Synthesis of C2-Symmetric Benzimidazolium Salts and Their Application in Palladium-Catalyzed Enantioselective Intramolecular α-Arylation of Amides
Abstract
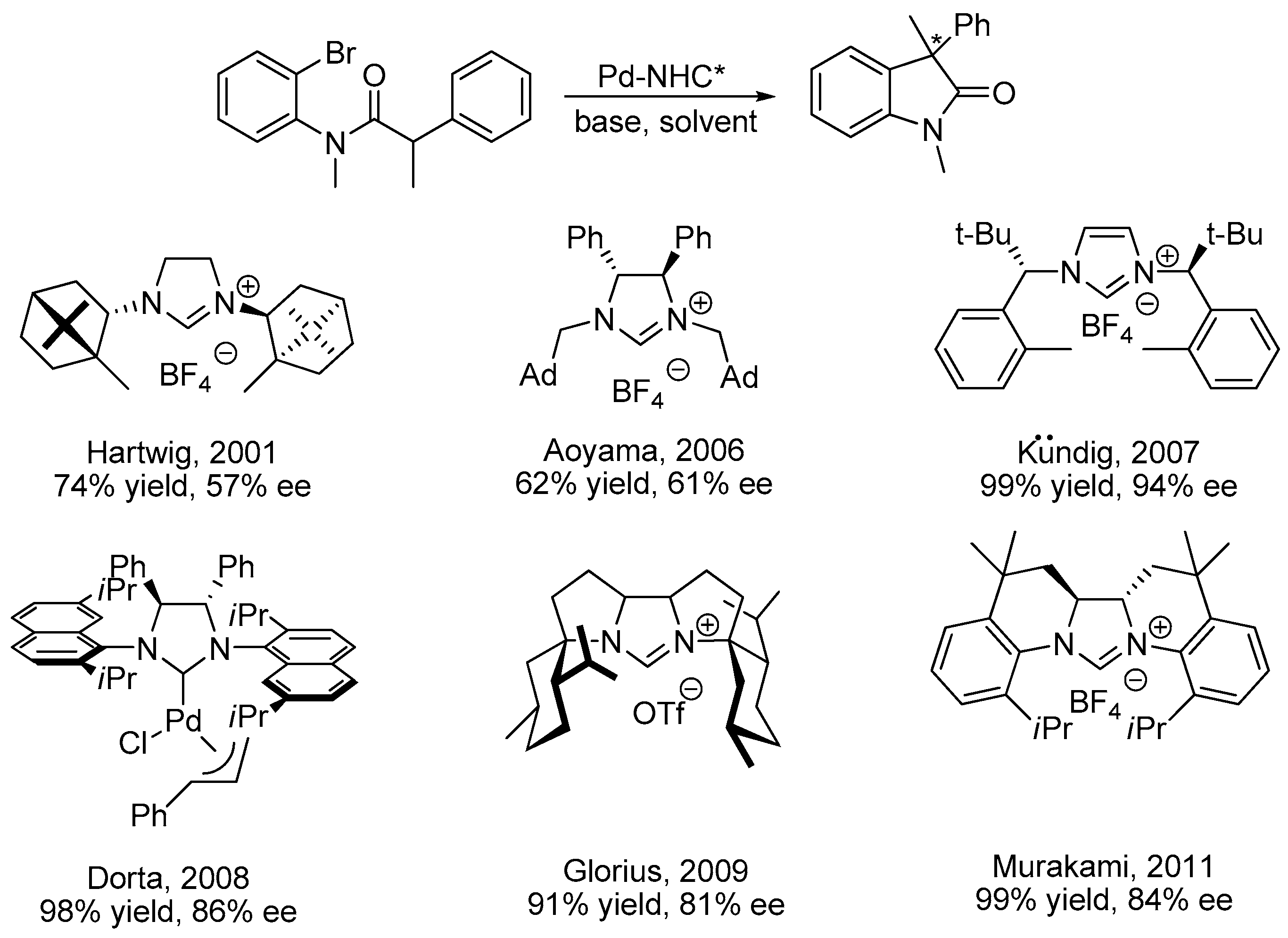
:1. Introduction
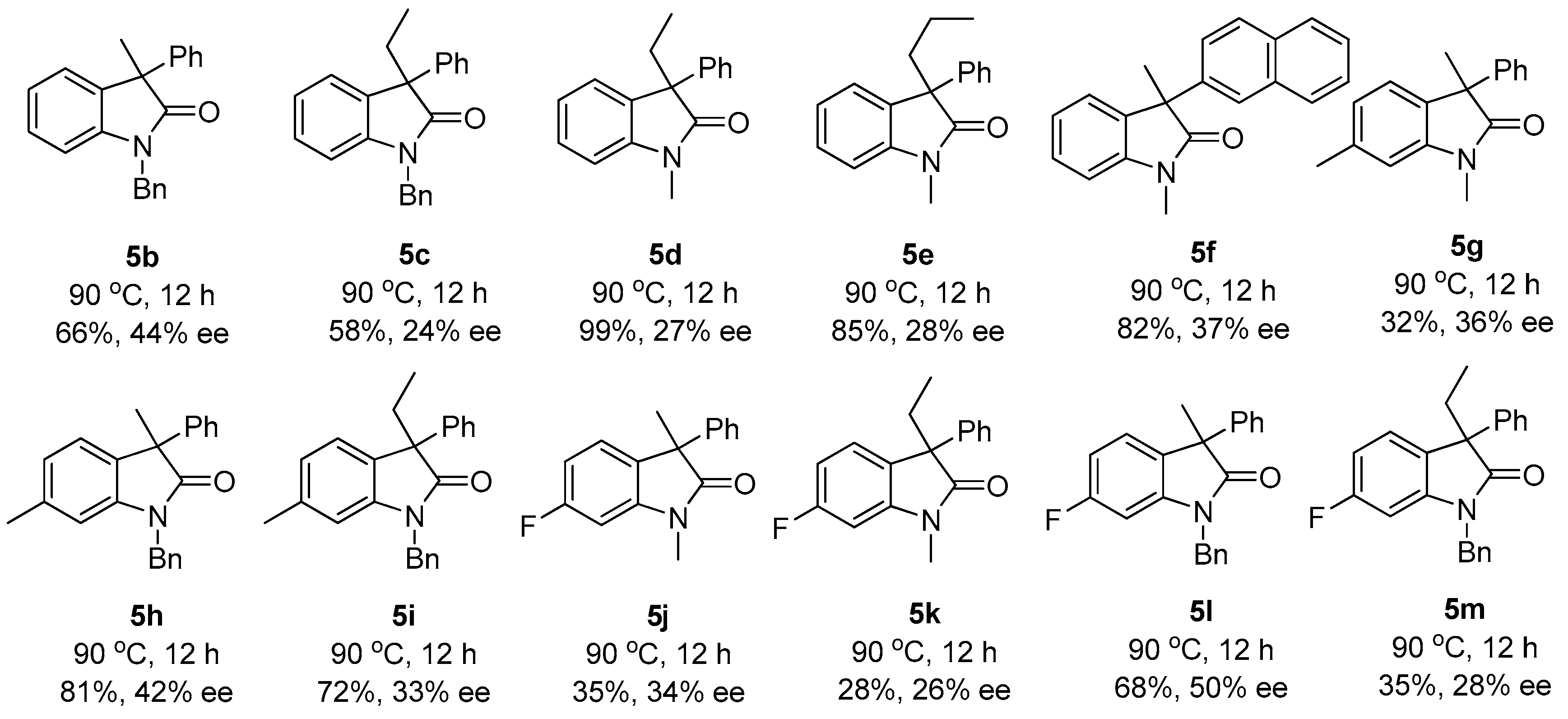
2. Results
3. Experimental Section
3.1. General
3.2. Procedure for the Synthesis of Compounds 1a–d
3.3. Procedure for the Synthesis of Benzimidazolium Salts 3a–d
3.4. Representative Procedure for the Pd-Catalyzed Intramolecular α-Arylation of Amides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jensen, B.S. BMS-204352: Apotassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2006, 8, 353–360. [Google Scholar]
- Danishefsky, S.J. Gelsemine: A thought-provoking target for total synthesis. Angew. Chem. Int. Ed. 2003, 42, 36–51. [Google Scholar]
- Marti, C.; Carreira, E.M. Construction of spiro[pyrrolidine-3,3′-oxindoles]-recent applications to the synthesis of oxindole alkaloids. Eur. J. Org. Chem. 2003, 2003, 2209–2219. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-Spirooxindole Natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar]
- Fensome, A.; Adams, W.R.; Adams, A.L.; Berrodin, T.J.; Cohen, J.; Huselton, C.; Illenberger, A.; Kern, J.C.; Hudak, V.A.; Marella, M.A.; et al. Design, synthesis, and SAR of new pyrrole-oxindole progesterone receptor modulators leading to 5-(7-fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile (WAY-255348). J. Med. Chem. 2008, 51, 1861–1873. [Google Scholar] [PubMed]
- Trost, B.M.; Brennan, M.K. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis 2009, 18, 3003–3025. [Google Scholar]
- Dounay, A.B.; Hatanaka, K.; Kodanko, J.J.; Oestreich, M.; Overman, L.E.; Pfeifer, L.A.; Weiss, M.M. Stability of thin-film solid-state electroluminescent devices based on tris(2,2′-bipyridine)ruthenium(II) complexes. J. Am. Chem. Soc. 2003, 125, 6261–6283. [Google Scholar] [CrossRef] [PubMed]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2963. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Overman, L.E. Total synthesis of complex cyclotryptamine alkaloids: Stereocontrolled construction of quaternary carbon stereocenters. Angew. Chem. Ind. Ed. 2007, 46, 5488–5508. