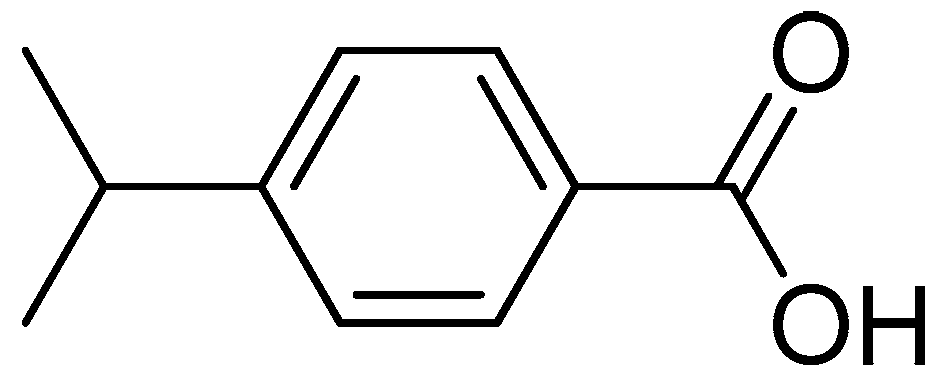
Antifungal Activity and Biochemical Response of Cuminic Acid against Phytophthora capsici Leonian
Abstract
:1. Introduction
2. Results
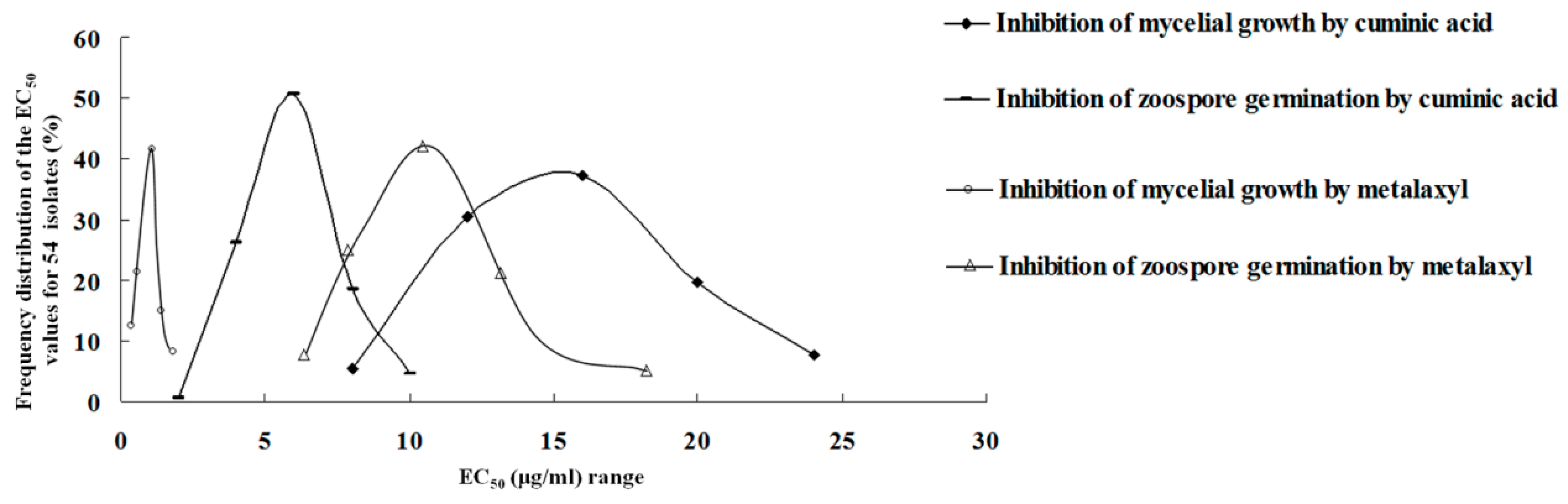
2.1. Sensitivity to Cuminic Acid and Metalaxyl
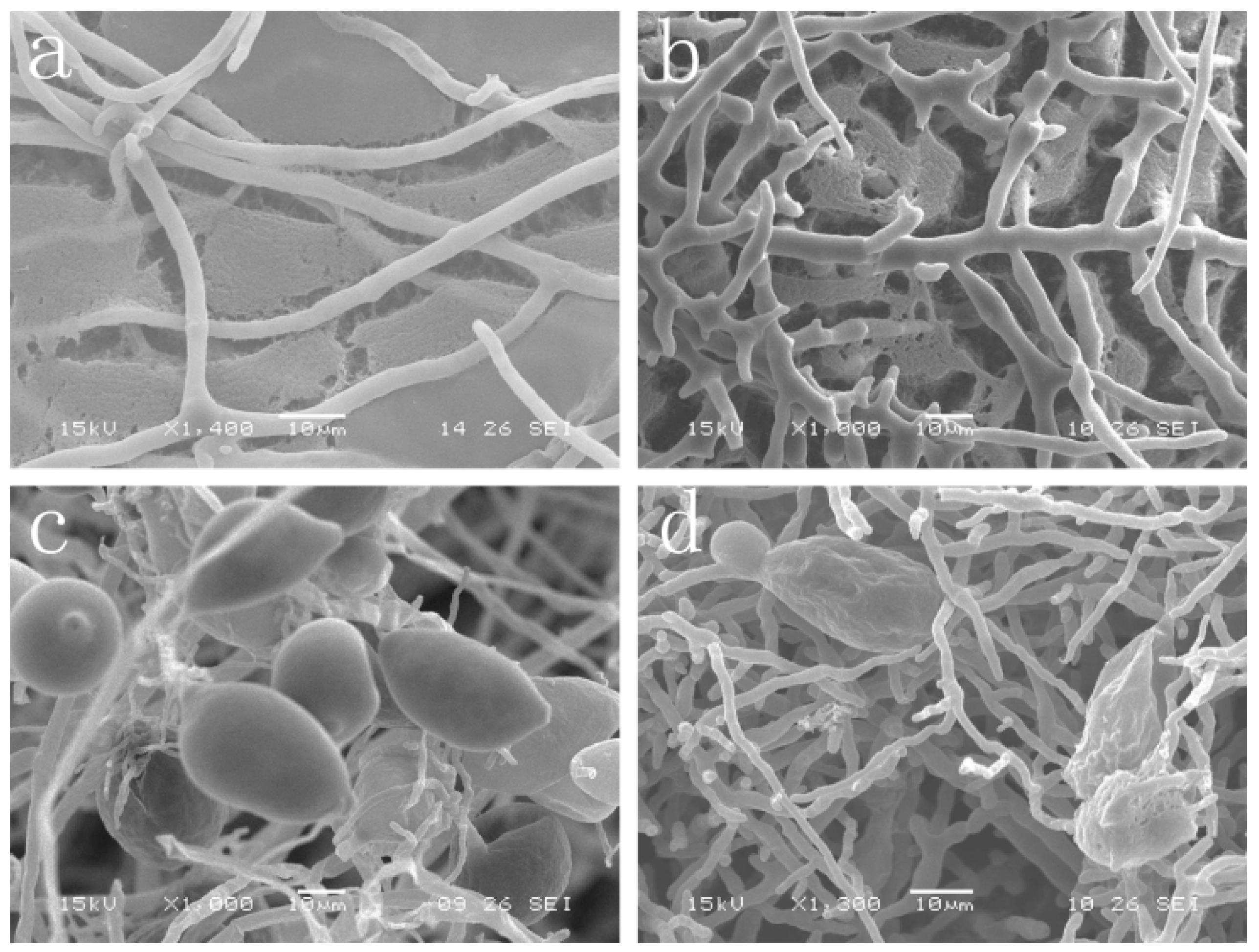
2.2. Effect of Cuminic Acid on Mycelial Morphology and Sporangium Formation
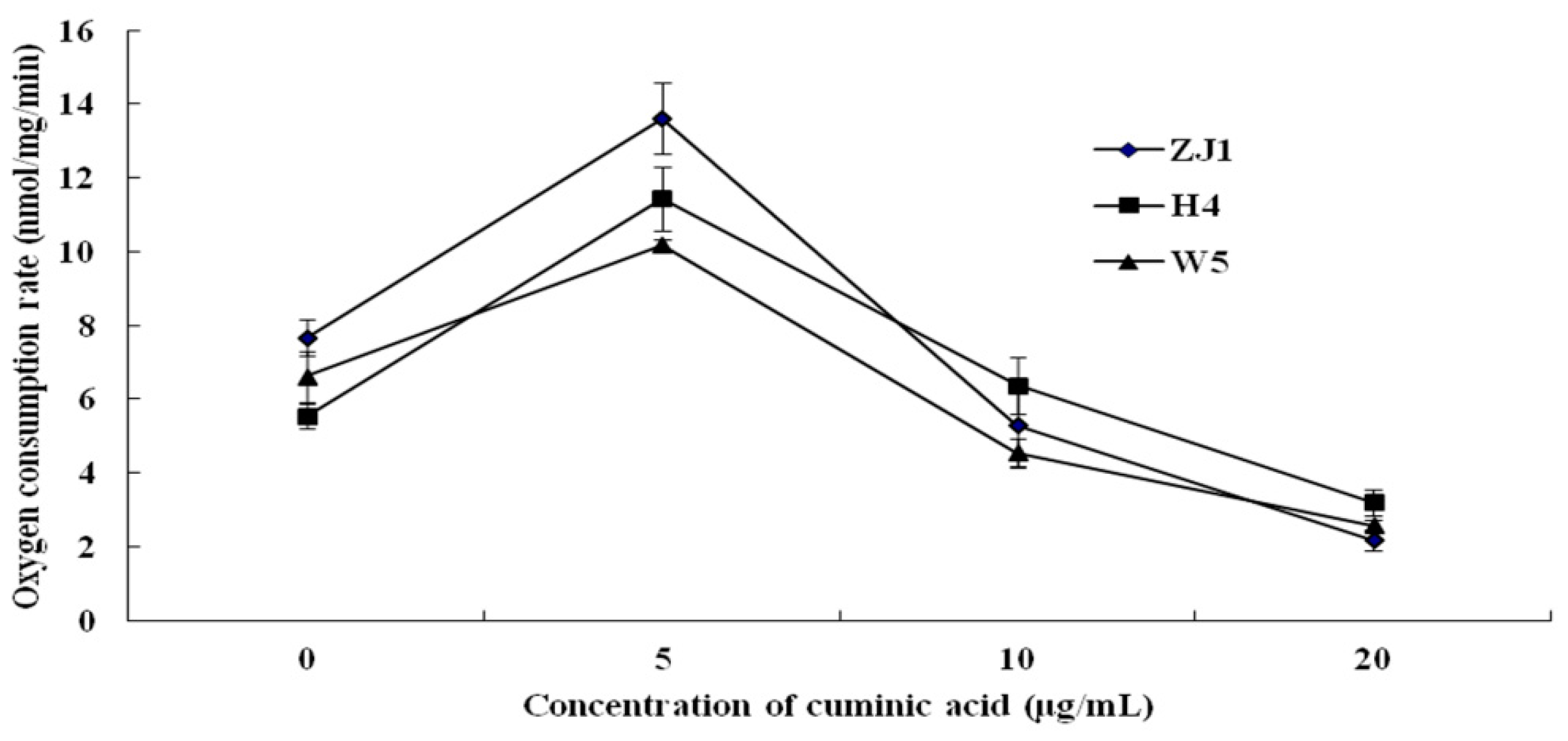
2.3. Mycelial Respiration
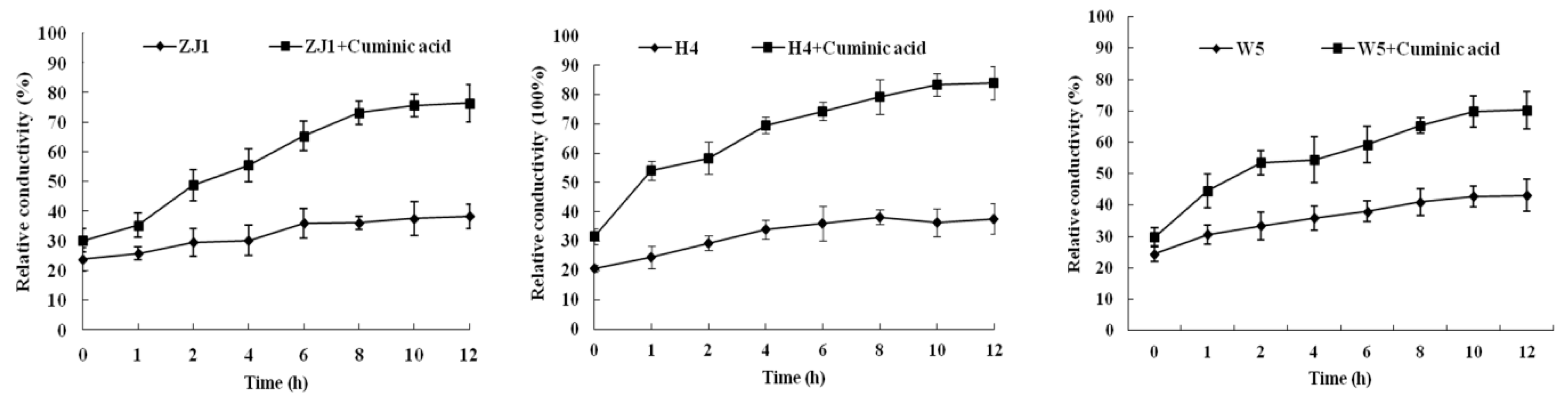
2.4. Cell Membrane Permeability
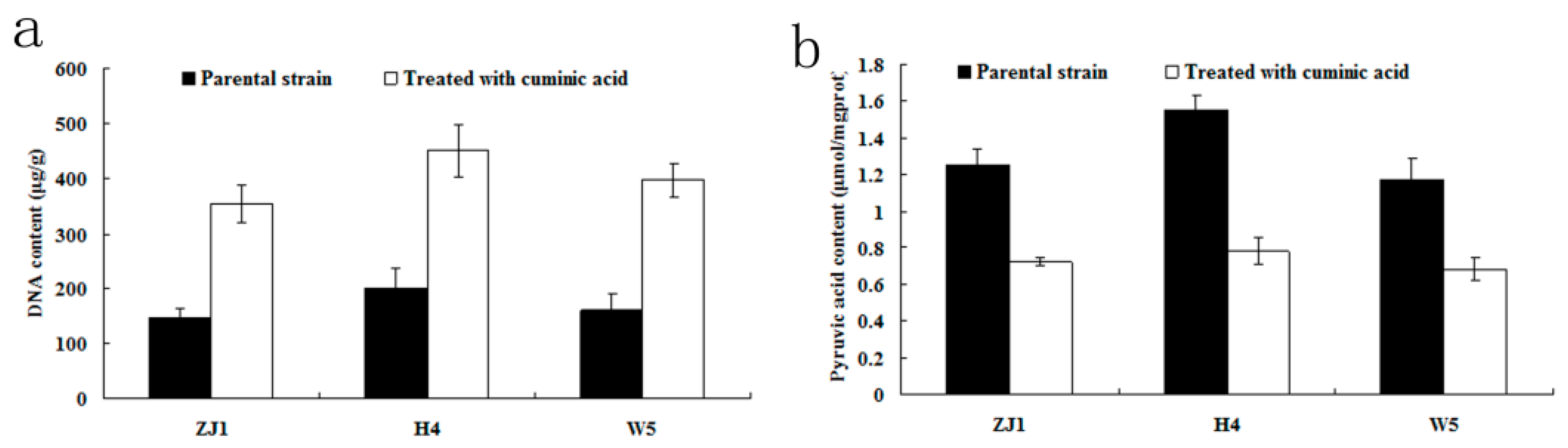
2.5. DNA and Pyruvic Acid Content
2.6. ATP Content and ATPase Activity
2.7. Protective and Curative Activity of Cuminic Acid
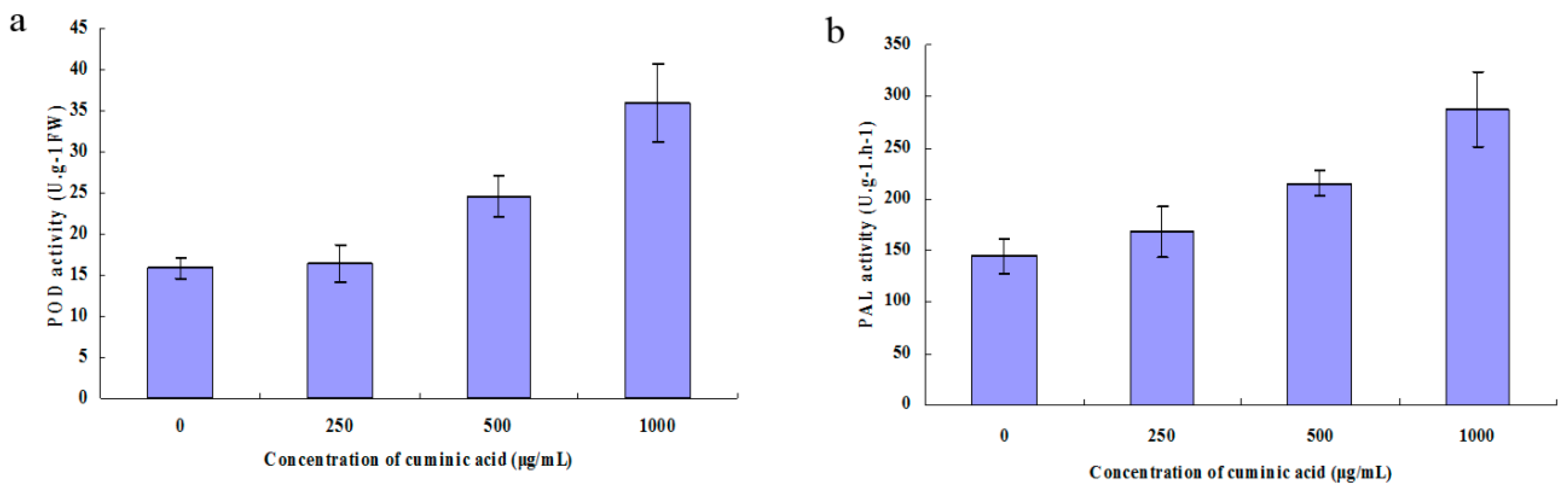
2.8. Peroxidase (POD) and Phenylalanine Ammonia-Lyase (PAL) Activity
3. Discussion
4. Materials and Methods
4.1. Media, Pathogen and Fungicides
4.2. Determination of Sensitivity of Mycelial Growth to Cuminic Acid
4.3. Determination of Sensitivity of Zoospore Germination to Cuminic Acid
4.4. Determination of Sensitivity of Mycelial Growth and Zoospore Germination to Metalaxyl
4.5. Effect of Cuminic Acid on Mycelial Morphology and Sporangium Formation of P. capsici
4.6. Effect of Cuminic Acid on Mycelial Respiration
4.7. Effect of Cuminic Acid on Cell Membrane Permeability
4.8. Pyruvic Acid and DNA Content of Mycelia
4.9. ATP Content and ATPase Activity
4.10. Protective and Curative Activity of Cuminic Acid in Pot Experiments
4.11. Peroxidase (POD) and Phenylalanine Ammonia-Lyase (PAL) Activity
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.S. Research progress in resistance of Phytophthora capsici Leonian to fungicide. Agrochem. Res. Appl. 2011, 15, 11–14. [Google Scholar]
