Capsaicin Synthesis Requires in Situ Phenylalanine and Valine Formation in in Vitro Maintained Placentas from Capsicum chinense
Abstract
:1. Introduction
2. Results
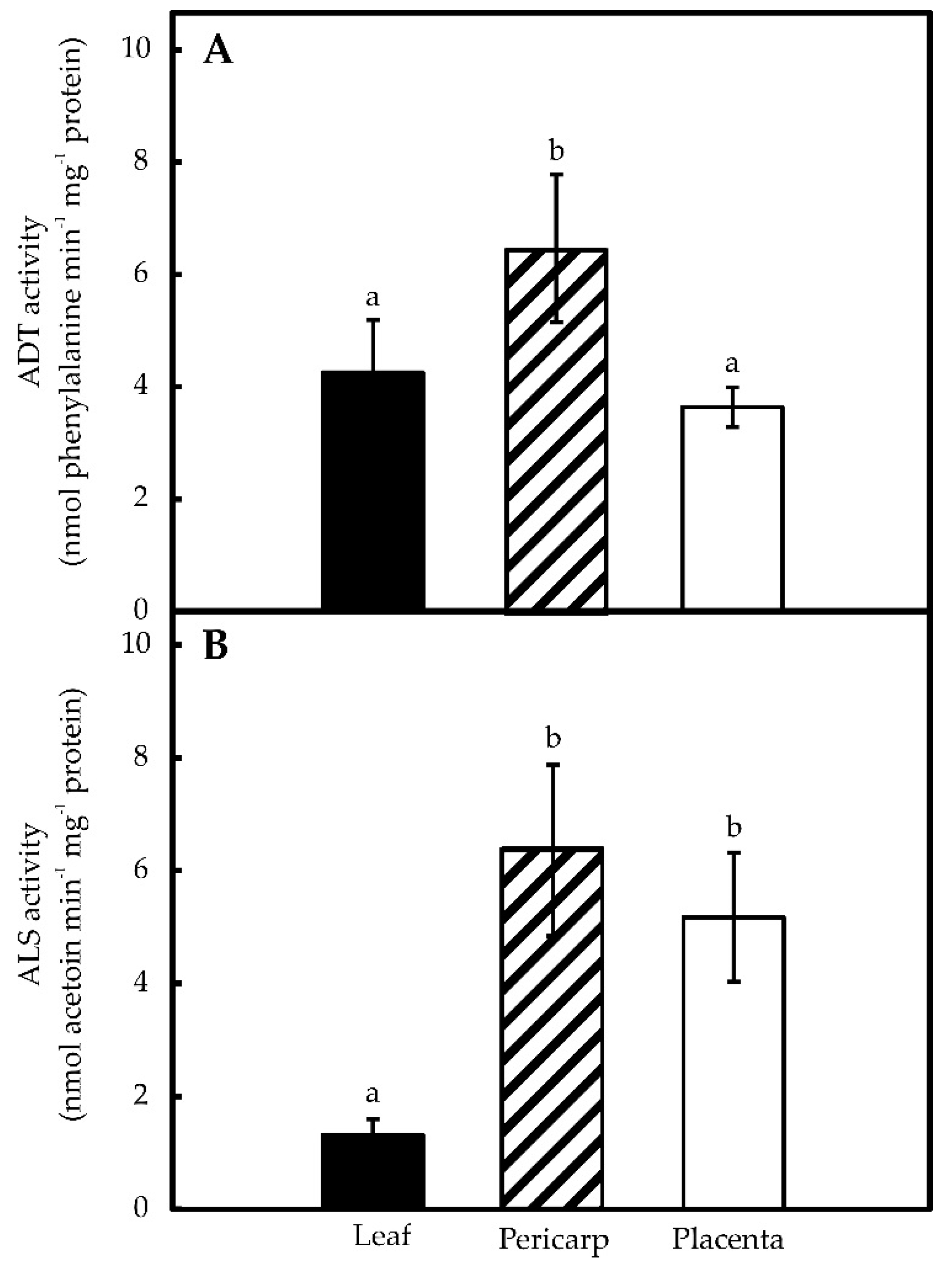
2.1. Pepper Placentas Are Able to Synthesize both Phe and Val in Situ
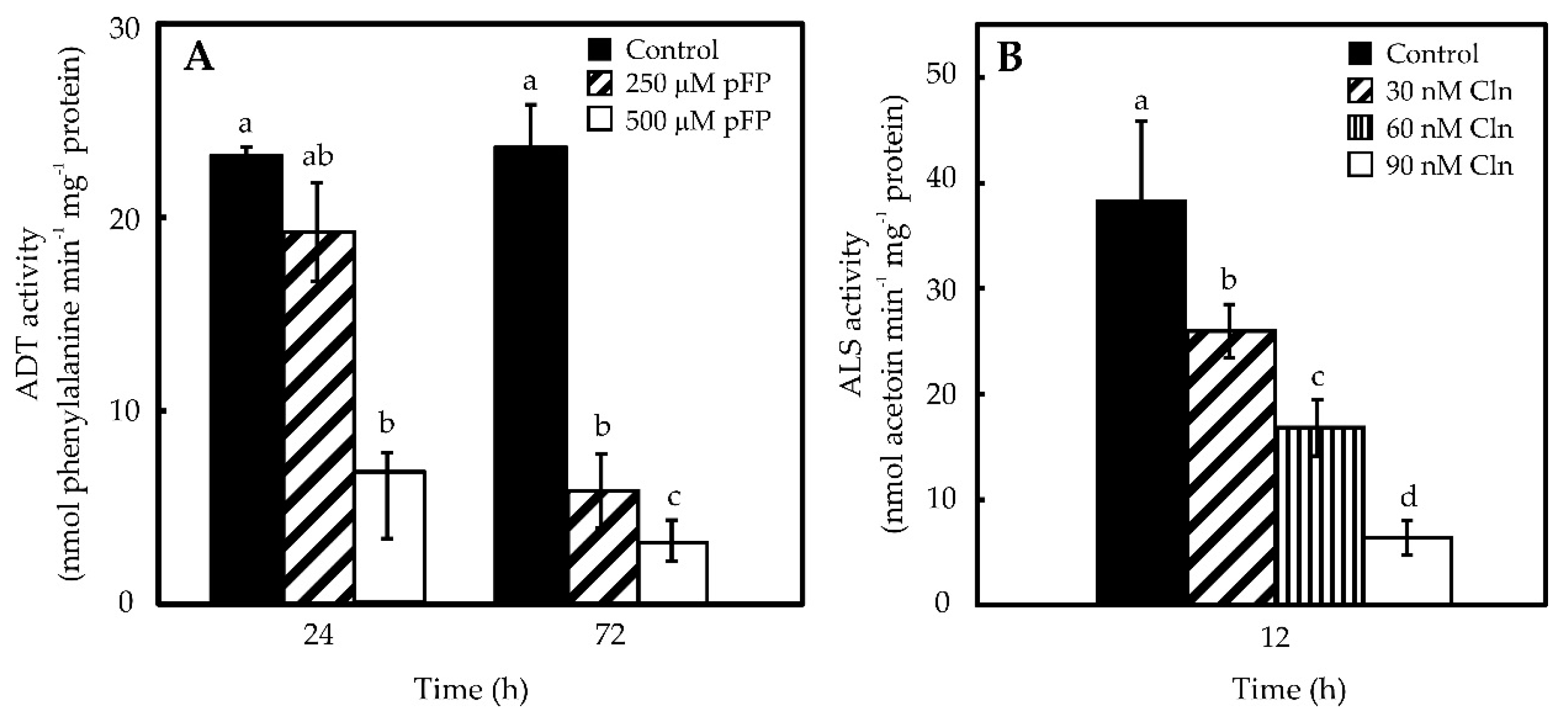
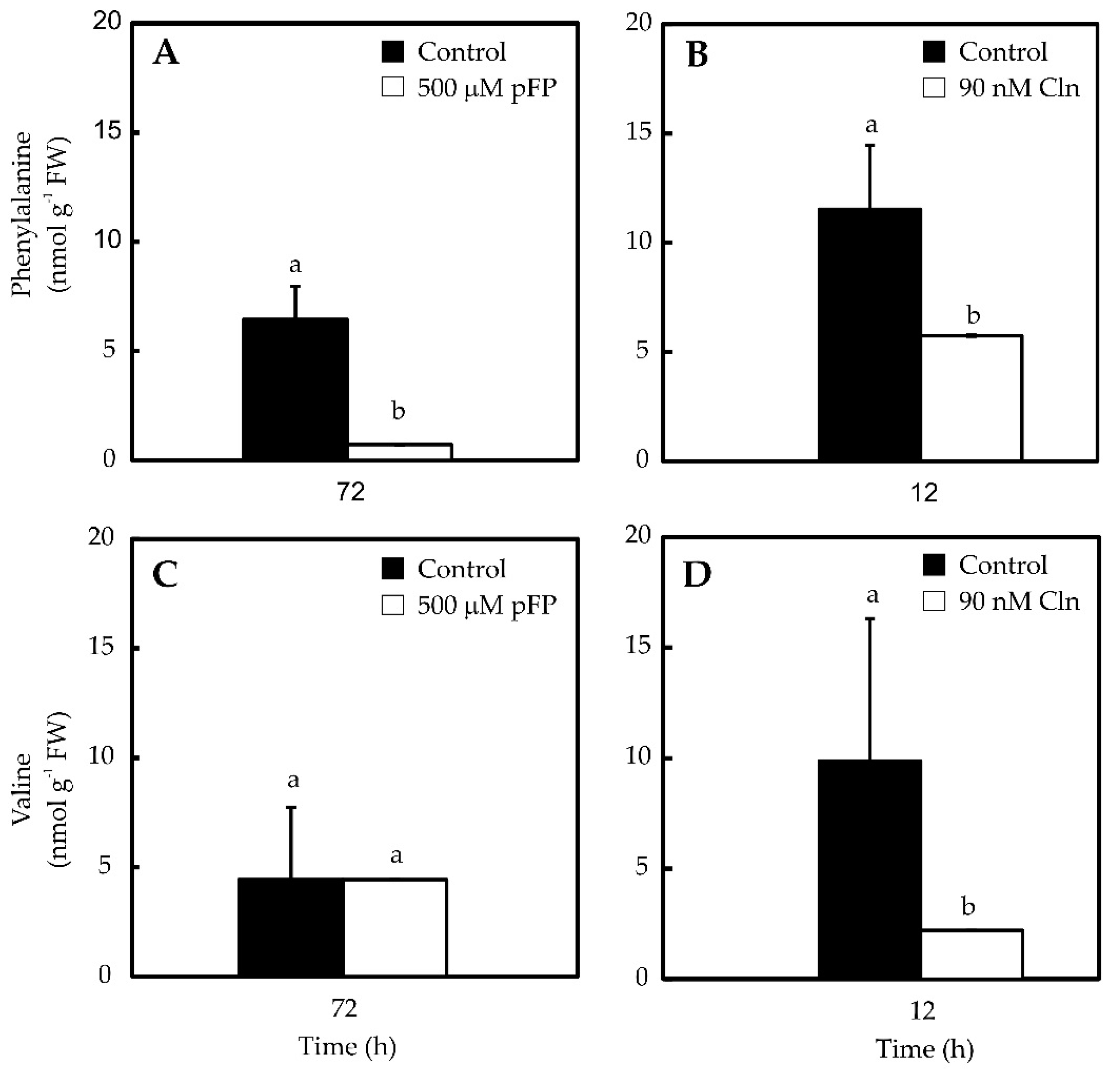
2.2. Phe and Val Accumulation in Pepper Placentas Are Sensitive to p-Fluorophenylalanine (p-FP) and Chlorsulfuron (Cln)
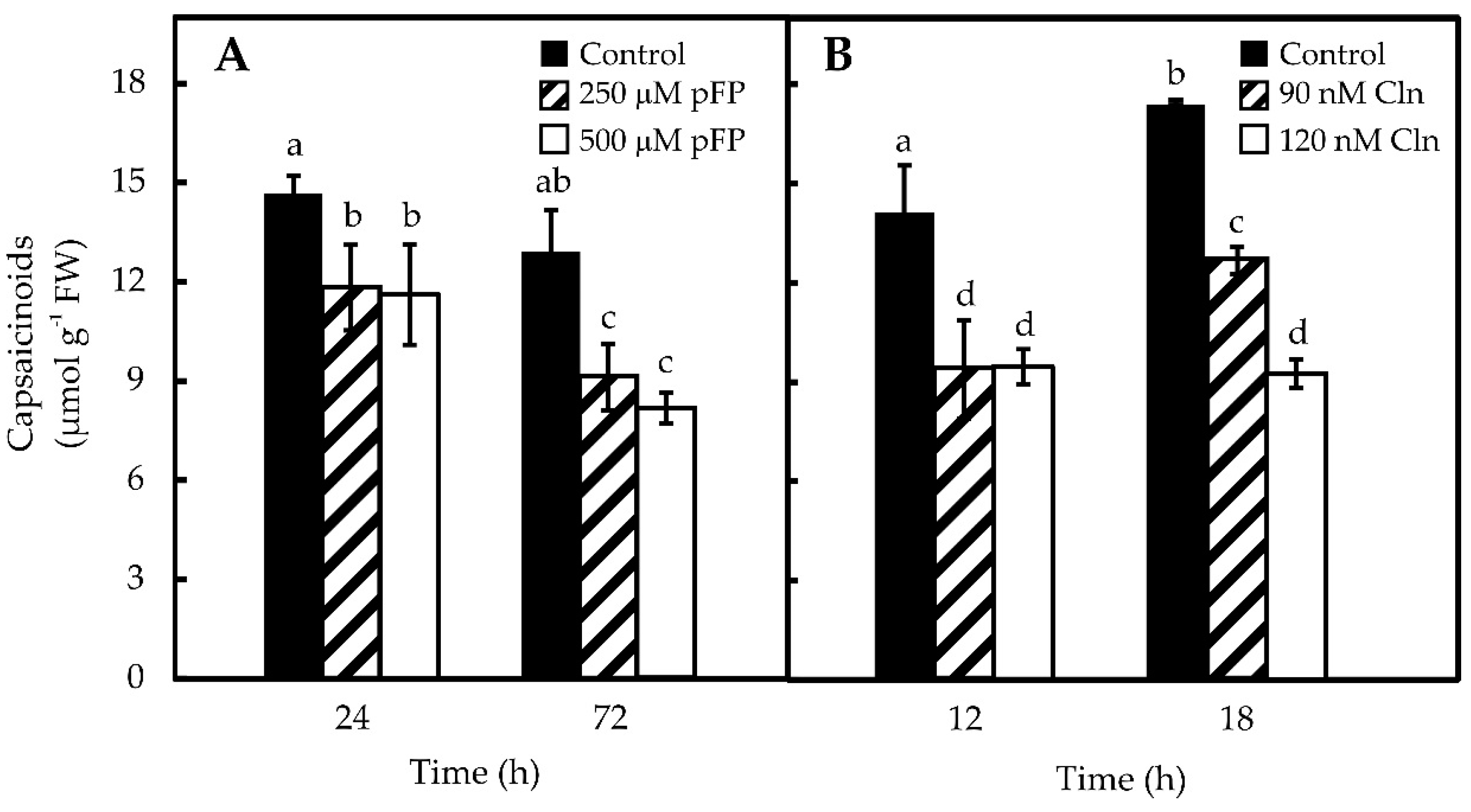
2.3. Blocking Phe and Val Synthesis Reduces CAP Accumulation in Pepper Placentas
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Inhibition of Phe and Val Synthesis
4.3. Determination of ADT and ALS Specific Activities
4.4. Determination of Metabolites in Placental Tissues
4.5. ADT and ALS mRNAs’ Relative Abundance
4.6. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castro-Concha, L.A.; Canché-Chuc, I.; Miranda-Ham, M.L. Determination of antioxidants in fruit tissues from three accessions of habanero pepper (Capsicum chinense Jacq.). J. Mex. Chem. Soc. 2012, 56, 15–18. [Google Scholar]
- Silva, L.R.; Acevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Kang, B.C.; Liu, K.; Mazourek, M.; Moore, S.L.; Yoo, E.Y.; Kim, B.D.; Paran, I.; Jahn, M.M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Murota, K.; Shimura, H.; Furuya, M.; Togawa, Y.; Matsumura, T.; Masuta, C. Evidence of capsaicin synthase activity of the Pun1-encoded protein and its role as a determinant of capsaicinoid accumulation in pepper. BMC Plant Biol. 2015, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of different Capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography. Determination of pungency and effect of high temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of environments on the accumulation of capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar]
- Johnson, C.D.; Decoteau, D.R. Nitrogen and potassium fertility affects Jalapeño pepper plant growth, pod yield, and pungency. HortScience 1996, 31, 1119–1123. [Google Scholar]
- Monforte-González, M.; Guzmán-Antonio, A.; Uuh-Chim, F.; Vázquez-Flota, F. Capsaicin accumulation is related to nitrate content in placentas of habanero peppers (Capsicum chinense Jacq.). J. Sci. Food Agric. 2010, 90, 764–768. [Google Scholar] [PubMed]
- Aldana-Iuit, J.G.; Sauri-Duch, E.; Miranda-Ham, M.L.; Castro-Concha, L.A.; Cuevas-Glory, L.F.; Vázquez-Flota, F.A. Nitrate promotes capsaicin accumulation in Capsicum chinense immobilized placentas. BioMed. Res. Int. 2015, 2015, 794084. [Google Scholar] [CrossRef] [PubMed]
- Ancona-Escalante, W.R.; Baas-Espinola, F.M.; Castro-Concha, L.A.; Vázquez-Flota, F.A.; Zamudio-Maya, M.; Miranda-Ham, M.L. Induction of capsaicinoid accumulation in placental tissues of Capsicum chinense Jacq. requieres primary ammonia assimilation. Plant Cell Tiss. Org. 2013, 113, 565–570. [Google Scholar] [CrossRef]
- Salgado-Garciglia, R.; Ochoa-Alejo, N. Increased capsaicin content in PFP-resistant cells of chili pepper (Capsicum annuum L.). Plant Cell Rep. 1990, 8, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.C.N.; Gururaj, H.B.; Kumar, V.; Giridhar, P.; Parimalan, R.; Sharma, A.; Ravishankar, G.A. Influence of 8-methyl-nonenoic acid on capsaicin biosynthesis