.; Sun, B.; Sun, Q.; Grundy, D.; Zhang, G.; Rong, W. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol. Motil. 2013, 25, e429–e440. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.Y.; Kim, S.A.; Kim, Y.H.; Lee, M.K.; Ahn, D.K.; Kim, H.J.; Kim, J.S.; Jung, S.J.; Oh, S.B. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J. Dent. Res. 2010, 89, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Gosu, V.; Basith, S.; Hong, S.; Choi, S. Polymodal Transient Receptor Potential Vanilloid Type 1 Nocisensor: Structure, Modulators, and Therapeutic Applications. Adv. Protein Chem. Struct. Biol. 2016, 104, 81–125. [Google Scholar] [PubMed]
- Brandt, M.R.; Beyer, C.E.; Stahl, S.M. TRPV1 Antagonists and Chronic Pain: Beyond Thermal Perception. Pharmaceuticals 2012, 5, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Chizh, B.A.; O'Donnell, M.B.; Napolitano, A.; Wang, J.; Brooke, A.C.; Aylott, M.C.; Bullman, J.N.; Gray, E.J.; Lai, R.Y.; Williams, P.M.; et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Simone, D.A.; Ngeow, J.Y.; Putterman, G.J.; LaMotte, R.H. Hyperalgesia to heat after intradermal injection of capsaicin. Brain Res. 1987, 418, 201–203. [Google Scholar] [CrossRef]
- Nagy, J.I.; van der Kooy, D. Effects of neonatal capsaicin treatment on nociceptive thresholds in the rat. J. Neurosci. 1983, 3, 1145–1150. [Google Scholar] [PubMed]
- Kissin, I. Vanilloid-induced conduction analgesia: Selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth. Analg. 2008, 107, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; de Novellis, V.; Marabese, I.; Cuomo, D.; Rossi, F.; Berrino, L.; Rossi, F.; Maione, S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur. J. Pharmacol. 2002, 439, 69–75. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [PubMed]
- Lee, S.S.; Sohn, Y.W.; Yoo, E.S.; Kim, K.H. Neurotoxicity and long lasting analgesia induced by capsaicinoids. J. Toxicol. Sci. 1991, 16 (Suppl. S1), 3–20. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of depletion of substance P by capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar] [PubMed]
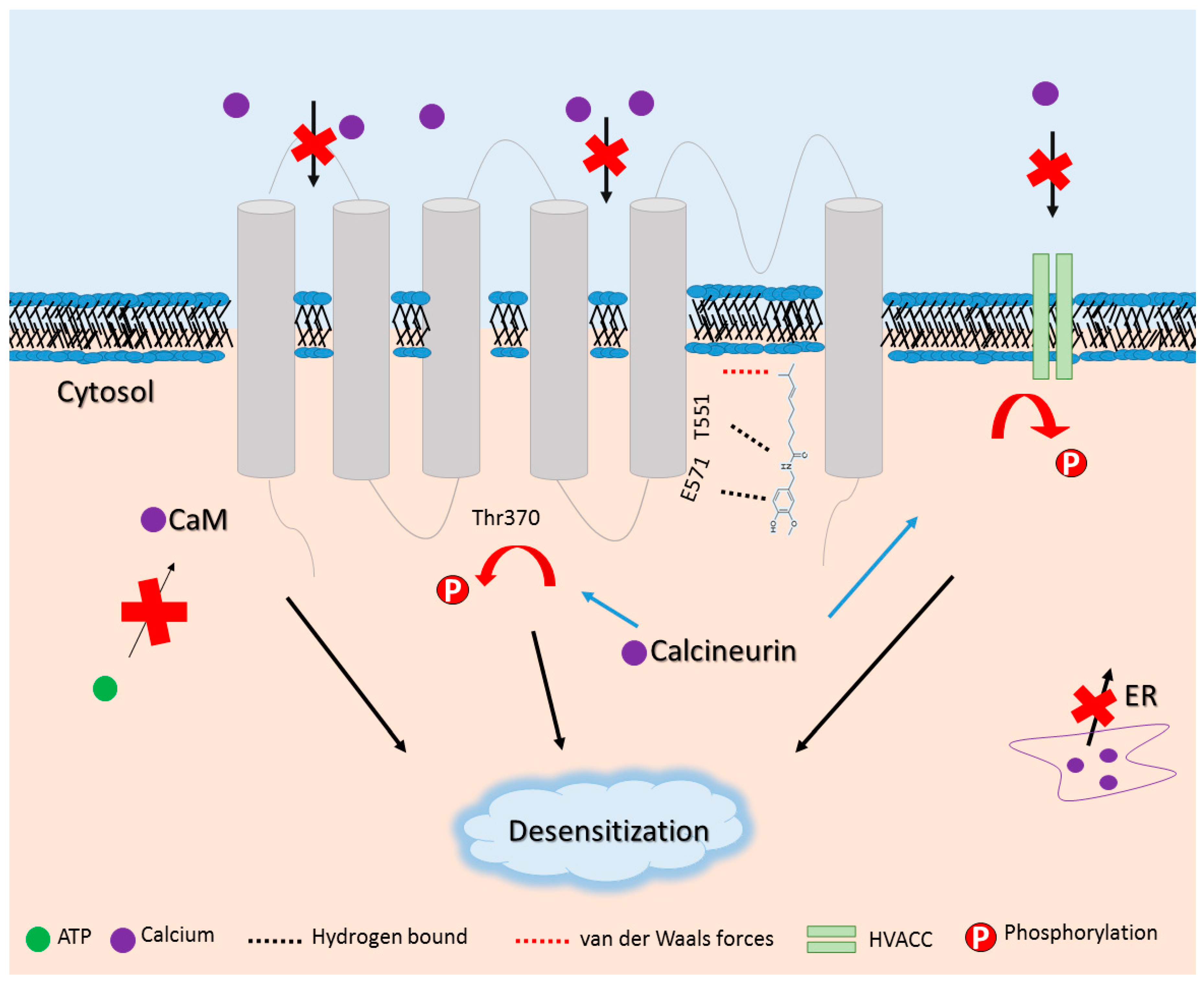
- Comunanza, V.; Carbone, E.; Marcantoni, A.; Sher, E.; Ursu, D. Calcium-dependent inhibition of T-type calcium channels by TRPV1 activation in rat sensory neurons. Pflugers Arch. 2011, 462, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Koplas, P.A.; Rosenberg, R.L.; Oxford, G.S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 1997, 17, 3525–3537. [Google Scholar] [PubMed]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Chen, S.R.; Pan, H.L. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J. Biol. Chem. 2005, 280, 18142–18151. [Google Scholar] [CrossRef] [PubMed]
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Hiura, A.; Ishizuka, H. Changes in features of degenerating primary sensory neurons with time after capsaicin treatment. Acta Neuropathol. 1989, 78, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Paik, K.S.; Kim, J.S.; Nam, S.C.; Kim, K.J.; Oh, U.T.; Hasegawa, T.; Chung, K.; Willis, W.D. Chronic effects of topical application of capsaicin to the sciatic nerve on responses of primate spinothalamic neurons. Pain 1993, 53, 311–321. [Google Scholar] [CrossRef]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [PubMed]
- Holzer, P.; Jurna, I.; Gamse, R.; Lembeck, F. Nociceptive threshold after neonatal capsaicin treatment. Eur. J. Pharmacol. 1979, 58, 511–514. [Google Scholar] [CrossRef]
- Shin, C.Y.; Shin, J.; Kim, B.M.; Wang, M.H.; Jang, J.H.; Surh, Y.J.; Oh, U. Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol. Cell Neurosci. 2003, 24, 57–68. [Google Scholar] [CrossRef]
- Pecze, L.; Blum, W.; Schwaller, B. Mechanism of capsaicin receptor TRPV1-mediated toxicity in pain-sensing neurons focusing on the effects of Na+/Ca2+ fluxes and the Ca2+-binding protein calretinin. Biochim. Biophys. Acta 2013, 1833, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Baamonde, A.; Lastra, A.; Juarez, L.; Hidalgo, A.; Menendez, L. TRPV1 desensitisation and endogenous vanilloid involvement in the enhanced analgesia induced by capsaicin in inflamed tissues. Brain Res. Bull. 2005, 67, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Menendez, L.; Lastra, A.; Hidalgo, A.; Baamonde, A. The analgesic effect induced by capsaicin is enhanced in inflammatory states. Life Sci. 2004, 74, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- Orliac, M.L.; Peroni, R.N.; Abramoff, T.; Neuman, I.; Podesta, E.J.; Adler-Graschinsky, E. Increases in vanilloid TRPV1 receptor protein and CGRP content during endotoxemia in rats. Eur. J. Pharmacol. 2007, 566, 145–152. [Google Scholar] [CrossRef] [PubMed]