- Huang, B.K.; Kim, C.H. Phytophthora blight of pepper and its control in Korea. Plant Dis. 1995, 79, 221–227. [Google Scholar]
- Ristaino, J.B.; Johnston, S.A. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 1999, 83, 1080–1089. [Google Scholar] [CrossRef]
- Hausbeck, M.K.; Lamour, K.H. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef]
- Babadoos, M. Outbreak of Phytophthora foliar blight and fruit rot in processing pumpkin fields in Illinois. Plant Dis. 2000, 84, 1345. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Disease Worldwide; American Phytopathological Society: St. Paul, MN, USA, 1996. [Google Scholar]
- Lee, B.K.; Kim, B.S.; Chang, S.W.; Hwang, B.K. Aggressiveness to pumpkin cultivars of isolates of Phytophthora capsici from pumpkin and bell pepper. Plant Dis. 2001, 85, 497–500. [Google Scholar] [CrossRef]
- Leonian, L.H. Stem and fruit blight of bell pepper caused by Phytophthora capsici. Phytopathology 1922, 12, 401–408. [Google Scholar]
- Tamietli, G.; Valentino, D. Physiological characterization of a population of Phytophthora capsici Lcon. from northern Italy. J. Plant Pathol. 2001, 62, 127–129. [Google Scholar]
- Black, L.L.; Green, S.K.; Hartman, G.L.; Poulos, J.M. Phytophthora blight. In Pepper Disease: A Field Guide; Asian Vegetable Research and Development Center: Taiwan, China, 1991; pp. 1–50. [Google Scholar]
- Jiang, Z.Q.; Guo, Y.H.; Li, S.M.; Qi, H.Y.; Guo, J.H. Evaluation of biocontrol efficiency of different Bacillus preparations and field application methods against Phytophthora blight of bell pepper. Biol. Control 2006, 36, 216–223. [Google Scholar] [CrossRef]