in in-vivo and in-vitro cell cultures of Capsicum spp. J. Agric. Food Chem. 2006, 54, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Castro-Concha, L.; Baas-Espinola, F.; Ancona-Escalante, W.; Vázquez-Flota, F.; Miranda-Ham, M.L. Phenylalanine biosynthesis and its relationship to accumulation of capsaicinoids during Capsicum chinense fruit development. Biol. Plantarum 2016, 60, 579–584. [Google Scholar] [CrossRef]
- Ray, T.B. Site of action of chlorsulfuron. Inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984, 75, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Hareven, D.; Gutfinger, T.; Ken-Dror, S.; Lifschitz, E. Biosynthetic threonine deaminase gene of tomato: Isolation, structure, and upregulation in floral organs. PNAS 1991, 88, 2678–2682. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-H.; Corea, O.R.; Yang, H.; Bedgar, D.L.; Laskar, D.D.; Anterola, A.M.; Moog-Anterola, F.A.; Hood, R.L.; Kohalmi, S.E.; Bernards, M.A. Phenylalanine biosynthesis in Arabidopsis thaliana identification and characterization of arogenate dehydratases. J. Biol. Chem. 2007, 282, 30827–30835. [Google Scholar] [CrossRef] [PubMed]
- Lancien, M.; Lea, P.J.; Azevedo, R.A. Amino acid synthesis in plastids. In The Structure and Function of Plastids; Wise, R.R., Hoober, J.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 23, pp. 355–385. [Google Scholar]
- Maeda, H.; Shasany, A.K.; Schnepp, J.; Orlova, I.; Taguchi, G.; Cooper, B.R.; Rhodes, D.; Pichersky, E.; Dudareva, N. RNAi suppression of arogenate dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals. Plant Cell 2010, 22, 832–849. [Google