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Frederiksen, M.U. Palladium-catalyzed asymmetric allylation of prochiral nucleophiles: Synthesis of 3-allyl-3-aryl oxindoles. Angew. Chem. Ind. Ed. 2005, 44, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Zhang, Y. Molybdenum-catalyzed asymmetric allylation of 3-alkyloxindoles: Application to the formal total synthesis of (−)-physostigmine. J. Am. Chem. Soc. 2006, 128, 4590–4591. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Zhang, Y. Mo-catalyzed regio-, diastereo-, and enantioselective allylic alkylation of 3-aryloxindoles. J. Am. Chem. Soc. 2007, 129, 14548–14549. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hartwig, J.F. Improved catalysts for the palladium-catalyzed synthesis of oxindoles by amide α-arylation. Rate acceleration, use of aryl chloride substrates, and a new carbene ligand for asymmetric transformations. J. Org. Chem. 2001, 66, 3402–3415. [Google Scholar] [CrossRef] [PubMed]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as chiral building blocks for imidazolium salts and N-heterocyclic carbene ligands. Chem. Commun. 2002, 2704–2705. [Google Scholar] [CrossRef]
- Arao, T.; Kondo, K.; Aoyama, T. Development of an N-heterocyclic carbene ligand based on concept of chiral mimetic. Tetrahedron Lett. 2006, 47, 1417–1420. [Google Scholar] [CrossRef]
- Arao, T.; Sato, K.; Kondo, K.; Aoyama, T. Function of an N-heterocyclic carbene ligand based on concept of chiral mimetic. Chem. Pharm. Bull. 2006, 54, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Kündig, E.P.; Seidel, T.M.; Jia, Y.X.; Bernardinelli, G. Bulky chiral carbene ligands and their application in the palladium-catalyzed asymmetric intramolecular α-arylation of amides. Angew. Chem. Ind. Ed. 2007, 46, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Hillgren, M.; Watson, E.L.; Marsden, S.P.; Kündig, E.P. Chiral N-heterocyclic carbene ligands for asymmetric catalytic oxindole synthesis. Chem. Commun. 2008, 4040–4042. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Katayev, D.; Bernardinelli, G.; Seidel, T.M.; Kündig, E.P. New chiral N-heterocyclic carbene ligands in palladium-catalyzed α-arylations of amides: Conformational locking through allylic strain as a device for stereocontrol. Chem. Eur. J. 2010, 16, 6300–6309. [Google Scholar] [CrossRef] [PubMed]
