A New One-Pot Synthesis of Quinoline-2-carboxylates under Heterogeneous Conditions
Abstract
:1. Introduction
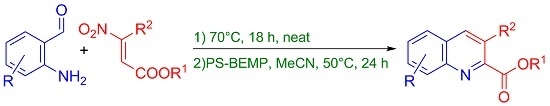
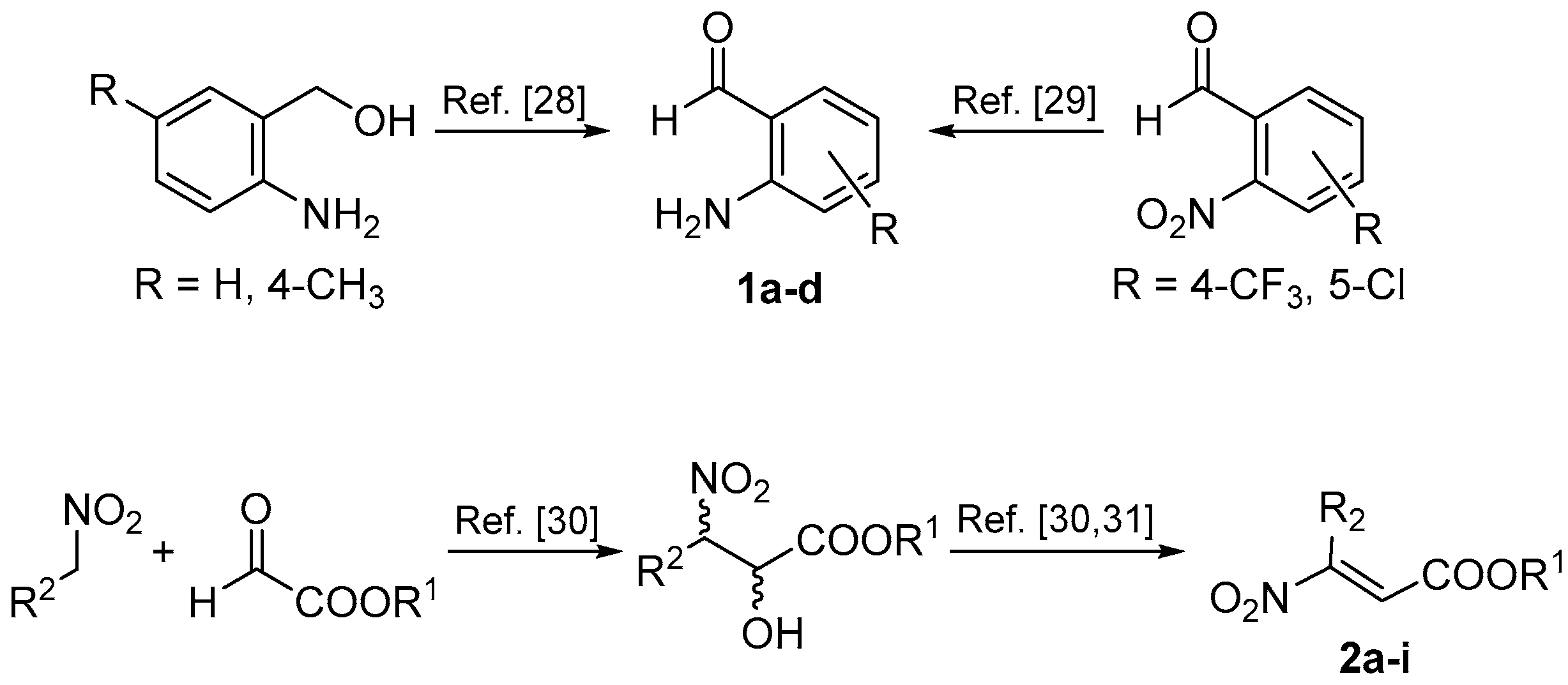
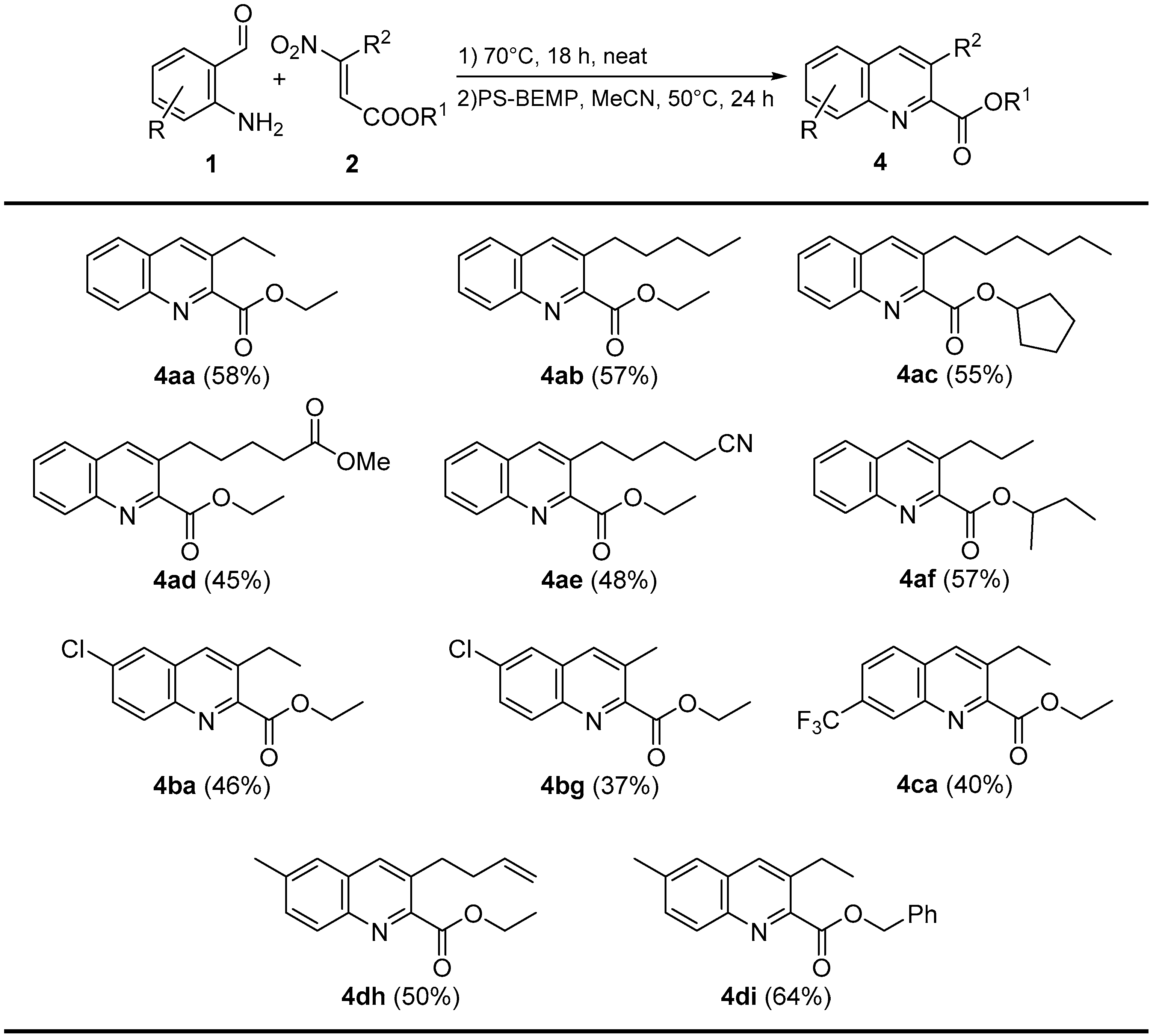
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General Section
4.2. Chemistry Section
Acknowledgments
Conflicts of Interest
Abbreviations
| TMG | 1,1,3,3-Tetramethylguanidine |
| DBU | 1,5-Diazabiciclo[5.4.0]undec-5-ene |
| BEMP | 2-tert-Butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine |
| TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene |
References and Note
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Ciufolini, M.A.; Roschangar, F. Practical total synthesis of (+)-camptothecin: The full story. Tetrahedron 1997, 53, 11049–11060. [Google Scholar] [CrossRef]
- Manfredini, S.; Vertuani, S.; Pavan, B.; Vitali, F.; Scaglianti, M.; Bortolotti, F.; Biondi, C.; Scatturin, A.; Prasad, P.; Dalpiaz, A. Design, synthesis and in vitro evaluation on HRPE cells of ascorbic and 6-bromoascorbic acid conjugates with neuroactive molecules. Bioorg. Med. Chem. 2004, 12, 5453–5463. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Monga, V.; Malde, A.; Coutinhob, E.; Jaina, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolines. Bioorg. Med. Chem. 2007, 15, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Horchler, C.L.; McCauley, J.P.; Hall, J.E.; Snyder, D.H.; Moore, W.C.; Hudzik, T.J.; Chapdelaine, M.J. Synthesis of novel quinoline and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: A late-stage diversification approach to potent 5HT1B antagonists. Bioorg. Med. Chem. 2007, 15, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Contour-Galcéra, M.-O.; Sidhu, A.; Plas, P.; Roubert, P. 3-Thio-1,2,4-triazoles, novel somatostatin sst2/sst5 agonists. Bioorg. Med. Chem. Lett. 2005, 15, 3555–3559. [Google Scholar] [CrossRef] [PubMed]
