The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis
Abstract
:1. Introduction
2. Results
2.1. Thymol Significantly Inhibited the Growth of F. graminearum
2.2. Thymol Affected Conidia Production and Conidia Germination of F. graminearum
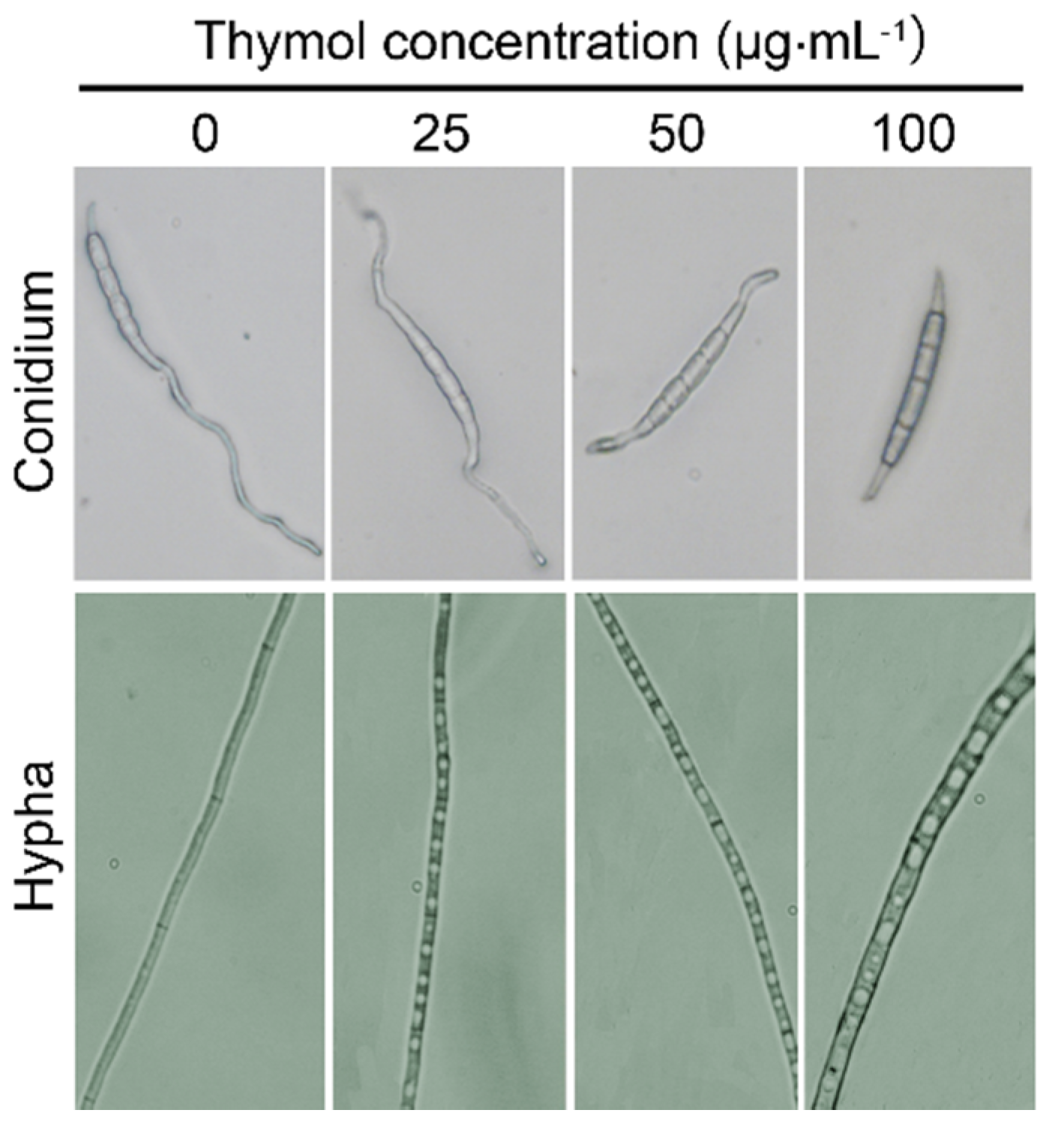
2.3. Thymol Changed the Morphology of F. graminearum
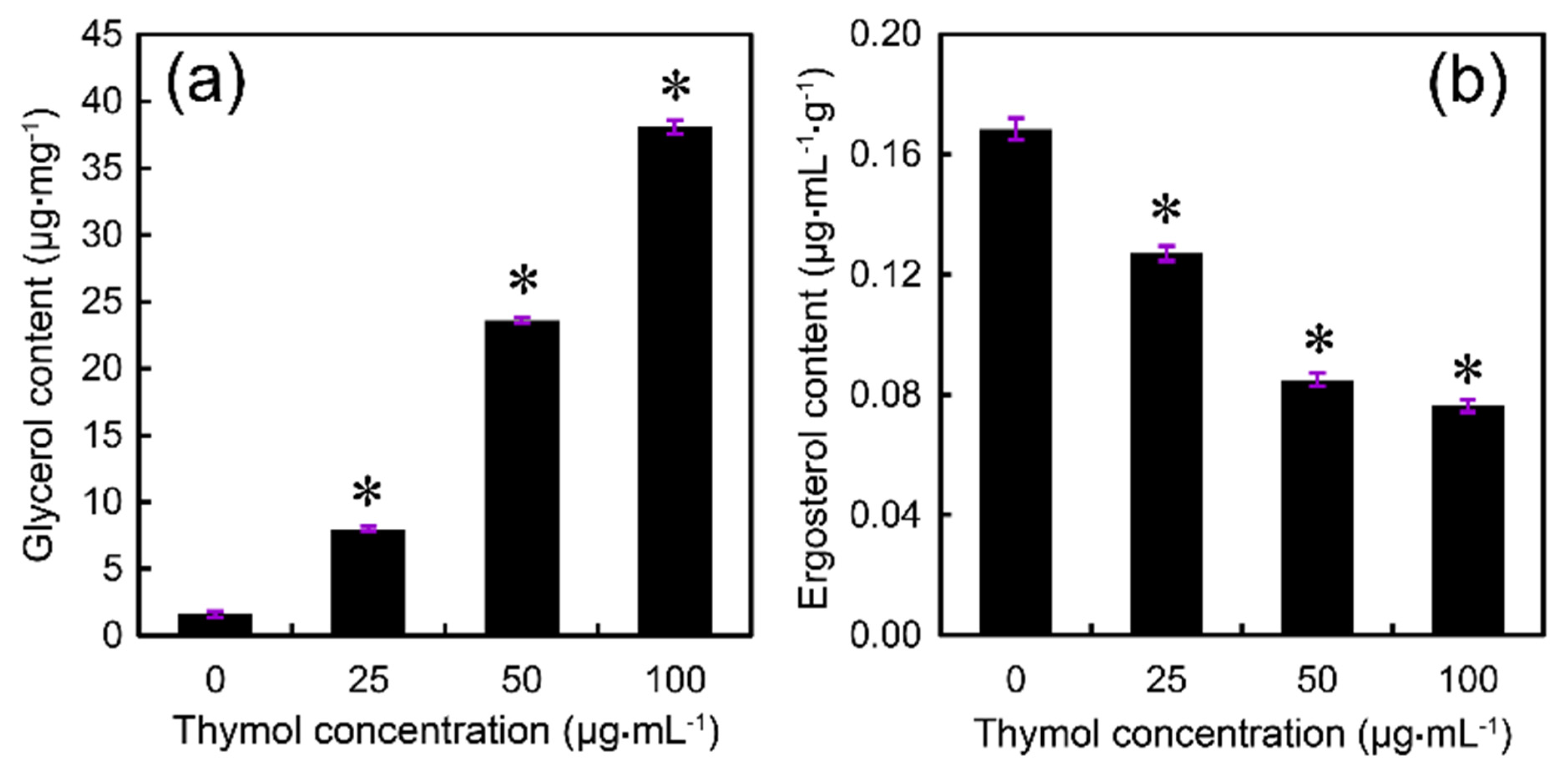
2.4. Thymol Induced Cell Membrane Injury of F. graminearum
3. Discussion
4. Materials and Methods
4.1. Media, Strains, and Chemicals
4.2. Determination of Baseline Sensitivity of F. graminearum to Thymol
4.3. Measurement of Conidiation Production, Conidiation Germination, and Mycelial Morphology of F. graminearum
4.4. Scanning Electron Microscopy (SEM)
4.5. Determination of Relative Conductivity
4.6. Determination of Glycerol Content
4.7. Determination of Lipid Peroxidation
4.8. Determination of Ergosterol Content
4.9. Histochemical Detection Cell Membrane Permeability
4.10. Analysis of Gene Expression
4.11. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.C.; Geiser, D.M.; Suga, H.; Toth, B.; Varga, J.; O’Donnell, K. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 2007, 44, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, M.-G. Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide JS399-19. Phytopathology 2009, 99, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Xu, J.; Shi, J. Molecular characterization of the Fusarium graminearum species complex in Eastern China. Eur. J. Plant Pathol. 2014, 139, 811–823. [Google Scholar] [CrossRef]