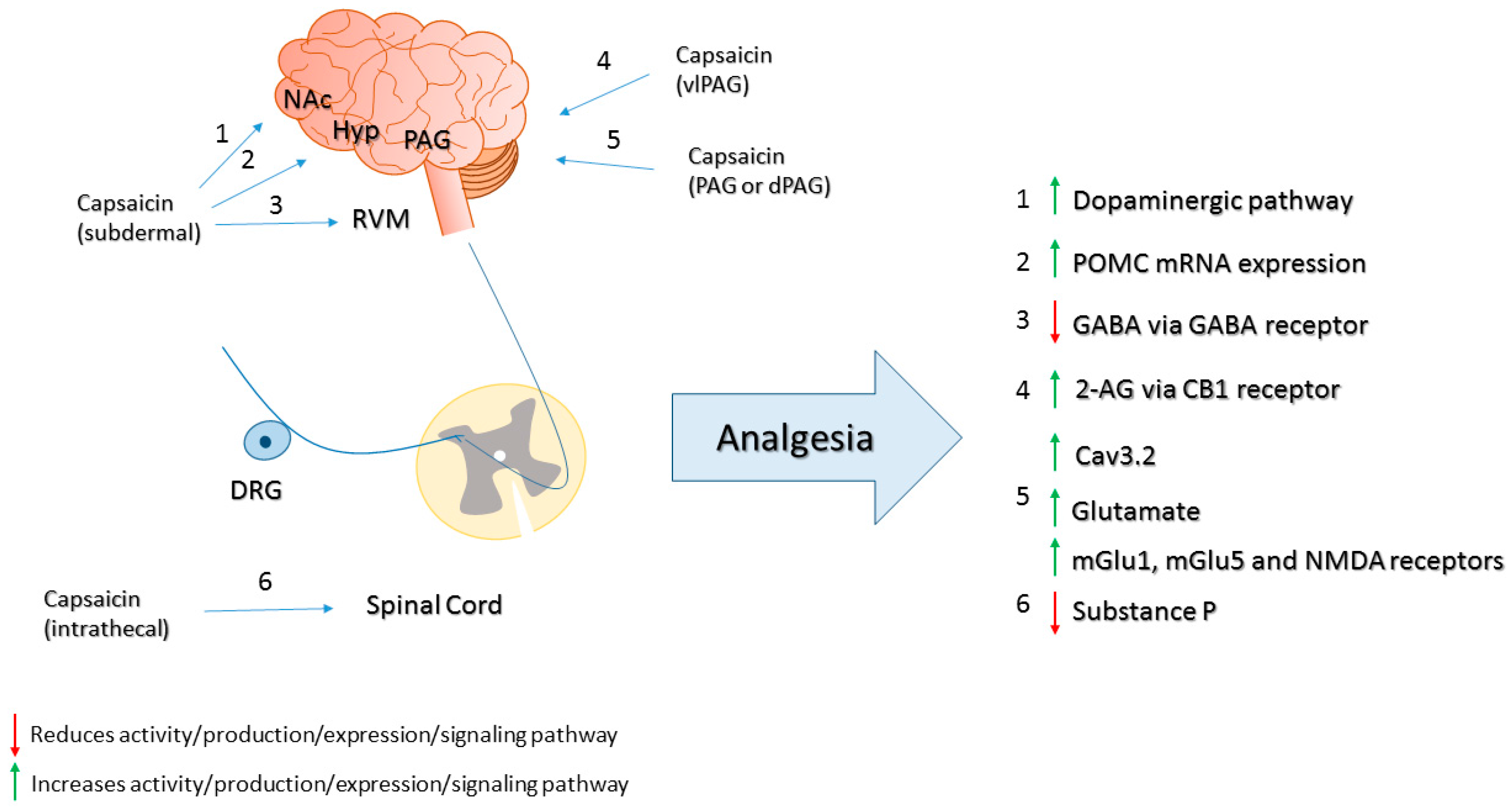
- Gear, R.W.; Aley, K.O.; Levine, J.D. Pain-induced analgesia mediated by mesolimbic reward circuits. J. Neurosci. 1999, 19, 7175–7181. [Google Scholar] [PubMed]
- Tambeli, C.H.; Levine, J.D.; Gear, R.W. Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord. Pain 2009, 143, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Baek, S.Y.; Kim, C.M. Acute effects of capsaicin on proopioimelanocortin mRNA levels in the arcuate nucleus of Sprague-Dawley rats. Psychiatry Investig. 2012, 9, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Eimerl, D.; Papir-Kricheli, D. Epidural capsaicin produces prolonged segmental analgesia in the rat. Exp. Neurol. 1987, 97, 169–178. [Google Scholar] [CrossRef]
- Szabo, T.; Olah, Z.; Iadarola, M.J.; Blumberg, P.M. Epidural resiniferatoxin induced prolonged regional analgesia to pain. Brain Res. 1999, 840, 92–98. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Farb, D.H.; Leeman, S.E.; Jessell, T.M. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science 1979, 206, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Mallet, C.; Francois, A.; Boudes, M.; Chemin, J.; Voets, T.; Bourinet, E.; Alloui, A.; Eschalier, A. Cav3.2 calcium channels: The key protagonist in the supraspinal effect of paracetamol. Pain 2014, 155, 764–772. [Google Scholar] [CrossRef] [PubMed]
- McGaraughty, S.; Chu, K.L.; Bitner, R.S.; Martino, B.; El Kouhen, R.; Han, P.; Nikkel, A.L.; Burgard, E.C.; Faltynek, C.R.; Jarvis, M.F. Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J. Neurophysiol. 2003, 90, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Farani, A.; Sahebgharani, M.; Sepehrizadeh, Z.; Jaberi, E.; Ghazi-Khansari, M. Diabetic thermal hyperalgesia: Role of TRPV1 and CB1 receptors of periaqueductal gray. Brain Res. 2010, 1328, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Maione, S.; Cristino, L.; Palazzo, E.; Marabese, I.; Rossi, F.; de Novellis, V.; di Marzo, V. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J. Neurosci. 2007, 27, 13739–13749. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Lee, H.J.; Ho, Y.C.; Chiou, L.C. Capsaicin in the periaqueductal gray induces analgesia via metabotropic glutamate receptor-mediated endocannabinoid retrograde disinhibition. Br. J. Pharmacol. 2011, 163, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Starowicz, K.; Cristino, L.; Guida, F.; Palazzo, E.; Luongo, L.; Rossi, F.; Marabese, I.; de Novellis, V.; di Marzo, V. Functional interaction between TRPV1 and mu-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J. Neurophysiol. 2009, 101, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Martins, D.; Charrua, A.; Piscitelli, F.; Tavares, I.; Morgado, C.; Di Marzo, V. Endovanilloid control of pain modulation by the rostroventromedial medulla in an animal model of diabetic neuropathy. Neuropharmacology 2016, 107, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kingery, W.S. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain 1997, 73, 123–139. [Google Scholar] [CrossRef]
- Robbins, W.R.; Staats, P.S.; Levine, J.; Fields, H.L.; Allen, R.W.; Campbell, J.N.; Pappagallo, M. Treatment of intractable pain with topical large-dose capsaicin: Preliminary report. Anesth. Analg. 1998, 86, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Ellison, N.; Loprinzi, C.L.; Kugler, J.; Hatfield, A.K.; Miser, A.; Sloan, J.A.; Wender, D.B.; Rowland, K.M.; Molina, R.; Cascino, T.L.; et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J. Clin. Oncol. 1997, 15, 2974–2980. [Google Scholar] [PubMed]
- Zis, P.; Apsokardos, A.; Isaia, C.; Sykioti, P.; Vadalouca, A. Posttraumatic and postsurgical neuropathic pain responsive to treatment with capsaicin 8% topical patch. Pain Phys. 2014, 17, E213–E218. [Google Scholar]
- Watson, C.P.; Evans, R.J.; Watt, V.R.; Birkett, N. Post-herpetic neuralgia: 208 cases. Pain 1988, 35, 289–297. [Google Scholar] [CrossRef]
- Watson, C.P.; Tyler, K.L.; Bickers, D.R.; Millikan, L.E.; Smith, S.; Coleman, E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin. Ther. 1993, 15, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Kiani, J.; Sajedi, F.; Nasrollahi, S.A.; Esna-Ashari, F. A randomized clinical trial of efficacy and safety of the topical clonidine and capsaicin in the treatment of painful diabetic neuropathy. J. Res. Med. Sci. 2015, 20, 359–363. [Google Scholar] [PubMed]