- Saburi, H.; Tanaka, S.; Kitamura, M. Catalytic dehydrative allylation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Saburi, H.; Ishibashi, Y.; Kitamura, M. CpRuIIPF6/Quinaldic Acid-Catalyzed Chemoselective Allyl Ether Cleavage. A Simple and Practical Method for Hydroxyl Deprotection. Org. Lett. 2004, 6, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, Y.; Yamashita, K.; Higuchi, Y.; Goto, Y. Improved oxidation of active methyl group of N-heteroaromatic compounds by selenium dioxide in the presence of tert-butyl hydroperoxide. Heterocycles 2003, 60, 953–957. [Google Scholar] [CrossRef]
- George, I.R.; Lewis, W.; Moody, C.J. Synthesis of iodopyridone. Tetrahedron 2013, 69, 8209–8215. [Google Scholar] [CrossRef]
- Shrader, W.D.; Celebuski, J.; Kline, S.J.; Johnson, D. Synthesis of a novel hexadentate chelating agent based on8-hydroxyquinoline. Tetrahedron Lett. 1988, 29, 1351–1354. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, H.; Chen, K.; Liu, H. A Simple and convenient copper-catalyzed tandem synthesis of quinoline-2-carboxylates at room temperature. J. Org. Chem. 2009, 74, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, R.; Stopka, T.; Richter, H.; Mancheño, O.G. Iron-catalyzed oxidative tandem reactions with TEMPO oxoammonium salts: Synthesis of dihydroquinazolines and quinolines. J. Org. Chem. 2013, 78, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.Y.; Caravella, J.A.; Chen, L.; Creech, K.L.; Deaton, D.N.; Madauss, K.P.; Marr, H.B.; McFadyen, R.B.; Miller, A.B.; Mills, W.Y.; et al. Conformationally constrained farnesoid X receptor (FXR) agonists: Heteroaryl replacements of the naphthalene. Bioorg. Med. Chem. Lett. 2011, 21, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Ciufolini, M.A.; Mitchell, J.W.; Rosehangar, F. Facile palladium-mediated substitution of chlorine in 2-chloroquinolines. Tetrahedron Lett. 1996, 37, 8281–8284. [Google Scholar] [CrossRef]