- Zitter, T.A. Phytophthora Blight of Cucurbits, Pepper, Tomato, and Eggplant; New York State Agricultural Experiment Station: Geneva, NY, USA, 1989. [Google Scholar]
- Ozgonen, H.; Erkilic, A. Growth enhancement and Phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. Crop Prot. 2007, 26, 1682–1688. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hausbeck, M.K. Investigating the spatiotemporal genetic structure of Phytophthora capsici in Michigan. Phytopathology 2001, 91, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Matheron, M.E.; Porchas, M. Impact of azoxystrobin, dimethomorph, fluazinam, fosetyl-Al, and metalaxyl on growth sporulation and zoospore cyst germination of three Phytophthora spp. Plant Dis. 2000, 84, 454–458. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hausbeck, M.K. Mefenoxam insensitivity and the sexual stage of Phytophthora capsici in Michigan cucurbit fields. Phytopathology 2000, 90, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.; Ristaino, J.B. Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Dis. 2001, 85, 1069–1075. [Google Scholar] [CrossRef]
- EPPO/OPPE. Solanaceous crops under protected cultivation. In Bulletin OPPE/EPPO Bulletin; European and Mediterranean Plant Protection Organisation, Wiley: Paris, France, 2004; Volume 34, pp. 65–67. [Google Scholar]
- Ma, Z.; Felts, D.; Michailides, T.J. Resistance to azoxystrobin in Alternaria isolates from pistachio in California. Pestic. Biochem. Phys. 2003, 77, 66–74. [Google Scholar] [CrossRef]