- Burness, C.B.; McCormack, P.L. Capsaicin 8% Patch: A Review in Peripheral Neuropathic Pain. Drugs 2016, 76, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fusco, B.M.; Marabini, S.; Maggi, C.A.; Fiore, G.; Geppetti, P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain 1994, 59, 321–325. [Google Scholar] [CrossRef]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Backonja, M.M.; Malan, T.P.; Vanhove, G.F.; Tobias, J.K.; Group, C.S. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010, 11, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.A.; Backonja, M.M.; Dunteman, E.; Blonsky, E.R.; Vanhove, G.F.; Lu, S.P.; Tobias, J.; Group, N.C.S. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011, 12, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Saberski, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef]
- Turnbull, J.H.; Gebauer, S.L.; Miller, B.L.; Barbaro, N.M.; Blanc, P.D.; Schumacher, M.A. Cutaneous nerve transection for the management of intractable upper extremity pain caused by invasive squamous cell carcinoma. J. Pain Symptom Manag. 2011, 42, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, K.; Li, H.Y.; Chung, G.; Park, C.K.; Kim, J.S.; Jung, S.J.; Lee, M.K.; Ahn, D.K.; Hwang, S.J.; et al. Selectively targeting pain in the trigeminal system. Pain 2010, 150, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Ririe, D.; Houle, T.T.; Aschenbrenner, C.A.; Eisenach, J.C. Nociceptor-selective peripheral nerve block induces delayed mechanical hypersensitivity and neurotoxicity in rats. Anesthesiology 2014, 120, 976–986. [Google Scholar] [CrossRef] [PubMed]
- McCleane, G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: A randomized, double-blind, placebo-controlled study. Br. J. Clin. Pharmacol. 2000, 49, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, Y.; Yu, Y.; Li, Y.; Zhao, S.; Chen, Y.; Waqar, A.B.; Fan, J.; Liu, E. Expression of TRPV1 in rabbits and consuming hot pepper affects its body weight. Mol. Biol. Rep. 2012, 39, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Hagihara, K.; Iwai, K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J. Nutr. 1986, 116, 1272–1278. [Google Scholar] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Mattes, R.D. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol. Behav. 2011, 102, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Smeets, A.; Lejeune, M.P. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int. J. Obes. 2005, 29, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [PubMed]
- Smeets, A.J.; Janssens, P.L.; Westerterp-Plantenga, M.S. Addition of capsaicin and exchange of carbohydrate with protein counteract energy intake restriction effects on fullness and energy expenditure. J. Nutr. 2013, 143, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Reinbach, H.C.; Smeets, A.; Martinussen, T.; Moller, P.; Westerterp-Plantenga, M.S. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clin. Nutr. 2009, 28, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawada, T.; Kurosawa, M.; Sato, A.; Iwai, K. Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am. J. Physiol. 1988, 255, E23–E27. [Google Scholar] [PubMed]
- Watanabe, T.; Kawada, T.; Yamamoto, M.; Iwai, K. Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem. Biophys. Res. Commun. 1987, 142, 259–264. [Google Scholar] [CrossRef]
- Russek, M.; Vega, C.; Barrera, J.; Soto-Mora, L.M.; Lanzagorta, A.; Racotta, R. Anorexia elicited by different catecholamines in rats. Appetite 1987, 9, 119–126. [Google Scholar] [CrossRef]
- Smeets, A.J.; Westerterp-Plantenga, M.S. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur. J. Nutr. 2009, 48, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite 2014, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Miyawaki, C.; Ue, H.; Yuasa, T.; Miyatsuji, A.; Moritani, T. Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J. Nutr. Sci. Vitaminol. 2000, 46, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Saito, M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Lim, K.; Kikuzato, S.; Kiyonaga, A.; Tanaka, H.; Shindo, M.; Suzuki, M. Effects of red-pepper diet on the energy metabolism in men. J. Nutr. Sci. Vitaminol. 1995, 41, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Watanabe, T.; Takaishi, T.; Tanaka, T.; Iwai, K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc. Soc. Exp. Biol. Med. 1986, 183, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Murtaza, N.; Jagtap, S.; Singh, D.P.; Karmase, A.; Kaur, J.; Bhutani, K.K.; Boparai, R.K.; Premkumar, L.S.; Kondepudi, K.K.; et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J. Nutr. Biochem. 2014, 25, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.B.; O'Rahilly, S. Regulation of adipose cell number in man. Clin. Sci. 1997, 92, 3–11. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Mandrup, S. Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 2002, 13, 5–11. [Google Scholar] [CrossRef]