- Itoh, S.; Fukui, Y.; Haranou, S.; Ogino, M.; Komatau, M.; Ohshiro, Y. Synthesis and characterization of dimethyl 9,10-dihydro-9,10-dioxobenzo[f]quinoline-2,4-dicarboxylate. Effect of the pyrrole nucleus on the reactivity of coenzyme PQQ. J. Org. Chem. 1992, 57, 4452–4457. [Google Scholar] [CrossRef]
- Ballini, R.; Palmieri, A.; Talaq, M.A.; Gabrielli, S. Preparation of 2H-1,4-benzoxazin-2-one derivatives under heterogeneous conditions via domino process. Adv. Synth. Catal. 2009, 351, 2611–2614. [Google Scholar] [CrossRef]
- Ballini, R.; Gabrielli, S.; Palmieri, A. β-Nitroacrylates as key starting materials for the uncatalysed one-pot synthesis of polyfunctionalized dihydroquinoxalinone derivatives, via an anti-Michael reaction. Synlett 2009, 2009, 965–967. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Gabrielli, S.; Palmieri, A. β -Nitroacrylates and silyl enol ethers as key starting materials for the synthesis of polyfunctionalized β-nitro esters and 1,2-oxazine-2-oxides. Tetrahedron 2009, 65, 2916–2920. [Google Scholar] [CrossRef]
- Ballini, R.; Gabrielli, S.; Palmieri, A. β-Nitroacrylates as precursors of tetrasubstituted furans in a one-pot process and under acidic solvent-free conditions. Synlett 2010, 2010, 2468–2470. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Lanari, D.; Vaccaro, L.; Ballini, R. A new one-pot synthesis of polysubstituted indoles from pyrroles and β-nitroacrylates. Adv. Synth. Catal. 2011, 353, 1425–1428. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Cimarelli, C.; Ballini, R. Fast, mild, eco-friendly synthesis of polyfunctionalized pyrroles from β-nitroacrylates and β-enaminones. Green Chem. 2011, 13, 3333–3336. [Google Scholar] [CrossRef]