- Ishii, H.; Fraaije, B.A.; Sugiyama, T.; Noguchi, K.; Nishimura, K.; Takeda, T.; Amano, T.; Hollomon, D.W. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 2001, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Butters, J.A.; Coelho, J.M.; Jones, D.R.; Hollomon, D.W. Following the dynamics of strobilurin resistance in Blumeria graminis f.sp. Tritici using quantitative allele-specific real-time PCR measurements with the fluorescent dye SYBR Green I. Plant Pathol. 2002, 51, 45–54. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.B.; Zhou, M.G. Control of Sclerotinia sclerotiorum infection in oilseed rape with strobilurin fungicide SYP-7017. Can. J. Plant Pathol. 2014, 36, 354–359. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Hashinaga, F.; Nakatani, M. Antifungal activity of limonoids from Khaya ivorensis. Pest Manag. Sci. 2005, 61, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, M.; Saikia, B.; Mathur, R.K.; Goswami, B.N. 1,2-Dihydro-6α-acetoxyazadirone, a new antifungal meliacin from the fruit of Chisochton paniculatus. Phytochemistry 1993, 34, 583–584. [Google Scholar] [CrossRef]
- Yano, Y.; Satomi, M.; Oikawa, H. Antimicrobial effect of species and herbs on Vibrio parahaemolyticus. Int. J. Food Microbiol. 2006, 111, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Feng, J.T.; Zhang, X.; Zhang, Y.L. Isolation and Structure Detection of Fungicidal Components from Cuminum cyminum Seed. Chin. J. Pestic. Sci. 2007, 9, 330–334. [Google Scholar]
- Feng, J.T.; Han, L.R.; Fan, R.J.; Chen, C.Z.; Zhang, X. Effects of cuminic acid on the growth and development of Phytophthora capsici Leonian. Agric. Sci. China 2012, 45, 2628–2635. [Google Scholar]
- Hu, L.F.; Chen, C.Z.; Yi, X.H.; Feng, J.T.; Zhang, X. Inhibition of p-isopropyl Benzaldehyde and p-isopropyl Benzoic Acid extracted from Cuminum cyminum against Plant Pathogens. Acta Bot. Boreal. Occident. Sin. 2008, 28, 2349–2354. [Google Scholar]