- Feng, Z.; Hai-ning, Y.; Xiao-man, C.; Zun-chen, W.; Sheng-rong, S.; Das, U.N. Effect of yellow capsicum extract on proliferation and differentiation of 3T3-L1 preadipocytes. Nutrition 2014, 30, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Jang, M.; Park, M.; Gobianand, K.; You, S.; Yeon, S.H.; Park, S.; Kim, M.J.; Lee, H.J. Capsaicin inhibits the adipogenic differentiation of bone marrow mesenchymal stem cells by regulating cell proliferation, apoptosis, oxidative and nitrosative stress. Food Funct. 2015, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Browne, G.J.; Finn, S.G.; Proud, C.G. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J. Biol. Chem. 2004, 279, 12220–12231. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Lee, J.; Ha, J.; Kim, S.S.; Cho, Y.H.; Baik, H.H.; Kang, I. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neurosci. Lett. 2004, 354, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Fact. 2011, 10 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 2003, 90, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.; Thompson, D.; Edelsberg, J.; Bird, A.P.; Colditz, G.A. Lifetime health and economic benefits of weight loss among obese persons. Am. J. Public Health 1999, 89, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Kanungo, A.; Toth, C. Equivalency of tricyclic antidepressants in open-label neuropathic pain study. Acta Neurol. Scand. 2014, 129, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am. J. Clin. Nutr. 2006, 84, 63–69. [Google Scholar] [PubMed]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thail. 2009, 92, 108–113. [Google Scholar] [PubMed]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Tolan, I.; Ragoobirsingh, D.; Morrison, E.Y. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother. Res. 2001, 15, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Scheurink, A.J.; Steffens, A.B.; Ahren, B. Involvement of capsaicin-sensitive nerves in regulation of insulin secretion and glucose tolerance in conscious mice. Am. J. Physiol. 1994, 267, R1071–R1077. [Google Scholar] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Goto, T.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Razavi, R.; Chan, Y.; Afifiyan, F.N.; Liu, X.J.; Wan, X.; Yantha, J.; Tsui, H.; Tang, L.; Tsai, S.; Santamaria, P.; et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 2006, 127, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Kato, S.; Katsube, K.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal. Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Okajima, K. Effects of capsaicin and isoflavone on blood pressure and serum levels of insulin-like growth factor-I in normotensive and hypertensive volunteers with alopecia. Biosci. Biotechnol. Biochem. 2009, 73, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.H. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J. Hypertens. 2003, 21, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.J. The vanilloid receptor TRPV1: role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zvara, A.; Bencsik, P.; Fodor, G.; Csont, T.; Hackler, L., Jr.; Dux, M.; Furst, S.; Jancso, G.; Puskas, L.G.; Ferdinandy, P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: A DNA microarray study. FASEB J. 2006, 20, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Li, Y.J. Calcitonin gene-related peptide and hypertension. Peptides 2005, 26, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Mehrotra, S.; Jan Danser, A.H.; Schoemaker, R.G. The role of calcitonin gene-related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur. J. Pharmacol. 2006, 531, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Peng, J. The cardioprotection of calcitonin gene-related peptide-mediated preconditioning. Eur. J. Pharmacol. 2002, 442, 173–177. [Google Scholar] [CrossRef]
- Zhou, F.W.; Li, Y.J.; Deng, H.W. Early and delayed protection by capsaicin against reperfusion injury in rat hearts. Zhongguo Yao Li Xue Bao 1999, 20, 912–916. [Google Scholar] [PubMed]
- Peng, J.; Lu, R.; Deng, H.W.; Li, Y.J. Involvement of alpha-calcitonin gene-related peptide in monophosphoryl lipid A-induced delayed preconditioning in rat hearts. Eur. J. Pharmacol. 2002, 436, 89–96. [Google Scholar] [CrossRef]