Scholar] [CrossRef] [PubMed]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A dynamic interface for capsaicinoid systems biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Sukrasno, N.; Yeoman, M. Phenylpropanoid metabolism during growth and development of Capsicum frutescens fruits. Phytochemistry 1993, 32, 839–844. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuum var. annuum cv. karayatsubusa at different growth stages after flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar]
- Zamudio-Moreno, E.; Echevarría-Machado, I.; Medina-Lara, M.F.; Calva-Calva, G.; Miranda-Ham, M.L.; Martínez-Estévez, M. Role of peroxidases in capsaicinoids degradation in habanero pepper (Capsicum chinense Jacq.) plants grown under water deficit conditions. Aust. J. Crop Sci. 2014, 8, 448–454. [Google Scholar]
- Díaz, J.; Pomar, F.; Bernal, A.; Merino, F. Peroxidases and the metabolism of capsaicin in Capsicum annuum L. Phytochem. Rev. 2004, 3, 141–157. [Google Scholar] [CrossRef]
- Nuñez-Palenius, H.; Ochoa-Alejo, N. Effect of phenylalanine and phenylpropanoids on the accumulation of capsaicinoids and lignin in cell cultures of chili pepper (Capsicum annuum L.). In Vitro Cell. Dev. Biol. Plant 2005, 41, 801–805. [Google Scholar] [CrossRef]
- Liu, S.; Chen, C.; Chen, G.; Cao, B.; Chen, Q.; Lei, J. RNA-sequencing tag profiling of the placenta and pericarp of pungent pepper provides robust candidates contributing to capsaicinoid biosynthesis. Plant Cell Tiss. Org. 2012, 110, 111–121. [Google Scholar] [CrossRef]