- Zhang, J.; Wang, C.H.; Cheng, L.G.; Chen, H.; Shi, Z.Q. Inhibition activity of eugenol to Botrytis cinerea. Chin. J. Pestic. Sci. 2008, 10, 68–74. [Google Scholar]
- Gao, Z.B.; Yu, B.; Diao, H.; Yan, H.L.; Chen, D.W. The use of Benzoic acid in diets of swine and poultry. Chin. J. Anim. Nutr. 2014, 26, 1127–1133. [Google Scholar]
- Howard, H.T.H.; Clarke, A.H. A role for ATPase in the mechanism of ATP-dependent Ca and Phosphate deposition by isolated rachitic matrix vesicles. Int. J. Biochem. Cell Biol. 1995, 27, 1349–1356. [Google Scholar]
- Duniway, J.M. Role of physical factors in the development of Phytophthora diseases. In Phytophthora: Its Biology, Taxonomy, Ecology, and Pathology; Erwin, D.C., Bartnicki-Garcia, S., Tsao, P.H., Eds.; APS: St. Paul, MN, USA, 1983; pp. 175–187. [Google Scholar]
- Wang, S.W.; Gong, H.; Gao, A.; Zhao, T. Pharmaco toxicological study of preservative sodium benzoate. J. Anhui Agric. Sci. 2010, 38, 16724–16846. [Google Scholar]
- Donald, V.; Judith, G.V.; Charlotte, W.P. Fundamentals of Biochemistry, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 547–556. [Google Scholar]
- Stryer, L. Fatty acid metabolism. In Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 1995; pp. 603–628. [Google Scholar]
- Stryer, L. Citric acid cycle. In Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 1995; pp. 569–579, 614–616, 739–748, 770–773. [Google Scholar]
- Duan, Y.B.; Ge, C.Y.; Liu, S.M.; Feng, X.J.; Chen, C.J.; Zhou, M.G. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Phys. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Swait, T. Secondary compounds of protective agents. Annu. Rev. Plant Physiol. 1997, 28, 479–501. [Google Scholar]