- Peng, J.; Lu, R.; Xiao, L.; Deng, H.W.; Li, Y.J. Involvement of alpha-calcitonin gene-related peptide in heat stress-induced delayed preconditioning in rat hearts. Clin. Exp. Pharmacol. Physiol. 2002, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H. Transient receptor potential vanilloid channels in hypertension, inflammation, and end organ damage: an imminent target of therapy for cardiovascular disease? Curr. Opin. Cardiol. 2008, 23, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, P.; Zhao, Z.; Cao, T.; He, H.; Luo, Z.; Zhong, J.; Gao, F.; Zhu, Z.; Li, L.; et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke 2011, 42, 3245–3451. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Walter, S.; Rapoport, A.M. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013, 53, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.H.; Yin, Y.W.; Liu, Y.; Pi, Y.; Guo, L.; Cao, X.J.; Gao, C.Y.; Zhang, L.L.; Li, J.C. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death. Dis. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Kunde, D.A.; Ball, M.J.; Geraghty, D.P. Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids. J. Agric. Food Chem. 2006, 54, 6436–6439. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, H.; Srinivasan, K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006, 273, 4528–4537. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of dietary curcumin and capsaicin in induced hypercholesterolemic rats. Lipids 2007, 42, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Ahuja, K.D.; Geraghty, D.P. Effect of capsaicin and dihydrocapsaicin on in vitro blood coagulation and platelet aggregation. Thromb. Res. 2009, 124, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Mittelstadt, S.W.; Nelson, R.A.; Daanen, J.F.; King, A.J.; Kort, M.E.; Kym, P.R.; Lubbers, N.L.; Cox, B.F.; Lynch, J.J., 3rd. Capsaicin-induced inhibition of platelet aggregation is not mediated by transient receptor potential vanilloid type 1. Blood Coagul. Fibrinolysis 2012, 23, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Topol, E.J. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2003, 2, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.J.; Villalain, J.; Gomez-Fernandez, J.C. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim. Biophys. Acta 1995, 1234, 225–234. [Google Scholar] [CrossRef]
- Meddings, J.B.; Hogaboam, C.M.; Tran, K.; Reynolds, J.D.; Wallace, J.L. Capsaicin effects on non-neuronal plasma membranes. Biochim. Biophys. Acta 1991, 1070, 43–50. [Google Scholar] [CrossRef]
- Harper, A.G.; Brownlow, S.L.; Sage, S.O. A role for TRPV1 in agonist-evoked activation of human platelets. J. Thromb. Haemost 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Poston, G.J. Global cancer surgery: The Lancet Oncology review. Eur. J. Surg. Oncol. 2015, 41, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Ballarini, P.; Caprodossi, S.; Nabissi, M.; Morelli, M.B.; Lucciarini, R.; Cardarelli, M.A.; Mammana, G.; Santoni, G. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis 2009, 30, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Chen, Y.L.; Yang, J.S.; Yang, Y.Y.; Liu, J.Y.; Hsu, S.C.; Lai, K.C.; Chung, J.G. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J. Agric. Food Chem. 2010, 58, 12999–3005. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Lee, S.S. Capsaicin in hot chili pepper: Carcinogen, co-carcinogen or anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Z.; Wang, Y.; Zhu, G.; Wang, X. Capsaicin induces cycle arrest by inhibiting cyclin-dependent-kinase in bladder carcinoma cells. Int. J. Urol. 2012, 19, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L. Capsaicin causes inactivation and degradation of the androgen receptor by inducing the restoration of miR-449a in prostate cancer. Oncol. Rep. 2015, 34, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Seevasant, I.; Mohamad, J.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Weber, N.; Lou, Y.J.; Wang, Z.Q.; Chovolou, Y.; Kampkotter, A.; Kahl, R.; Proksch, P. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem. Toxicol. 2007, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells-Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, S.U.; Oh, U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J. Immunol. 2006, 177, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoennissen, N.H.; O'Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Han, K.Y.; Kim, E.C.; Kim, Y.M.; Lee, S.W.; Kim, O.H.; Kim, K.W.; Gho, Y.S.; Kwon, Y.G. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004, 64, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Adhikary, A.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Guha, D.; Choudhuri, T.; Chattopadhyay, S.; Sa, G.; Sen, A.; et al. Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Hoch-Ligeti, C. Production of liver tumours by dietary means; effect of feeding chilies [Capsicum frutescens and annuum (Linn.)] to rats. Acta Unio Int. Contra Cancrum. 1951, 7, 606–611. [Google Scholar]