- Prat, N.; Buerstmayr, M.; Steiner, B.; Robert, O.; Buerstmayr, H. Current knowledge on resistance to Fusarium head blight in tetraploid wheat. Mol. Breed. 2014, 34, 1689–1699. [Google Scholar] [CrossRef]
- De Ackermann, M.; Kohli, M. Chemical control of Fusarium head blight of wheat. In Fusarium Head Blight in Latin America; Alconada Magliano, T.M., Chulze, S.N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 175–189. [Google Scholar]
- Liu, N.; Fan, F.; Qiu, D.; Jiang, L. The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 2013, 58–59, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Zhu, Y.F.; Liu, Y.Y.; Deng, Y.Y.; Li, W.; Zhang, A.X.; Chen, H.G. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australas. Plant Pathol. 2014, 43, 631–638. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, X.; Ge, C.; Wang, Y.; Cao, J.; Jia, X.; Wang, J.; Zhou, M. Development and application of loop-mediated isothermal amplification for detection of the F167Y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luo, Q.; Chen, C.; Zhou, M. Application of tetra primer ARMS-PCR approach for detection of Fusarium graminearum genotypes with resistance to carbendazim. Australas. Plant Pathol. 2013, 42, 73–78. [Google Scholar] [CrossRef]
- Zheng, Z.; Hou, Y.; Cai, Y.; Zhang, Y.; Li, Y.; Zhou, M. Whole-genome sequencing reveals that mutations in myosin-5 confer resistance to the fungicide phenamacril in Fusarium graminearum. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Issues Bio. Sci. Pharm. Res. 2014, 2, 1–7. [Google Scholar]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine Hazardous Substances Data Bank. Available online: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB (accessed on 7 November 2014).
- Navarro, D.; Diaz-Mula, H.M.; Guillen, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martinez-Romero, D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Pérez-Alfonso, C.O.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 2014, 35, 132–136. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Ansorena, M.; Viacava, G.; Roura, S.; Ayala-Zavala, J. Plant Essential Oils as Antifungal Treatments on the Postharvest of Fruit and Vegetables. In Antifungal Metabolites from Plants; Razzaghi-Abyaneh, M., Rai, M., Eds.; Springer Berlin: Heidelberg, Germany, 2013; pp. 429–446. [Google Scholar]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and Recovers Infected Macrophages from Oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef] [PubMed]
- De Lira Mota, K.S.; de Oliveira Pereira, F.; de Oliveira, W.A.; Lima, I.O.; de Oliveira Lima, E. Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- USEPA/IRIS Integrated Risk Information System. Available online: http://www.epa.gov/iris/ (accessed on 7 November 2014).