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M.; Morsy, K.M. Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Prot. 2011, 30, 185–191. [Google Scholar] [CrossRef]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity of leaf extracts from South African trees against plant pathogens. Crop Prot. 2010, 29, 1529–1533. [Google Scholar] [CrossRef]
- Jin, H.; Geng, Y.C.; Yu, Z.Y.; Tao, K.; Hou, T.P. Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme L. Pestic. Biochem. Phys. 2009, 93, 133–137. [Google Scholar] [CrossRef]
- Akila, R.; Rajendran, L.; Harish, S.; Saveetha, K.; Raguchander, T.; Samiyappan, R. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biol. Control 2011, 57, 175–183. [Google Scholar] [CrossRef]
- Becker, W.F.; von Jagow, G.; Anke, T.; Steglich, W. Oudemansin, strobilurin A, strobilurin B and myxothiazol: New inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS Lett. 1981, 132, 329–333. [Google Scholar] [CrossRef]
- Fujimura, M. Mechanism of action of dicarboximide and phenylpyrrole on the stress-response signal transduction pathway. J. Pestic. Sci. 2010, 35, 351–353. [Google Scholar] [CrossRef]
- Sujkowski, L.S.; Parra, G.R.; Gumpertz, M.L.; Ristaino, J.B. Temporal dynamics of Phytophthora blight on bell pepper in relation to the mechanisms of dispersal of primary inoculum of Phytophthora capsici in soil. Phytopathology 2000, 90, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Hou, Y.P.; Wang, J.X.; Zhou, M.G. Sensitivity of Sclerotinia sclerotiorum to fludioxonil: In vitro determination of baseline sensitivity and resistance risk. Crop Prot. 2011, 30, 876–882. [Google Scholar] [CrossRef]