- Toth, B.; Gannett, P. Carcinogenicity of lifelong administration of capsaicin of hot pepper in mice. In Vivo 1992, 6, 59–63. [Google Scholar] [PubMed]
- Liu, Z.; Zhu, P.; Tao, Y.; Shen, C.; Wang, S.; Zhao, L.; Wu, H.; Fan, F.; Lin, C.; Chen, C.; et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem. Toxicol. 2015, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.C.; Wiessler, M.; Hecker, E.; Bhide, S.V. Tumour-promoting effect of chilli extract in BALB/c mice. Int. J. Cancer 1986, 38, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Park, J.G.; Lee, M.D.; Han, M.D.; Park, S.T.; Lee, B.H.; Jung, S.E. Co-carcinogenic effects of several Korean foods on gastric cancer induced by N-methyl-N′-nitro-N-nitrosoguanidine in rats. Jpn. J. Surg. 1985, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Boyer, P.J.; Bonneau, R.H.; Clawson, G.A.; Welch, D.R. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004, 24, 1003–1009. [Google Scholar] [PubMed]
- Serra, I.; Yamamoto, M.; Calvo, A.; Cavada, G.; Baez, S.; Endoh, K.; Watanabe, H.; Tajima, K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cancer 2002, 102, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrillo, L.; Hernandez Avila, M.; Dubrow, R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am. J. Epidemiol. 1994, 139, 263–271. [Google Scholar] [PubMed]
- Talbot, S.; Abdulnour, R.E.; Burkett, P.R.; Lee, S.; Cronin, S.J.; Pascal, M.A.; Laedermann, C.; Foster, S.L.; Tran, J.V.; Lai, N.; et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 2015, 87, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.; Bhat, Y.A.; Panda, L.; Mabalirajan, U. TRPV1 inhibition attenuates IL-13 mediated asthma features in mice by reducing airway epithelial injury. Int. Immunopharmacol. 2013, 15, 597–605. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Settipane, R.A.; Lieberman, P. Update on nonallergic rhinitis. Ann. Allergy Asthma Immunol. 2001, 86, 494–507; quiz 507–508. [Google Scholar] [CrossRef]
- Stjärne, P.; Lundblad, L.; Änggard, A.; Lunderberg, J.M. Local capsaicin treatment of the nasal mucosa reduces symptoms in patients with nonallergic nasal hyperreactivity. Am. J. Rhinol. 1991, 5, 145–151. [Google Scholar] [CrossRef]
- Van Rijswijk, J.B.; Boeke, E.L.; Keizer, J.M.; Mulder, P.G.; Blom, H.M.; Fokkens, W.J. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: A double-blind randomized application regimen study. Allergy 2003, 58, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.M.; Van Rijswijk, J.B.; Garrelds, I.M.; Mulder, P.G.; Timmermans, T.; Gerth van Wijk, R. Intranasal capsaicin is efficacious in non-allergic, non-infectious perennial rhinitis. A placebo-controlled study. Clin. Exp. Allergy 1997, 27, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Gerth Van Wijk, R.; Terreehorst, I.T.; Mulder, P.G.; Garrelds, I.M.; Blom, H.M.; Popering, S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo-controlled study. Clin. Exp. Allergy 2000, 30, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, L.; Alpizar, Y.A.; Wouters, M.M.; Hox, V.; Hauben, E.; Jorissen, M.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 1332–1339. e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Barrett, L.B.; Williams, E.K.; Strochlic, D.E.; Lee, S.; Weyer, A.D.; Lou, S.; Bryman, G.S.; Roberson, D.P.; Ghasemlou, N.; et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Roberson, D.P.; Gudes, S.; Sprague, J.M.; Patoski, H.A.; Robson, V.K.; Blasl, F.; Duan, B.; Oh, S.B.; Bean, B.P.; Ma, Q.; et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 2013, 16, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Riol-Blanco, L.; Ordovas-Montanes, J.; Perro, M.; Naval, E.; Thiriot, A.; Alvarez, D.; Paust, S.; Wood, J.N.; von Andrian, U.H. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 2014, 510, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.N.; Berberian, B.; Sulica, V.I.; Dodd, W.A.; Jarratt, M.T.; Katz, H.I.; Prawer, S.; Krueger, G.; Rex, I.H., Jr.; Wolf, J.E. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J. Am. Acad. Dermatol. 1993, 29, 438–442. [Google Scholar] [CrossRef]
- Bernstein, J.E.; Parish, L.C.; Rapaport, M.; Rosenbaum, M.M.; Roenigk, H.H., Jr. Effects of topically applied capsaicin on moderate and severe psoriasis vulgaris. J. Am. Acad. Dermatol. 1986, 15, 504–507. [Google Scholar] [CrossRef]