- Martínez-López, L.A.; Ochoa-Alejo, N.; Martínez, O. Dynamics of the chili pepper transcriptome during fruit development. BMC Genomics 2014, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.S.; Ravishankar, G.; Venkataraman, L. In vitro capsaicin production by immobilized cells and placental tissues of Capsicum annuum L. grown in liquid medium. Plant Sci. 1990, 70, 223–229. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Salgado-Garciglia, R. Phenylalanine ammonia-lyase activity and capsaicin-precursor compounds in p-fluorophenylalanine-resistant and-sensitive variant cells of chili pepper (Capsicum annuum). Physiol. Plant. 1992, 85, 173–179. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Org. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid-associated redox status and defense responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary-secondary metabolism interface. Curr. Opin. Biotech. 2011, 22, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M.; Vázquez-Flota, F. An introduction to plant cell culture. In Plant Cell Culture Protocols, 2nd ed.; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 318, pp. 3–8. [Google Scholar]
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. PNAS 2010, 107, 15287–15292. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Eberlein, C.V.; Guttieri, M.J.; Mallory-Smith, C.A.; Thill, D.C.; Baerg, R.J. Altered acetolactate synthase activity in ALS-inhibitor resistant prickly lettuce (Lactuca serriola). Weed Sci. 1997, 45, 212–217. [Google Scholar]
- Connelly, J.A.; Siehl, D.L. Purification of chorismate, prephenate, and arogenate by HPLC. Method Enzymol. 1987, 142, 422–431. [Google Scholar]