- Katayev, D.; Kündig, E.P. Catalytic enantioselective synthesis of a 3-aryl-3-benzyloxindole (=3-aryl-3-benzyl-1,3-dihydro-2H-indol-2-one) exhibiting antitumor activity. Helv. Chim. Acta 2012, 95, 2287–2295. [Google Scholar] [CrossRef]
- Katayev, D.; Jia, Y.X.; Sharma, A.K.; Banerjee, D.; Besnard, C.; Sunoj, R.B.; Kündig, E.P. Synthesis of 3,3-disubstituted oxindoles by palladium-catalyzed asymmetric intramolecular α-arylation of amides: Reaction development and mechanistic studies. Chem. Eur. J. 2013, 19, 11916–11927. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.J.; Mariz, R.; Robert, C.; Gatti, M.; Blumentritt, S.; Linden, A.; Dorta, R. Matching the chirality of monodentate N-Heterocyclic carbene ligands: A case study on well-defined palladium complexes for the asymmetric α-arylation of amides. Org. Lett. 2008, 10, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.J.; Wu, L.L.; Drinkel, E.; Mariz, R.; Gatti, M.; Dorta, R. Highly chemo- and enantioselective synthesis of 3-allyl-3-aryl oxindoles via the direct palladium-catalyzed α-arylation of amides. Org. Lett. 2010, 12, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Falivene, L.; Drinkel, E.; Grant, S.; Linden, A.; Cavallo, L.; Dorta, R. Synthesis of 3-fluoro-3-aryl oxindoles: Direct enantioselective α-arylation of amides. Angew. Chem. Ind. Ed. 2012, 51, 2870–2873. [Google Scholar] [CrossRef] [PubMed]
- Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. IBiox[(–)-menthyl]: A sterically demanding chiral NHC ligand. J. Am. Chem. Soc. 2009, 131, 8344–8345. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.T.; Ishida, N.; Ashida, S.; Murakami, M. Synthesis of chiral N-heterocyclic carbene ligands with rigid backbones and application to the palladium-catalyzed enantioselective intramolecular α-arylation of amides. Org. Lett. 2011, 13, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.M.; Riaz, U.; Giessert, A.; Smulik, J.A.; Diver, S.T. A versatile synthesis of substituted benzimidazolium salts by an amination/ring closure sequence. Org. Lett. 2001, 3, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3a–d are available from the authors.