- Chen, X.R.; Xing, Y.P.; Li, Y.P.; Tong, Y.H.; Xu, J.R. RNA-Seq Reveals Infection-Related Gene Expression Changes in Phytophthora capsici. PLoS ONE 2013, 8, e74588. [Google Scholar] [CrossRef] [PubMed]
- Randall, T.A.; Dwyer, R.A.; Huitema, E.; Beyer, K.; Cvitanich, C. Large-scale gene discovery in the Oomycete Phytophthora infestans reveals likely components of phytopathogenicity shared with true fungi. Mol. Plant Microbe Interact. 2005, 18, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.S. Ultrastructure of Plant Pathogenic Fungi; China Science and Technology Press: Beijing, China, 1996. [Google Scholar]
- Wang, Y.; Duan, Y.B.; Zhou, M.G. Molecular and biochemical characterization of boscalid resistance in laboratory mutants of Sclerotinia sclerotiorum. Plant Pathol. 2015, 64, 101–108. [Google Scholar] [CrossRef]
- Duan, Y.B.; Liu, S.M.; Ge, C.Y.; Feng, X.J.; Chen, C.J.; Zhou, M.G. In vitro inhibition of Sclerotinia sclerotiorum by mixtures of azoxystrobin, SHAM, and thiram. Pestic. Biochem. Phys. 2012, 103, 101–107. [Google Scholar] [CrossRef]
- Bacon, J.R.; Moates, G.K.; Ng, A.; Rhodes, M.J.C.; Smith, A.C.; Waldron, K.W. Quantitative analysis of flavour precursors and pyruvate levels in different tissues and cultivars of onion (Allium cepa). Food Chem. 1999, 64, 257–261. [Google Scholar] [CrossRef]
- Doohan, F.M.; Parry, D.W.; Jenkinson, P.; Nicholson, P. The use of species-specific PCR-based assays to analyse Fusarium ear blight of wheat. Plant Pathol. 1998, 47, 197–205. [Google Scholar] [CrossRef]