- Rippert, P.; Matringe, M. Molecular and biochemical characterization of an Arabidopsis thaliana arogenate dehydrogenase with two highly similar and active protein domains. Plant Mol. Biol. 2002, 48, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Jensen, R. Arogenate dehydratase. Method Enzymol. 1987, 142, 495–502. [Google Scholar]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Collins, M.D.; Wasmund, L.M.; Bosland, P.W. Improved method for quantifying capsaicinoids in Capsicum using high-performance liquid chromatography. HortScience 1995, 30, 137–139. [Google Scholar]
- Rubio-Piña, J.A.; Vázquez-Flota, F.A. Isolation of functional total RNA from Argemone mexicana tissues. Electron. J. Biotechnol. 2008, 11, 15–16. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are commercially available.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baas-Espinola, F.M.; Castro-Concha, L.A.; Vázquez-Flota, F.A.; Miranda-Ham, M.L. Capsaicin Synthesis Requires in Situ Phenylalanine and Valine Formation in in Vitro Maintained Placentas from Capsicum chinense. Molecules 2016, 21, 799. https://doi.org/10.3390/molecules21060799
Baas-Espinola FM, Castro-Concha LA, Vázquez-Flota FA, Miranda-Ham ML. Capsaicin Synthesis Requires in Situ Phenylalanine and Valine Formation in in Vitro Maintained Placentas from Capsicum chinense. Molecules. 2016; 21(6):799. https://doi.org/10.3390/molecules21060799
Chicago/Turabian StyleBaas-Espinola, Fray M., Lizbeth A. Castro-Concha, Felipe A. Vázquez-Flota, and María L. Miranda-Ham. 2016. "Capsaicin Synthesis Requires in Situ Phenylalanine and Valine Formation in in Vitro Maintained Placentas from Capsicum chinense" Molecules 21, no. 6: 799. https://doi.org/10.3390/molecules21060799