- Oliva, M.L.; Carezzano, M.; Giuliano, M.; Daghero, J.; Zygadlo, J.; Bogino, P.; Giordano, W.; Demo, M. Antimicrobial activity of essential oils of Thymus vulgaris and Origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. (Stuttg.) 2015, 17, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Firstencel, H.; Butt, T.M.; Carruthers, R.I. A fluorescence microscopy method for determining the viability of entomophthoralean fungal spores. J. Invertebr. Pathol. 1990, 55, 258–264. [Google Scholar] [CrossRef]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in Fungi. J. Steroid Biochem. Mol. Biol. 2012, 129, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, F.; Schnabel, G.; Wu, J.; Wang, Z.; Ma, Z. Paralogous cyp51 genes in Fusarium graminearum mediate differential sensitivity to sterol demethylation inhibitors. Fungal Genet. Biol. 2011, 48, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Brown, J.L.; Sheraton, J.; Fortin, N.; Bussey, H. A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein. Yeast 1994, 10, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.T.; Rine, J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J. Cell Sci. 2004, 117, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro Antifungal Activity of Terpinen-4-ol, Eugenol, Carvone, 1,8-cineole (eucalyptol) and Thymol against Mycotoxigenic Plant Pathogens. Food Addit. Contam. A 2011, 29, 415–422. [Google Scholar]
- Becher, R.; Miedaner, T.; Wirsel, S.R. Biology, Diversity, and Management of FHB-Causing Fusarium Species in Small-Grain Cereals. In Agricultural Applications; Kempken, F., Ed.; Springer Berlin: Heidelberg, Germany, 2013; Volume 11, pp. 199–241. [Google Scholar]
- Forrer, H.R.; Musa, T.; Schwab, F.; Jenny, E.; Bucheli, T.D.; Wettstein, F.E.; Vogelgsang, S. Fusarium head blight control and prevention of mycotoxin contamination in wheat with botanicals and tannic acid. Toxins 2014, 6, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.; Veses, V.; Gow, N.A.R. Vacuole dynamics in fungi. Fungal Biol. Rev. 2010, 24, 93–105. [Google Scholar] [CrossRef]
- Lew, R.R. Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora crassa. Fungal Genet. Biol. 2010, 47, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hansberg, W.; Navarro, R. Fungal responses to reactive oxygen species. Med. Mycol. 2006, 44, S101–S107. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS involves the fungicidal actions of thymol against spores of Aspergillus flavus via the Induction of nitric oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Jakhar, R.; Paul, S.; Kang, S.C. Potentiation of macrophage activity by thymol through augmenting phagocytosis. Int. Immunopharmacol. 2014, 18, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Iefuji, H.; Hiraga, Y.; Hosomi, A.; Morita, T.; Giga-Hama, Y.; Takegawa, K. Multiple functions of ergosterol in the fission yeast Schizosaccharomyces pombe. Microbiology 2008, 154, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H.; Tkacz, J.S. The fungal cell wall as a drug target. Trends Microbiol. 1995, 3, 98–104. [Google Scholar] [CrossRef]
- Warrilow, A.G.; Parker, J.E.; Kelly, D.E.; Kelly, S.L. Azole affinity of sterol 14α-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 2013, 57, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Curwin, A.J.; Stefan, C.J.; McMaster, C.R. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc. Natl. Acad. Sci. USA 2007, 104, 15352–15357. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Luo, Q.; Yuan, S.; Zhou, M. Characterization and fitness of carbendazim-resistant strains of Fusarium graminearum (wheat scab). Pest Manag. Sci. 2007, 63, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Thomulka, K.W.; Abbas, C.G.; Young, D.A.; Lange, J.H. Evaluating median effective concentrations of chemicals with bioluminescent bacteria. Bull. Environ. Contam. Toxicol. 1996, 56, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Xu, J.; Yu, J.; Bi, C.; Chen, C.; Zhou, M. Localisation of the benzimidazole fungicide binding site of Gibberella zeae β2-tubulin studied by site-directed mutagenesis. Pest Manag. Sci. 2011, 67, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Ge, C.; Liu, S.; Chen, C.; Zhou, M. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, T.; Zhang, Y.; Hou, Y.; Wang, J.; Zhou, M. FgFim, a key protein regulating resistance to the fungicide JS399-19, asexual and sexual development, stress responses and virulence in Fusarium graminearum. Mol. Plant Pathol. 2014, 15, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, F.; Zhuo, R.; Ma, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X. Expression of the laccase gene from a white rot fungus in Pichia pastoris can enhance the Resistance of this yeast to H2O2-mediated oxidative stress by stimulating the glutathione-based antioxidative system. Appl. Environ. Microbiol. 2012, 78, 5845–5854. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.L.; Nybakken, L.; Kauserud, H.; Ohlson, M. Fungal biomass associated with the phyllosphere of bryophytes and vascular plants. Mycol. Res. 2009, 113, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds thymol are available from the authors.






© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. https://doi.org/10.3390/molecules21060770
Gao T, Zhou H, Zhou W, Hu L, Chen J, Shi Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules. 2016; 21(6):770. https://doi.org/10.3390/molecules21060770
Chicago/Turabian StyleGao, Tao, Hao Zhou, Wei Zhou, Liangbin Hu, Jian Chen, and Zhiqi Shi. 2016. "The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis" Molecules 21, no. 6: 770. https://doi.org/10.3390/molecules21060770