- Shao, W.Y.; Zhang, Y.; Ren, W.C.; Chen, C.J. Physiological and biochemical characteristics of laboratory induced mutants of Botrytis cinerea with resistance to fluazinam. Pestic. Biochem. Phys. 2015, 117, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.X.; He, H.; Ruan, H.C.; Guan, X.; Hu, F.P. Biological control of pepper Phytophthora blight by endophytic TB2 (Bacillus sp.). Acta Phytopathol. Sin. 2004, 34, 173–179. [Google Scholar]
- Sample Availability: Samples of the compounds cuminic acid are available from the authors.


| Treatment | Protective Activity | Curative Activity | ||
|---|---|---|---|---|
| Disease Index | Control Efficacy (%) | Disease Index | Control Efficacy (%) | |
| Cuminic acid (250 μg/mL) | 62.78b a | 32.00c | 71.36b | 21.97d |
| Cuminic acid (500 μg/mL) | 49.25c | 46.65b | 51.54c | 43.64c |
| Cuminic acid (1000 μg/mL) | 26.87d | 70.89a | 30.28d | 66.89b |
| Metalaxyl (250 μg/mL) | 25.78d | 72.08a | 24.35e | 73.37a |
| Water control | 92.32a | - | 91.45a | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, Y.; Zhang, Y.; Zhang, X.; Feng, J. Antifungal Activity and Biochemical Response of Cuminic Acid against Phytophthora capsici Leonian. Molecules 2016, 21, 756. https://doi.org/10.3390/molecules21060756
Wang Y, Sun Y, Zhang Y, Zhang X, Feng J. Antifungal Activity and Biochemical Response of Cuminic Acid against Phytophthora capsici Leonian. Molecules. 2016; 21(6):756. https://doi.org/10.3390/molecules21060756
Chicago/Turabian StyleWang, Yong, Yang Sun, Ying Zhang, Xing Zhang, and Juntao Feng. 2016. "Antifungal Activity and Biochemical Response of Cuminic Acid against Phytophthora capsici Leonian" Molecules 21, no. 6: 756. https://doi.org/10.3390/molecules21060756