- Andersen, H.H.; Sand, C.; Elberling, J. Considerable Variability in the Efficacy of 8% Capsaicin Topical Patches in the Treatment of Chronic Pruritus in 3 Patients with Notalgia Paresthetica. Ann. Dermatol. 2016, 28, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Erfan, N.; Castela, E.; Brenaut, E.; Lanteri-Minet, M.; Lacour, J.P.; Passeron, T. Successful treatment of refractory neuropathic pruritus with capsaicin 8% patch: A bicentric retrospective study with long-term follow-up. Acta Derm. Venereol. 2015, 95, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Pabst, M.A. Visceral Afferent Neurons: Role in Gastric Mucosal Protection. News Physiol. Sci. 1999, 14, 201–206. [Google Scholar] [PubMed]
- Holzer, P.; Sametz, W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology 1986, 91, 975–981. [Google Scholar] [CrossRef]
- Takeuchi, K.; Niida, H.; Matsumoto, J.; Ueshima, K.; Okabe, S. Gastric motility changes in capsaicin-induced cytoprotection in the rat stomach. Jpn. J. Pharmacol. 1991, 55, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sobue, M.; Joh, T.; Oshima, T.; Suzuki, H.; Seno, K.; Kasugai, K.; Nomura, T.; Ohara, H.; Yokoyama, Y.; Itoh, M. Contribution of capsaicin-sensitive afferent nerves to rapid recovery from ethanol-induced gastric epithelial damage in rats. J. Gastroenterol. Hepatol. 2003, 18, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Mozsik, G.; Szolcsanyi, J.; Racz, I. Gastroprotection induced by capsaicin in healthy human subjects. World J. Gastroenterol. 2005, 11, 5180–5184. [Google Scholar] [PubMed]
- Fukushima, K.; Aoi, Y.; Kato, S.; Takeuchi, K. Gastro-protective action of lafutidine mediated by capsaicin-sensitive afferent neurons without interaction with TRPV1 and involvement of endogenous prostaglandins. World J. Gastroenterol. 2006, 12, 3031–3017. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, M.N. Capsaicin and gastric ulcers. Crit. Rev. Food Sci. Nutr. 2006, 46, 275–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.O.; Lee, K.H.; Pyo, J.H.; Kim, J.H.; Choi, Y.J.; Lee, Y.C. Anti-inflammatory effect of capsaicin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter 2007, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Shabib, S.; Sherman, P.M. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol. Lett. 1997, 146, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Teng, C.H.; Wee, A.; Chen, F.C. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in the rat. Gut 1995, 36, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.S.; Ricardo, I.; Azliyati, A.; Laxminarayan, R.; Amol, B.; Santosh, W.; Boo, K. Assessment of risk factors of helicobacter pylori infection and peptic ulcer disease. J. Glob. Infect. Dis. 2013, 5, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.E.; Lake, A.G. Use of Vanilloids in Urologic Disorders. In Capsaicin as a Therapeutic Molecule; Abdel-Salam, E.O.M., Ed.; Springer Basel: Basel, Switzerland, 2014; pp. 307–317. [Google Scholar]
- Haab, F. Chapter 1: The conditions of neurogenic detrusor overactivity and overactive bladder. Neurourol. Urodyn. 2014, 33 (Suppl. S3), S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.T.; Casagrande, R.A.; Wouters, F.; Watanabe, T.T.; Boabaid, F.M.; Cruz, C.E.; Driemeier, D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J. Vet. Diagn. Investig. 2013, 25, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Wadie, B.S. Management of refractory OAB in the non-neurogenic patient. Curr. Urol. Rep. 2014, 15, 438. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Nishizawa, O.; Takeda, M.; Yokoyama, O.; Homma, Y.; Kakizaki, H.; Obara, K.; Gotoh, M.; Igawa, Y.; Seki, N.; et al. Clinical guidelines for overactive bladder. Int. J. Urol. 2009, 16, 126–142. [Google Scholar] [CrossRef] [PubMed]
- De Seze, M.; Wiart, L.; Joseph, P.A.; Dosque, J.P.; Mazaux, J.M.; Barat, M. Capsaicin and neurogenic detrusor hyperreflexia: A double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol. Urodyn. 1998, 17, 513–523. [Google Scholar] [CrossRef]
- Wiart, L.; Joseph, P.A.; Petit, H.; Dosque, J.P.; de Seze, M.; Brochet, B.; Deminiere, C.; Ferriere, J.M.; Mazaux, J.M.; N’Guyen, P.; et al. The effects of capsaicin on the neurogenic hyperreflexic detrusor. A double blind placebo controlled study in patients with spinal cord disease. Preliminary results. Spinal Cord 1998, 36, 95–99. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Tharion, G.; Richar, J.; Macaden, A.S.; Thomas, R.; Bhattacharji, S. The effectiveness of intravesical oxybutynin, propantheline, and capsaicin in the management of neuropathic bladder following spinal cord injury. Sci. World J. 2007, 7, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Soontrapa, S.; Ruksakul, W.; Nonthasood, B.; Tappayuthpijarn, P. The efficacy of Thai capsaicin in management of overactive bladder and hypersensitive bladder. J. Med. Assoc. Thail. 2003, 86, 861–867. [Google Scholar] [PubMed]