- Gabrielli, S.; Ballini, R.; Palmieri, A. β-Nitroacrylates as key building blocks for the synthesis of alkyl 3-substituted 5-oxopiperazine-2-carboxylates under fully heterogeneous conditions. Mon. Chem. 2013, 144, 509–514. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Maggi, R.; Ballini, R. β-Nitroacrylates as useful building blocks for the synthesis of alkyl indole-2-carboxylates. Synlett 2014, 25, 128–132. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Parlapiano, M.; Ballini, R. One-pot synthesis of alkyl pyrrole-2-carboxylates starting from β-nitroacrylates and primary amines. RSC Adv. 2015, 5, 4210–4213. [Google Scholar] [CrossRef]
- Castaing, M.; Wason, S.L.; Estepa, B.; Hooper, J.F.; Willis, M.C. 2-Aminobenzaldehydes as versatile substrates for rhodium-catalyzed alkyne hydroacylation: Application to dihydroquinoline synthesis. Angew. Chem. Int. Ed. 2013, 52, 13280–13283. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, M.J.; Bou Zerdan, R.M.; Kurth, M.J.; Fettinger, J.C. Efficient syntheses of the unknown quinolino[2,3-c]cinnolines; synthesis of neocryptolepines. Org. Lett. 2010, 12, 5502–5505. [Google Scholar] [CrossRef] [PubMed]
- Ballini, R.; Fiorini, D.; Palmieri, A. Nitroalkanes and ethyl glyoxalate as common precursors for the preparation of both β-keto esters and α,β-unsaturated esters. Tetrahedron Lett. 2004, 45, 7027–7029. [Google Scholar] [CrossRef]
- Palmieri, A.; Gabrielli, S.; Ballini, R. Low impact synthesis of β-nitroacrylates under fully heterogeneous conditions. Green Chem. 2013, 15, 2344–2348. [Google Scholar] [CrossRef]
- The BEMP on polymer support was purchased from Sigma–Aldrich (code 536490) and used directly without any manipulation.
- Sample Availability: Samples of the compounds 4 are available from the authors.