| Entry a | Ligand | [Pd] | T (°C) | Solvent | Base | Yield (%) b | ee (%) c |
|---|---|---|---|---|---|---|---|
| 1 | 3a | Pd2(dba)3 | 90 | DME | NaOtBu | 51 | 14 |
| 2 | 3b | Pd2(dba)3 | 90 | DME | NaOtBu | 63 | 9 |
| 3 | 3c | Pd2(dba)3 | 90 | DME | NaOtBu | 98 | 40 |
| 4 | 3d | Pd2(dba)3 | 90 | DME | NaOtBu | 95 | 9 |
| 5 | 3c | Pd2(dba)3 | 90 | dioxane | NaOtBu | 98 | 25 |
| 6 | 3c | Pd2(dba)3 | 90 | toluene | NaOtBu | 98 | 17 |
| 7 | 3c | Pd2(dba)3 | 90 | THF | NaOtBu | 96 | 30 |
| 8 | 3c | Pd2(dba)3 | 90 | DME | KOtBu | 72 | 25 |
| 9 | 3c | Pd2(dba)3 | 90 | DME | LiOtBu | − | − |
| 10 | 3c | Pd2(dba)3 | 90 | DME | KOH | 99 | 38 |
| 11 | 3c | Pd2(dba)3 | 90 | DME | LiOH | − | − |
| 12 | 3c | Pd(OAc)2 | 90 | DME | NaOtBu | 99 | 37 |
| 13 | 3c | PdCl2 | 90 | DME | NaOtBu | 96 | 40 |
| 14 | 3c | [Pd(allyl)Cl]2 | 90 | DME | NaOtBu | 99 | 46 |
| 15 | 3c | Pd[P(C6H5)3]4 | 90 | DME | NaOtBu | 54 | 29 |
| 16 | 3c | [Pd(allyl)Cl]2 | 50 | DME | NaOtBu | 41 | 48 |
| 17 | 3c | [Pd(allyl)Cl]2 | rt | DME | NaOtBu | trace | − |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Zhao, W.; Zhou, B.; Liu, H.; Li, X.; Li, L.; Li, J.; Shi, J. Synthesis of C2-Symmetric Benzimidazolium Salts and Their Application in Palladium-Catalyzed Enantioselective Intramolecular α-Arylation of Amides. Molecules 2016, 21, 742. https://doi.org/10.3390/molecules21060742
He W, Zhao W, Zhou B, Liu H, Li X, Li L, Li J, Shi J. Synthesis of C2-Symmetric Benzimidazolium Salts and Their Application in Palladium-Catalyzed Enantioselective Intramolecular α-Arylation of Amides. Molecules. 2016; 21(6):742. https://doi.org/10.3390/molecules21060742
Chicago/Turabian StyleHe, Weiping, Wei Zhao, Bihui Zhou, Haifeng Liu, Xiangrong Li, Linlin Li, Jie Li, and Jianyou Shi. 2016. "Synthesis of C2-Symmetric Benzimidazolium Salts and Their Application in Palladium-Catalyzed Enantioselective Intramolecular α-Arylation of Amides" Molecules 21, no. 6: 742. https://doi.org/10.3390/molecules21060742