- MacDonald, R.; Monga, M.; Fink, H.A.; Wilt, T.J. Neurotoxin treatments for urinary incontinence in subjects with spinal cord injury or multiple sclerosis: A systematic review of effectiveness and adverse effects. J. Spinal Cord Med. 2008, 31, 157–165. [Google Scholar] [PubMed]
- De Seze, M.; Gallien, P.; Denys, P.; Labat, J.J.; Serment, G.; Grise, P.; Salle, J.Y.; Blazejewski, S.; Hazane, C.; Moore, N.; et al. Intravesical glucidic capsaicin versus glucidic solvent in neurogenic detrusor overactivity: A double blind controlled randomized study. Neurourol. Urodyn. 2006, 25, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Kim, J.H.; Torimoto, K.; Kwon, D.D.; Kim, Y.T.; Tyagi, P.; Yoshimura, N.; Chancellor, M.B. Early capsaicin intervention for neurogenic bladder in a rat model of spinal cord injury. Biomed. Res. 2007, 28, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Everaerts, W.; Gevaert, T.; Nilius, B.; De Ridder, D. On the origin of bladder sensing: Trips in urology. Neurourol. Urodyn. 2008, 27, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolidis, A.; Brady, C.M.; Yiangou, Y.; Davis, J.; Fowler, C.J.; Anand, P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 2005, 65, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Y.; Simard, J.M.; Chai, T.C. Increased transient receptor potential vanilloid type 1 (TRPV1) signaling in idiopathic overactive bladder urothelial cells. Neurourol. Urodyn. 2011, 30, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.A.; Wolf-Johnston, A.S.; Sun, Y.; Chai, T.C. Alteration in TRPV1 and Muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol. 2013, 207, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Bley, K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Lloyd, R.; Moore, R.A.; McQuay, H.J. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2009, CD007393. [Google Scholar] [CrossRef]
- Hempenstall, K.; Nurmikko, T.J.; Johnson, R.W.; A'Hern, R.P.; Rice, A.S. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005, 2, e164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormack, P.L. Capsaicin dermal patch: In non-diabetic peripheral neuropathic pain. Drugs 2010, 70, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Brown, S.; Tobias, J.K.; Vanhove, G.F.; Group, N.-C.S. NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: Results of a 52-week open-label study. Clin. J. Pain 2014, 30, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, C.; Patel, S.; Trueman, D.; Bentley, A.; Poole, C. Cost-Effectiveness of Capsaicin 8% Patch Compared with Pregabalin for the Treatment of Patients with Peripheral Neuropathic Pain in Scotland. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Haanpaa, M.; Cruccu, G.; Nurmikko, T.J.; McBride, W.T.; Docu Axelarad, A.; Bosilkov, A.; Chambers, C.; Ernault, E.; Abdulahad, A.K. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur. J. Pain 2016, 20, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.O.; Moritani, T. Alterations of autonomic nervous activity and energy metabolism by capsaicin ingestion during aerobic exercise in healthy men. J. Nutr. Sci. Vitaminol. 2007, 53, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Davis, B.P.; Picard, J.K.; Cooper, J.P.; Zheng, S.; Levin, L.S. A randomized, double-blind, parallel trial comparing capsaicin nasal spray with placebo in subjects with a significant component of nonallergic rhinitis. Ann. Allergy Asthma Immunol. 2011, 107, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ciabatti, P.G.; D’Ascanio, L. Intranasal Capsicum spray in idiopathic rhinitis: a randomized prospective application regimen trial. Acta Otolaryngol. 2009, 129, 367–371. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. https://doi.org/10.3390/molecules21070844
Fattori V, Hohmann MSN, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules. 2016; 21(7):844. https://doi.org/10.3390/molecules21070844
Chicago/Turabian StyleFattori, Victor, Miriam S. N. Hohmann, Ana C. Rossaneis, Felipe A. Pinho-Ribeiro, and Waldiceu A. Verri. 2016. "Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses" Molecules 21, no. 7: 844. https://doi.org/10.3390/molecules21070844