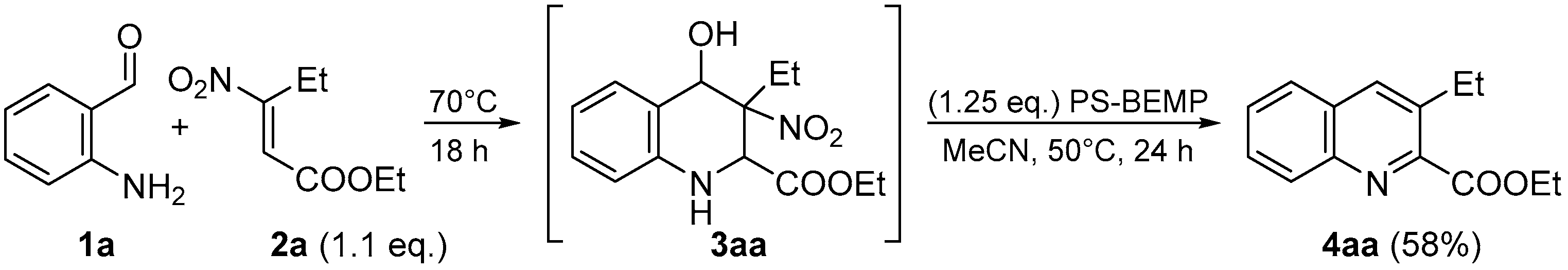
| Entry | 1a (mmol) | 2a (mmol) | Solvent | Temp. (°C) | Time (h) | Yield (%) 1 |
|---|---|---|---|---|---|---|
| Entry 1 | 1 | 1 | - | r.t. | 24 | Traces |
| Entry 2 | 1 | 1 | - | 50 | 24 | 44 |
| Entry 3 | 1 | 1 | - | 60 | 24 | 51 |
| Entry 4 | 1 | 1 | - | 70 | 18 | 61 |
| Entry 5 | 1 | 1 | - | 80 | 18 | 52 |
| Entry 6 | 1.1 | 1 | - | 70 | 18 | 59 |
| Entry 7 | 1 | 1.1 | - | 70 | 18 | 66 |
| Entry 8 | 1 | 1.2 | - | 70 | 18 | 64 |
| Entry 9 | 1 | 1.1 | MeCN | 70 | 18 | 11 |
| Entry 10 | 1 | 1.1 | EtOAc | 70 | 18 | 9 |
| Entry 11 | 1 | 1.1 | - | 70 | 18 | 21 2 |
| Entry 12 | 1 | 1.1 | - | 70 | 18 | 8 3 |
| Entry | Base (eq.) | Solvent | Temp. (°C) | Time (h) | Yield (%) 1 |
|---|---|---|---|---|---|
| Entry 1 | TMG (1) | MeCN | 50 | 24 | 47 |
| Entry 2 | DBU (1) | MeCN | 50 | 24 | 36 |
| Entry 3 | Amberlyst A21 (1) | MeCN | 50 | 24 | Traces |
| Entry 4 | TBD on polymer (1) | MeCN | 50 | 24 | 62 |
| Entry 5 | Carbonate on polymer (1) | MeCN | 50 | 24 | 57 |
| Entry 6 | BEMP on polymer (1) | MeCN | 50 | 24 | 79 |
| Entry 7 | BEMP on polymer (0.8) | MeCN | 50 | 24 | 67 |
| Entry 8 | BEMP on polymer (1.25) | MeCN | 50 | 24 | 86 |
| Entry 9 | BEMP on polymer (1.5) | MeCN | 50 | 24 | 82 |
| Entry 10 | BEMP on polymer (1.25) | MeCN | 40 | 24 | 69 |
| Entry 11 | BEMP on polymer (1.25) | MeCN | 60 | 24 | 85 |
| Entry 12 | BEMP on polymer (1.25) | EtOAc | 50 | 24 | 71 |
| Entry 13 | BEMP on polymer (1.25) | 2-MeTHF | 50 | 24 | 47 |
| Entry 14 | BEMP on polymer (1.25) | Toluene | 50 | 24 | 39 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielli, S.; Giardinieri, A.; Sampaolesi, S.; Ballini, R.; Palmieri, A. A New One-Pot Synthesis of Quinoline-2-carboxylates under Heterogeneous Conditions. Molecules 2016, 21, 776. https://doi.org/10.3390/molecules21060776
Gabrielli S, Giardinieri A, Sampaolesi S, Ballini R, Palmieri A. A New One-Pot Synthesis of Quinoline-2-carboxylates under Heterogeneous Conditions. Molecules. 2016; 21(6):776. https://doi.org/10.3390/molecules21060776
Chicago/Turabian StyleGabrielli, Serena, Alessandra Giardinieri, Susanna Sampaolesi, Roberto Ballini, and Alessandro Palmieri. 2016. "A New One-Pot Synthesis of Quinoline-2-carboxylates under Heterogeneous Conditions" Molecules 21, no. 6: 776. https://doi.org/10.3390/molecules21060776